Key Points
Invasive pneumococcal infection in children with SCD has declined significantly with PCV7/PCV13 but remains a life-threatening risk.
PPSV23 and new vaccines PCV15, PCV20, and PCV21 include 62%, 16%, 51%, and 92% of IPD serotypes not included in PCV13, respectively.
Abstract
Children with sickle cell disease (SCD) are at increased risk of invasive pneumococcal disease (IPD). Over 25 years, the Georgia Emerging Infections Program/Centers for Disease Control and Prevention Active Bacterial Core Surveillance network identified 104 IPD episodes among 3707 children with hemoglobin SS (HbSS) or HbSC aged <10 years, representing 6% of IPD in Black or African American children residing in Metropolitan Atlanta (reference population). Children with IPD and HbSS/SC were older than those with IPD in the reference population (P < .001). From 1994-1999 to 2010-2018, IPD declined by 87% in children with HbSS aged 0 to 4 years, and by 80% in those aged 5 to 9 years. However, IPD incidence rate ratios when comparing children with SCD with the reference population increased from 20.2 to 29.2 over these periods. Among children with HbSS and IPD, death declined from 14% to 3% after 2002, and meningitis declined from 16% to 8%. Penicillin resistance was more prevalent in children with SCD before 7-valent pneumococcal conjugate vaccine (PCV7) licensure. After 2010, all IPD serotypes were not included in the 13-valent PCV (PCV13). Within 3 years of vaccination, the effectiveness of the 23-valent pneumococcal polysaccharide vaccine (PPSV23) against non-PCV13 serotypes included in PPSV23 plus 15A/15C was 92% (95% confidence interval, 40.8- 99.0, P = .014; indirect-cohort effect adjusted for age and hydroxyurea). PPSV23 would cover 62% of non-PCV13 serotype IPD in children with SCD, whereas PCV15, PCV20, and PCV21/V116 (in development) could cover 16%, 51%, and 92%, respectively. Although less frequent, IPD remains a life-threatening risk in children with SCD. Effective vaccines with broader coverage could benefit these children.
Introduction
Sickle cell disease (SCD) is an inherited β-hemoglobinopathy that causes hemoglobin to polymerize and form rigid red blood cells and occlude the microvasculature.1 Children with SCD develop splenic dysfunction in infancy2,3 and, thus, are at risk of invasive bacterial infections including Streptococcus pneumoniae. Before the general adoption of empiric antibiotics for fever4 and penicillin prophylaxis,5 ∼1 in 10 children per year aged <5 years with hemoglobin SS (HbSS) developed invasive pneumococcal disease (IPD).4 IDP case fatality ranged from 12.5%6 to 25%7 or 27%,4 and in the Cooperative Study of Sickle Cell Disease, IPD accounted for 32% of all causes of death in individuals with SCD aged <20 years.8 Powars et al demonstrated that IPD-related deaths and meningitis were preventable by the rapid institution of parenteral antibiotic therapy in children with HbSS and fever.4 Systematic collection of blood cultures before the administration of antibiotics also increased the detection of bacteremia.4 The Prophylactic Penicillin Study (PROPS) in children with HbSS5 showed 85% reduction in IPD with penicillin prophylaxis compared with placebo,5 leading to the 1987 National Institutes of Health Consensus Conference statement recommending universal newborn screening for SCD and routine oral penicillin prophylaxis.9 In a subsequent trial (PROPS II), a 50% reduction in IPD incidence rate was noted in children with HbSS aged ≥5 years on penicillin prophylaxis. However, because the study was underpowered and did not reach statistical significance,10 routine use of penicillin prophylaxis in those aged ≥5 years was not recommended.11 After institution of penicillin prophylaxis, both adherence12 and the emergence of antibiotic resistance13 presented challenges to this IPD preventive strategy.
Children with SCD reportedly mount an adequate functional immune response after pneumococcal conjugated vaccination, as measured by opsonophagocytic assay.14-16 Within a single center, 3 years after the general licensure of the 7-valent pneumococcal conjugate vaccine (PCV7) in 2000, IPD rates decreased by 77% in children with SCD aged 0 to 10 years.17 However, by 2009, using US national surveillance data, IPD reduction in children with SCD aged <18 years was estimated at only 53%, compared with 74% in the general population.18 After PCV13 licensure in 2010, IPD incidence rates decreased further in the general population.19,20 Two additional PCVs were approved in 2021, PCV1521 and PCV20.22
Methods
Study population
The Georgia Emerging Infections Program23 identifies all IPD occurring in Metropolitan Atlanta since 1994 as part of a nationwide Centers for Disease Control and Prevention (CDC)–funded Active Bacterial Core Surveillance network. Initially the catchment area included 8 mostly urban counties (population of 3.1 million, with 34.5% Black or African American residents, per the 2000 census; thereafter a population of 4.2 million, with 42.7% Black or African American residents, per the 2020 census). Twelve additional counties were added after 1996 (population of 1 and then 1.7 million, with 10.9% and 19.4% Black or African Americans residents, per the 2000 and 2020 censuses, respectively).24 The reference population was defined as the total number of Black or African American children residing in counties included in the study by year and age, as provided by the Georgia Department of Public Health Online Analytical Statistical Information System.25
Population of children with SCD
Targeted newborn screening for hemoglobinopathies was mandated in Georgia in 197826,27 and had expanded to universal screening in 1998.26 This study used 2 databases of children with SCD aged 0 to 9 years seen once or more at any of the only 3 pediatric hospitals providing specialized care for children with hemoglobinopathies serving the region examined (Egleston, Scottish Rite, and Grady/Hughes Spalding Hospitals, merged since 1998 under Children’s Healthcare of Atlanta [CHOA]). The first database was used to support IPD surveillance from 1994 to 2002 and included clinical information starting in 1984, as previously described.13,17 The second data set (2002-2018) was derived from the SCD Clinical Database at CHOA, which, since 2004, has contributed data to statewide CDC-sponsored surveillance programs.28 Both data sets were merged with the Georgia Emerging Infections Program IPD surveillance database at the Atlanta Veteran Administration Medical Center.23 To assess whether the study cohort was representative of children with SCD in the region, data were compared to a cohort of newborn infants screened for SCD.28 Standard of care included penicillin prophylaxis29 and 23-valent pneumococcal polysaccharide vaccine (PPSV23) vaccination29 for the entire period, with the addition of PCV717,30 and PCV1331 after respective licensures (2000 and 2010, respectively), and hydroxyurea use.32,33
Vaccination data
The vaccination status of children with SCD and IPD was obtained from the state mandatory vaccination registry (Georgia Registry of Immunization Transactions and Services) and chart review.17,34 All serotypes not included in PCV13 were defined as non-PCV13 serotypes. Serotype 15BC indicates typing that could be either 15B or 15C. Vaccine-related serotype 6C was considered covered in PCV21/V116335; 15A covered in PPSV23335; and 15C covered in PPSV23,35,36 PCV20,37 and PCV21/V116.35
Laboratory data
Hemoglobinopathy diagnosis was previously determined by complete blood count, electrophoresis, or high-performance liquid chromatography separation as part of the databases of children with SCD,28 or established from laboratory records and chart review, as previously described.13,17 Children with HbSS and Sβ0 thalassemia were included in a single HbSS group and differentiated from children with HbSC. Consistent with previous publications,13,17 children with HbSβ+ thalassemia and those with rare SCD genotypes (eg, HbSD and Hb SO-Arab) were excluded. Invasive pneumococcal disease (IPD) is defined as S pneumoniae identified from a sterile, invasive body site. Antibiotic susceptibility and serotype testing were performed at the CDC.38 Oral penicillin minimal inhibitory concentration breakpoints were defined as follows: susceptible, ≤0.06 μg/mL; intermediate, 0.12 to 1 μg/mL; and resistant, ≥2 μg/mL.39 Serotype was ascertained by latex agglutination, Quellung reaction, polymerase chain reaction, or whole-genome sequencing.38
Pooled data
To compare serotype distribution and vaccination coverage of PPSV23, PCV15, PCV20, and PCV21 to other data sources, reports of IPD in children with SCD outside of Georgia were identified by PubMed, Google Scholar (terms sickl$ and pneumoc$ and/or bacter$ and/or infect$), crossreferences, or prior surveillance data.13,40 IPD with an unknown or indeterminate number of patients or serotypes were excluded. A study of nasopharyngeal carriage was examined separately.41
Statistical analysis
Aims
The principal aim of the study was to assess IPD incidence rate changes before and after the introduction of PCVs, by age group in children with HbSS or HbSC, and the reference population. Secondary aims included assessing changes in IPD penicillin susceptibility and serotype distribution before and after PCV licensure. The effectiveness of PPSV23 on non-PCV13 serotypes and the potential coverage by PPSV23, PCV15, PCV20, and PCV21 were evaluated.
Incidence rate, incidence rate ratios, and relative risk
The first and last dates of service were identified for each patient. Person-time for each patient was defined as the beginning of the study period or the date of the first health care visit. The last observation date was the last recorded visit date, or the end of the study period, whichever came first.17 If a patient moved or died, the last recorded visits counted as the date of censor. For the reference population, IPD incidence rates in Black or African American children were calculated by dividing the total number of IPD by the total number of children estimated living in the region examined for the same year, group of counties, and age. Data were grouped into 3 study periods: 1994-1999 (pre-PCV7 era), 2000-2009 (PCV7 era), and 2010-2018 (PCV13 era).
Age distribution differences were estimated by Wilcoxon signed-rank test; incidence rate and incidence rate ratios confidence intervals (CIs) were estimated by Poisson regression.42 Frequency distribution differences were estimated by relative risk, and the strength of association by Fisher exact test.
Indirect-cohort PPSV23 vaccine effect
PPSV23 vaccine effect for non-PCV13 serotypes was assessed by the indirect-cohort method.13,43 The indirect-cohort effect assumes that among individuals vaccinated with PPSV23, “a” is the number of IPD with serotypes included in PPSV23, and “b” is the number of IPD with serotypes not included in the vaccine. Among unvaccinated individuals, “c” is the number of IPD with serotypes included in PPSV23, and “d” is the number of IPD with serotypes not included in the vaccine. The effect is calculated as Ê = 1 − (ad/bc).43 This method allows estimation of PPSV23 effectiveness with data limited to individuals with IPD, without needing vaccination data from control individuals without infection.13,43,44 PPSV23 vaccine effect was assumed limited to 3 years after vaccination13,45 and estimated by logistic regression13 controlling for age and hydroxyurea use.
Strength of association was defined as significant at an α level <.001 and approaching significance at an α level <.050 to .001 (statistical package, SAS 9.4, Cary, NC). The Emory institutional review board reviewed and approved the study under data use agreements in place between Morehouse School of Medicine, the Atlanta Veteran Administration Medical Center, and CHOA.
Results
Population description
Between 1994 and 2018, 3707 children aged <10 years, 2628 with HbSS (71%) and 1079 with HbSC (29%), were seen at least once in ≥1 of the 3 pediatric hospitals serving the region examined, for a total of 16 144 person-years of observation, whereas the reference population represented 5 923 730 person-years of observation. Children with SCD in this study corresponded to 2.7 per 1000 person-years of observation among Black or African American children in the region during the period examined.
Approximately 2.9 of 1000 Black or African American infants born in the study region were diagnosed with HbSS/SC (2009-2019: 948 children born with HbSS 485 with HbSC, per the Georgia Sickle Cell Data Collection program of the CDC,28 unpublished, of 499 137 Black or African American newborn infants born in the region25). The calculated observation time represents 94.9% of the expected time for the entire birth cohort of children with HbSS/SC in the region. Among infants with SCD in GA, 97% were identified as African American or Black,46 and 89% in the 20 surveillance counties (2009-2019, the Georgia Sickle Cell Data Collection program,28 unpublished).
There were 104 cases of IPD in SCD (84% HbSS, 16% HbSC), representing 6% of the 1858 IPD cases in the reference population. Case distribution over time was 67 (64%) in the pre-PCV period (1994-1999), 20 (19%) in PCV7 period (2000-2009), and 17 (16%) in PCV13 period (2010-2018). Infections occurred in boys in 51%, 53%, and 56% of IPD in children with HbSS, HbSC, or the reference population, respectively. Overall children with SCD and IPD were older compared with the reference population (aged 5-9 years vs aged 0-4 years; relative risk, 2.9; 95% CI, 2.0-4.1; P < .001). Median patient age at IPD onset was as follows: HbSS, 3 years (interquartile range [IQR],1-5), HbSC, 2 years (IQR, 1-4), and reference population, 1 year (IQR, 0-2; reference population vs HbSS, P < .0001; vs HbSC, P = .0249). In the reference population, the number of children aged 5 to 9 years with IPD increased from 6% (66/1030) in the period 1994-1999 to 12% (101/828, P < .0001) in the period 2000-2018; in SCD, older age at IPD onset was 20% (10/50) between 1994 and 1999 and 31% (17/54, P = .2630) between 2000 and 2018.
Incidence rate and incidence rate ratios
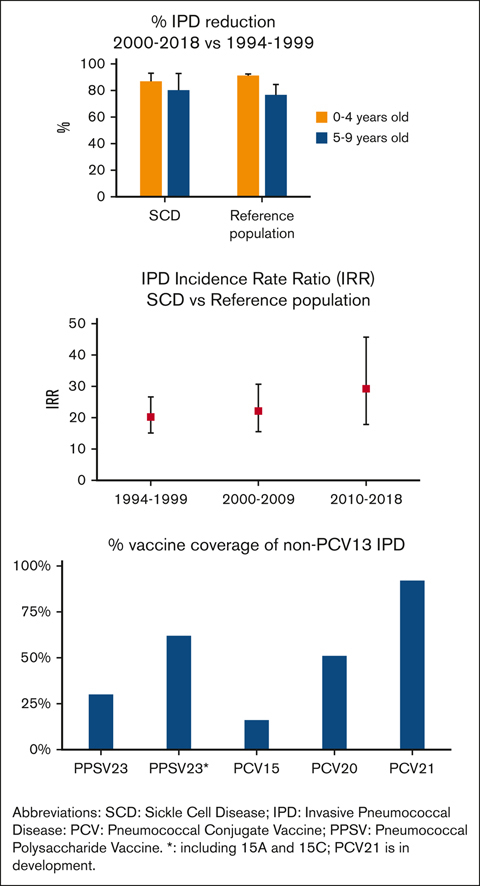
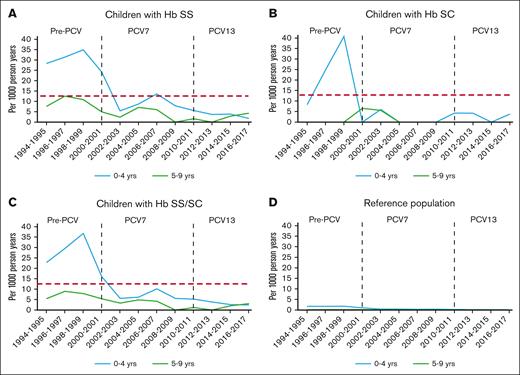
Table 1 shows IPD incidence rates for each era for children with HbSS or HbSC, and the reference population. Overall IPD incidence rates, meningitis, and death declined significantly in both age groups (aged 0-4 years and 5-9 years) of the SCD and reference populations after PCV7 (2000-2009) and PCV13 (2010-2018) licensure (Figure 1). Table 2 shows IPD incidence rate ratios for the PCV7 and the PCV13 eras, as compared with the pre-PCV era, for those with HbSS or HbSS/SC, and for reference populations, demonstrating substantial risk reductions overall for each complication (meningitis and death) in all populations, over time. However, when IPD incidence rates in children with SCD were compared with those in the reference population, IPD incidence rate ratios increased over time (Table 3).
IPD incidence rates in children with HbSS or SCD (HbSS/SC), or a reference population from Metropolitan Atlanta, GA
| Outcome/serotypes . | Population . | Age, y . | IPD/person-years . | IPD incidence rates (/1000 person-years) (95% CI) . | ||||
|---|---|---|---|---|---|---|---|---|
| Pre-PCV period, 1994-1999 . | PCV7 period, 2000-2009 . | PCV13 period, 2010-2018 . | Pre-PCV period, 1994-1999 . | PCV7 period, 2000-2009 . | PCV13 period, 2010-2018 . | |||
| All IPD | HbSS | 0-4 | 30/952 | 23/1 994 | 9/2 297 | 31.5 (22.0-45.1) | 11.5 (7.7-17.4) | 3.9 (2.0-7.5) |
| 5-9 | 10/949 | 9/2 312 | 6/2 867 | 10.5 (5.7-19.6) | 3.9 (2.0-7.5) | 2.1 (0.9-4.7) | ||
| 0-9 | 40/1 901 | 32/4 306 | 15/5 164 | 21.0 (15.4-28.7) | 7.4 (5.3-10.5) | 2.9 (1.8-4.8) | ||
| HbSC | 0-4 | 10/390 | 1/841 | 4/1 041 | 25.6 (13.8-47.7) | 1.2 (0.2-8.4) | 3.8 (1.4-10.2) | |
| 5-9 | 0/372 | 2/973 | 0/1 158 | - | 2.1 (0.5-8.2) | - | ||
| 0-9 | 10/761 | 3/1 814 | 4/2 198 | 13.1 (7.1-24.4) | 1.7 (0.5-5.1) | 1.8 (0.7-4.8) | ||
| HbSS/SC | 0-4 | 40/1 342 | 24/2 835 | 13/3 338 | 29.8 (21.9-40.6) | 8.5 (5.7-12.6) | 3.9 (2.3-6.7) | |
| 5-9 | 10/1 320 | 11/3 286 | 6/4 024 | 7.6 (4.1-14.1) | 3.3 (1.9-6.0) | 1.5 (0.7-3.3) | ||
| 0-9 | 50/2 662 | 35/6 120 | 19/7 362 | 18.8 (14.2-24.8) | 5.7 (4.1-8.0) | 2.6 (1.6-4.0) | ||
| Reference | 0-4 | 964/550 030 | 545/1 138 977 | 182/1 183 798 | 1.8 (1.6-1.9) | 0.5 (0.4-0.5) | 0.2 (0.1-0.2) | |
| 5-9 | 66/559 250 | 66/1 220 492 | 35/1 271 183 | 0.1 (0.1-0.2) | 0.1 (<.1-0.1) | <.1 (<.1 to <.1) | ||
| 0-9 | 1030/1 109 280 | 611/2 359 469 | 217/2 454 981 | 0.9 (0.9-1.0) | 0.3 (0.2-0.3) | 0.1 (0.1-0.1) | ||
| Meningitis | HbSS | 0-9 | 5/1 901 | 6/4 306 | 1/5 164 | 2.6 (1.1-6.3) | 1.4 (0.6-3.1) | 0.2 (<.1-1.4) |
| Reference | 0-9 | 32/1 109 280 | 34/2 359 469 | 17/2 454 981 | <.1 (<.1 to <.1) | <.1 (<.1 to <.1) | <.1 (<.1 to <.1) | |
| Death | HbSS | 0-9 | 6/1 901 | 1/4 306 | 1/5 164 | 3.2 (1.4-7.0) | 0.2 (<.1-1.6) | 0.2 (<.1-1.4) |
| Reference | 0-9 | 14/1 109 280 | 11/2 359 469 | 5/2 454 981 | <.1 (<.1 to <.1) | <.1 (<.1 to <.1) | <.1 (<.1 to <.1) | |
| PCV13∗ | HbSS/SC | 0-9 | 11/1 901 | 8/6 120 | 0/7 362 | 1.5 (0.7-2.7) | 1.1 (0.5-2.1) | - |
| Reference | 0-9 | 112/1 109 280 | 194/2 359 469 | 53/2 454 981 | <.1 (<.1-0.1) | 0.1 (0.1-0.1) | <.1 (<.1 to <.1) | |
| 6A | HbSS/SC | 0-9 | 11/1 901 | 1/6 120 | 0/7 362 | 1.5 (0.7-2.7) | 0.1 (<.1-0.8) | - |
| Reference | 0-9 | 71/1 109 280 | 36/2 359 469 | 9/2 454 981 | <.1 (<.1 to <.1) | <.1 (<.1 to <.1) | <.1 (<.1 to <.1) | |
| 19A | HbSS/SC | 0-9 | 0/1 901 | 7/6 120 | 0/7 362 | - | 1.0 (0.4-2.0) | - |
| Reference | 0-9 | 29/1 109 280 | 141/2 359 469 | 34/2 454 981 | <.1 (<.1-0.1) | 0.1 (0.0-0.1) | <.1 (<.1 to <.1) | |
| Non-PCV13 | HbSS/SC | 0-9 | 3/1 901 | 17/6 120 | 17/7 362 | 0.4 (0.1-1.2) | 2.3 (1.3-3.7) | 2.3 (1.3-3.7) |
| Reference | 0-9 | 39/1 109 280 | 136/2 359 469 | 121/2 454 981 | <.1 (<.1 to <.1) | 0.1 (<.1-0.1) | <.1 (<.1-0.1) | |
| 15B/15C | HbSS/SC | 0-9 | 2/1 901 | 3/6 120 | 4/7 362 | 0.3 (0.0-1.0) | 0.4 (0.1-1.2) | 0.5 (0.1-1.4) |
| Reference | 0-9 | 4/1 109 280 | 23/2 359 469 | 19/2 454 981 | <.1 (<.1 to <.1) | <.1 (<.1 to <.1) | <.1 (<.1 to <.1) | |
| Outcome/serotypes . | Population . | Age, y . | IPD/person-years . | IPD incidence rates (/1000 person-years) (95% CI) . | ||||
|---|---|---|---|---|---|---|---|---|
| Pre-PCV period, 1994-1999 . | PCV7 period, 2000-2009 . | PCV13 period, 2010-2018 . | Pre-PCV period, 1994-1999 . | PCV7 period, 2000-2009 . | PCV13 period, 2010-2018 . | |||
| All IPD | HbSS | 0-4 | 30/952 | 23/1 994 | 9/2 297 | 31.5 (22.0-45.1) | 11.5 (7.7-17.4) | 3.9 (2.0-7.5) |
| 5-9 | 10/949 | 9/2 312 | 6/2 867 | 10.5 (5.7-19.6) | 3.9 (2.0-7.5) | 2.1 (0.9-4.7) | ||
| 0-9 | 40/1 901 | 32/4 306 | 15/5 164 | 21.0 (15.4-28.7) | 7.4 (5.3-10.5) | 2.9 (1.8-4.8) | ||
| HbSC | 0-4 | 10/390 | 1/841 | 4/1 041 | 25.6 (13.8-47.7) | 1.2 (0.2-8.4) | 3.8 (1.4-10.2) | |
| 5-9 | 0/372 | 2/973 | 0/1 158 | - | 2.1 (0.5-8.2) | - | ||
| 0-9 | 10/761 | 3/1 814 | 4/2 198 | 13.1 (7.1-24.4) | 1.7 (0.5-5.1) | 1.8 (0.7-4.8) | ||
| HbSS/SC | 0-4 | 40/1 342 | 24/2 835 | 13/3 338 | 29.8 (21.9-40.6) | 8.5 (5.7-12.6) | 3.9 (2.3-6.7) | |
| 5-9 | 10/1 320 | 11/3 286 | 6/4 024 | 7.6 (4.1-14.1) | 3.3 (1.9-6.0) | 1.5 (0.7-3.3) | ||
| 0-9 | 50/2 662 | 35/6 120 | 19/7 362 | 18.8 (14.2-24.8) | 5.7 (4.1-8.0) | 2.6 (1.6-4.0) | ||
| Reference | 0-4 | 964/550 030 | 545/1 138 977 | 182/1 183 798 | 1.8 (1.6-1.9) | 0.5 (0.4-0.5) | 0.2 (0.1-0.2) | |
| 5-9 | 66/559 250 | 66/1 220 492 | 35/1 271 183 | 0.1 (0.1-0.2) | 0.1 (<.1-0.1) | <.1 (<.1 to <.1) | ||
| 0-9 | 1030/1 109 280 | 611/2 359 469 | 217/2 454 981 | 0.9 (0.9-1.0) | 0.3 (0.2-0.3) | 0.1 (0.1-0.1) | ||
| Meningitis | HbSS | 0-9 | 5/1 901 | 6/4 306 | 1/5 164 | 2.6 (1.1-6.3) | 1.4 (0.6-3.1) | 0.2 (<.1-1.4) |
| Reference | 0-9 | 32/1 109 280 | 34/2 359 469 | 17/2 454 981 | <.1 (<.1 to <.1) | <.1 (<.1 to <.1) | <.1 (<.1 to <.1) | |
| Death | HbSS | 0-9 | 6/1 901 | 1/4 306 | 1/5 164 | 3.2 (1.4-7.0) | 0.2 (<.1-1.6) | 0.2 (<.1-1.4) |
| Reference | 0-9 | 14/1 109 280 | 11/2 359 469 | 5/2 454 981 | <.1 (<.1 to <.1) | <.1 (<.1 to <.1) | <.1 (<.1 to <.1) | |
| PCV13∗ | HbSS/SC | 0-9 | 11/1 901 | 8/6 120 | 0/7 362 | 1.5 (0.7-2.7) | 1.1 (0.5-2.1) | - |
| Reference | 0-9 | 112/1 109 280 | 194/2 359 469 | 53/2 454 981 | <.1 (<.1-0.1) | 0.1 (0.1-0.1) | <.1 (<.1 to <.1) | |
| 6A | HbSS/SC | 0-9 | 11/1 901 | 1/6 120 | 0/7 362 | 1.5 (0.7-2.7) | 0.1 (<.1-0.8) | - |
| Reference | 0-9 | 71/1 109 280 | 36/2 359 469 | 9/2 454 981 | <.1 (<.1 to <.1) | <.1 (<.1 to <.1) | <.1 (<.1 to <.1) | |
| 19A | HbSS/SC | 0-9 | 0/1 901 | 7/6 120 | 0/7 362 | - | 1.0 (0.4-2.0) | - |
| Reference | 0-9 | 29/1 109 280 | 141/2 359 469 | 34/2 454 981 | <.1 (<.1-0.1) | 0.1 (0.0-0.1) | <.1 (<.1 to <.1) | |
| Non-PCV13 | HbSS/SC | 0-9 | 3/1 901 | 17/6 120 | 17/7 362 | 0.4 (0.1-1.2) | 2.3 (1.3-3.7) | 2.3 (1.3-3.7) |
| Reference | 0-9 | 39/1 109 280 | 136/2 359 469 | 121/2 454 981 | <.1 (<.1 to <.1) | 0.1 (<.1-0.1) | <.1 (<.1-0.1) | |
| 15B/15C | HbSS/SC | 0-9 | 2/1 901 | 3/6 120 | 4/7 362 | 0.3 (0.0-1.0) | 0.4 (0.1-1.2) | 0.5 (0.1-1.4) |
| Reference | 0-9 | 4/1 109 280 | 23/2 359 469 | 19/2 454 981 | <.1 (<.1 to <.1) | <.1 (<.1 to <.1) | <.1 (<.1 to <.1) | |
IPD trends (A-D). IPD in children with SCD Hb type and reference population, by age group. Red dashed line: IPD incidence rate level in children aged ≥5 years with HbSS before PCV licensure, an age group that was not recommended to take penicillin prophylaxis.9,10
IPD incidence rates ratio as % change in children with HbSS, SCD (HbSS/SC), or reference population, comparing periods
| Outcome/serotypes . | Population . | Age, y . | IPD incidence rate ratio as % change∗ (95% CI), P value . | ||
|---|---|---|---|---|---|
| PCV7 vs pre-PCV period . | PCV13 vs PCV7 period . | PCV13 vs pre-PCV period . | |||
| 2000-2009 vs 1994-1999 . | 2010-2018 vs 2000-2009 . | 2010-2018 vs 1994-1999 . | |||
| All IPD | HbSS | 0-4 | 63.4 (37.0-78.7), <.001 | 66.0 (26.6-84.3), .006 | 87.6 (73.8-94.1), <.001 |
| 5-9 | 63.1 (9.1-85.0), .030 | 46.2 (−33.8 to 80.9), .239 | 80.1 (45.4-92.8), .002 | ||
| 0-9 | 64.7 (43.8-77.8), <.001 | 60.9 (27.8-78.8), .003 | 86.2 (75.0-92.4), <.001 | ||
| HbSC | 0-4 | 95.4 (63.8-99.4), .003 | −69.1 (−96.5 to 63.9), .294 | 85.0 (52.2-95.3), .001 | |
| 0-9 | 87.4 (54.3-96.5), .002 | −9.1 (−79.7 to 75.4), .900 | 86.2 (55.8-95.7), <.001 | ||
| HbSS/SC | 0-4 | 71.6 (52.9-82.9), <.001 | 54.0 (9.7-76.6), .024 | 86.9 (75.6-93.0), <.001 | |
| 5-9 | 55.8 (−3.9 to 81.2), .062 | 55.5 (−17.0 to 83.5), .111 | 80.3 (45.8-92.8), .002 | ||
| 0-9 | 69.6 (53.1-80.2), <.001 | 54.9 (21.1-74.2), .005 | 86.3 (76.7-91.9), <.001 | ||
| Reference | 0-4 | 72.7 (69.7-75.4), <.001 | 67.9 (62.0-72.8), <.001 | 91.2 (89.7-92.5), <.001 | |
| 5-9 | 54.2 (35.5-67.4), <.001 | 49.1 (23.3-66.2), .001 | 76.7 (64.9-84.5), <.001 | ||
| 0-9 | 72.1 (69.2-74.8), <.001 | 65.9 (60.1-70.8), <.001 | 90.5 (89.0-91.8), <.001 | ||
| Meningitis | HbSS | 0-9 | 47.0 (−42.4 to 83.8), .294 | 86.1 (−13.4 to 98.3), .068 | 92.6 (37.0-99.1), .017 |
| Reference | 0-9 | 50.0 (19.1-69.2), .005 | 51.9 (14.0-73.2), .014 | 76.0 (56.8-86.7), <.001 | |
| Death | HbSS | 0-9 | 92.6 (38.9-99.1), .016 | 16.6 (−92.5 to 94.8), .898 | 93.9 (49.0-99.3), .010 |
| Reference | 0-9 | 63.1 (18.6-83.2), .013 | 56.3 (−20.5 to 84.8), .125 | 83.9 (55.2-94.2), <.001 | |
| PCV13∗ | HbSS/SC | 0-9 | 68.4 (21.4-87.3), .013 | 100.0 (62.2 to 100.0), ∗∗0.004 | 100.0 (91.9 to 100.0), ∗∗<.001 |
| Reference | 0-9 | 18.6 (−2.7 to 35.5), .084 | 73.7 (64.4-80.6), <.001 | 78.6 (70.4-84.6), <.001 | |
| Non-PCV13 | HbSS/SC | 0-9 | −59.4 (−88.1 to 27.8), .150 | 16.9 (−38.6 to 57.6), .590 | −51.2 (−85.7 to 40.0), .252 |
| Reference | 0-9 | −39.0 (−57.3 to 12.9), .006 | 14.5 (−8.5 to 33.1), .210 | −28.7 (−50.3 to 2.3), .067 | |
| 15B/15C | HbSS/SC | 0-9 | 34.8 (−74.4 to 89.1), .640 | −9.8 (−79.8 to 75.2), .893 | 27.7 (−74.7 to 86.8), .708 |
| Reference | 0-9 | −63.0 (−87.2 to 6.5), .066 | 20.6 (−31.4 to 56.8), .457 | −53.4 (−84.1 to 27.0), .165 | |
| Outcome/serotypes . | Population . | Age, y . | IPD incidence rate ratio as % change∗ (95% CI), P value . | ||
|---|---|---|---|---|---|
| PCV7 vs pre-PCV period . | PCV13 vs PCV7 period . | PCV13 vs pre-PCV period . | |||
| 2000-2009 vs 1994-1999 . | 2010-2018 vs 2000-2009 . | 2010-2018 vs 1994-1999 . | |||
| All IPD | HbSS | 0-4 | 63.4 (37.0-78.7), <.001 | 66.0 (26.6-84.3), .006 | 87.6 (73.8-94.1), <.001 |
| 5-9 | 63.1 (9.1-85.0), .030 | 46.2 (−33.8 to 80.9), .239 | 80.1 (45.4-92.8), .002 | ||
| 0-9 | 64.7 (43.8-77.8), <.001 | 60.9 (27.8-78.8), .003 | 86.2 (75.0-92.4), <.001 | ||
| HbSC | 0-4 | 95.4 (63.8-99.4), .003 | −69.1 (−96.5 to 63.9), .294 | 85.0 (52.2-95.3), .001 | |
| 0-9 | 87.4 (54.3-96.5), .002 | −9.1 (−79.7 to 75.4), .900 | 86.2 (55.8-95.7), <.001 | ||
| HbSS/SC | 0-4 | 71.6 (52.9-82.9), <.001 | 54.0 (9.7-76.6), .024 | 86.9 (75.6-93.0), <.001 | |
| 5-9 | 55.8 (−3.9 to 81.2), .062 | 55.5 (−17.0 to 83.5), .111 | 80.3 (45.8-92.8), .002 | ||
| 0-9 | 69.6 (53.1-80.2), <.001 | 54.9 (21.1-74.2), .005 | 86.3 (76.7-91.9), <.001 | ||
| Reference | 0-4 | 72.7 (69.7-75.4), <.001 | 67.9 (62.0-72.8), <.001 | 91.2 (89.7-92.5), <.001 | |
| 5-9 | 54.2 (35.5-67.4), <.001 | 49.1 (23.3-66.2), .001 | 76.7 (64.9-84.5), <.001 | ||
| 0-9 | 72.1 (69.2-74.8), <.001 | 65.9 (60.1-70.8), <.001 | 90.5 (89.0-91.8), <.001 | ||
| Meningitis | HbSS | 0-9 | 47.0 (−42.4 to 83.8), .294 | 86.1 (−13.4 to 98.3), .068 | 92.6 (37.0-99.1), .017 |
| Reference | 0-9 | 50.0 (19.1-69.2), .005 | 51.9 (14.0-73.2), .014 | 76.0 (56.8-86.7), <.001 | |
| Death | HbSS | 0-9 | 92.6 (38.9-99.1), .016 | 16.6 (−92.5 to 94.8), .898 | 93.9 (49.0-99.3), .010 |
| Reference | 0-9 | 63.1 (18.6-83.2), .013 | 56.3 (−20.5 to 84.8), .125 | 83.9 (55.2-94.2), <.001 | |
| PCV13∗ | HbSS/SC | 0-9 | 68.4 (21.4-87.3), .013 | 100.0 (62.2 to 100.0), ∗∗0.004 | 100.0 (91.9 to 100.0), ∗∗<.001 |
| Reference | 0-9 | 18.6 (−2.7 to 35.5), .084 | 73.7 (64.4-80.6), <.001 | 78.6 (70.4-84.6), <.001 | |
| Non-PCV13 | HbSS/SC | 0-9 | −59.4 (−88.1 to 27.8), .150 | 16.9 (−38.6 to 57.6), .590 | −51.2 (−85.7 to 40.0), .252 |
| Reference | 0-9 | −39.0 (−57.3 to 12.9), .006 | 14.5 (−8.5 to 33.1), .210 | −28.7 (−50.3 to 2.3), .067 | |
| 15B/15C | HbSS/SC | 0-9 | 34.8 (−74.4 to 89.1), .640 | −9.8 (−79.8 to 75.2), .893 | 27.7 (−74.7 to 86.8), .708 |
| Reference | 0-9 | −63.0 (−87.2 to 6.5), .066 | 20.6 (−31.4 to 56.8), .457 | −53.4 (−84.1 to 27.0), .165 | |
For incidence rate ratio (IRR) of <1: % change = (1 − IRR) × 100; for IRR of ≥1: % change = (1 − 1/IRR) × 100.
Non-PCV7 serotypes.
Conditional maximum likelihood estimate of Rate Ratio; https://www.openepi.com
IPD incidence rates ratio in children aged 0 to 9 years with HbSS or HbSC compared with the reference population
| Outcome . | Population . | IPD incidence rate ratio, SCD vs reference population, (95% CI), P value . | |||
|---|---|---|---|---|---|
| Pre-PCV period, 1994-1999 . | PCV7 period, 2000-2009 . | PCV13 period, 2010-2018 . | Overall period, 1994-2018 . | ||
| All IPD | HbSS | 22.7 (16.3-30.8), <.001 | 28.7 (19.8-40.4), <.001 | 32.9 (18.8-54.1), <.001 | 24.4 (19.6-30.1), <.001 |
| HbSC | 14.2 (7.6-26.4), <.001 | 6.4 (2.1-19.9), .001 | 20.6 (7.7-55.4), <.001 | 11.4 (7.0-18.3), <.001 | |
| HbSS/SC | 20.2 (15.1-26.6), <.001 | 22.1 (15.5-30.7), <.001 | 29.2 (17.8-45.7), <.001 | 20.5 (16.9-25.0), <.001 | |
| Meningitis | HbSS | 73.0 (22.0-191.0), <.001 | 96.7 (36.9-219.6), <.001 | 28.0 (1.3-154.1), .038 | 69.0 (35.1-125.6), <.001 |
| Death | HbSS | 250.1 (88.4-639.4), <.001 | 49.8 (2.3-291.8), .022 | 95.1 (4.0-686.7), .013 | 138.9 (59.7-294.2), <.001 |
| Outcome . | Population . | IPD incidence rate ratio, SCD vs reference population, (95% CI), P value . | |||
|---|---|---|---|---|---|
| Pre-PCV period, 1994-1999 . | PCV7 period, 2000-2009 . | PCV13 period, 2010-2018 . | Overall period, 1994-2018 . | ||
| All IPD | HbSS | 22.7 (16.3-30.8), <.001 | 28.7 (19.8-40.4), <.001 | 32.9 (18.8-54.1), <.001 | 24.4 (19.6-30.1), <.001 |
| HbSC | 14.2 (7.6-26.4), <.001 | 6.4 (2.1-19.9), .001 | 20.6 (7.7-55.4), <.001 | 11.4 (7.0-18.3), <.001 | |
| HbSS/SC | 20.2 (15.1-26.6), <.001 | 22.1 (15.5-30.7), <.001 | 29.2 (17.8-45.7), <.001 | 20.5 (16.9-25.0), <.001 | |
| Meningitis | HbSS | 73.0 (22.0-191.0), <.001 | 96.7 (36.9-219.6), <.001 | 28.0 (1.3-154.1), .038 | 69.0 (35.1-125.6), <.001 |
| Death | HbSS | 250.1 (88.4-639.4), <.001 | 49.8 (2.3-291.8), .022 | 95.1 (4.0-686.7), .013 | 138.9 (59.7-294.2), <.001 |
Clinical findings
Among 8 deaths attributed to IPD in children with SCD, all occurred in children with HbSS. Mortality of IPD in HbSS was 9% (8/87): 8% (5/62) of children aged 0 to 4 years and 12% (3/25) of those aged 5 to 9 years. Case fatality before 2002 was 14% (7/50), and 3% (1/37) thereafter (P = .1306). Among children with HbSS and IPD, meningitis occurred in 12 (14%) overall, 15% (9/62) in those aged 0 to 4 years and 12% (3/25) in those aged 5 to 9 years, with 9 cases (18%) and 4 (11%) before and after 2002, respectively (P = .5446). One child aged 0 to 4 years with HbSC developed meningitis. In the reference population case fatality before and after 2002 was 1.4% and 2.0% (P = .3312), respectively, and morbidity from meningitis increased (3.6% vs 6.4%, P = .0078, refer to supplemental Data).
After 2002, when PCV7 was generally introduced, clinical information was available in 93% (39/42) of IPD episodes. Antibiotic prophylaxis was prescribed in 77% (20/26) of children aged 0 to 4 years, and in 38% (5/13) aged 5 to 9 years. Hydroxyurea administration was recorded in 6 (23%) children aged 0 to 4 years and in 5 (38%) aged 5 to 9 years. Four (10%) children had a history of surgical splenectomy before IPD, and 2 (5%) had received bone marrow transplantation.
Penicillin susceptibility
Reference laboratory penicillin susceptibility measures were available in 94 (90%) IPIs from children with SCD, and from 1530 (83%) IPD in the reference population. Before PCV7 licensure, IPD in patients with SCD were less likely to be penicillin susceptible compared with the reference population. However, after PCV7 licensure there was no significant difference (supplemental Table 5). In the PCV13 era, no isolate from children with HbSS was resistant to penicillin at a minimal inhibitory concentration of ≥2 μg/mL.
IPD serotypes
Serotype data were available in 94% IPD in children with SCD, and in 84% IPD in the reference population. Compared with the reference population, children with HbSS were less likely to be infected with serotype 9V (0% vs 5%, P = .0299) and serotype 14 (8% vs 24%; P = .001); and more likely to be infected with serotype 23F (16% vs 7%; P = .008) or serotype 15BC (8% vs 3%; P = .011). After PCV13 licensure, no IPD with any PCV13 serotype occurred in children with SCD.
Among non-PCV13 IPD serotypes in children with SCD, 15BC was the most frequently identified in 9 cases (24%), followed by 15A, 22F, or 23B, in 4 (11%) cases each (Table 4). IPDs with non-PCV13 serotypes were less frequent in children with SCD aged 0 to 4 years compared with those aged 5 to 9 years (17% vs 42%, P = .011), similar to the reference population (18% vs 36%, P < .001). IPD incidence rate ratio for non-PCV13 IPD increased after PCV7 licensure in the reference population (1.5, 95% CI, 1.1-2.1; P = .015). A parallel trend was noted in children with SCD (2.2, 95% CI, 0.7-7.3; P = .181; Tables 1 and 2).
Non-PCV13 IPD in children with SCD and IPD, serotype coverage by PPSV23, PCV15, PCV20, and PCV21/V116
| Data . | IPD . | PPSV23 serotype . | PPSV23∗ serotype . | PCV15 serotype . | PCV20 serotype . | PCV21 serotype . | Total non-PCV13 serotypes . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | ||
| Present† | All | 11 | 30 | 23 | 62 | 6 | 16 | 19 | 51 | 34 | 92 | 37 | 100 |
| Meningitis | 2 | 50 | 4 | 100 | 0 | 0 | 3 | 75 | 4 | 100 | 4 | 100 | |
| Death | 1 | 50 | 2 | 100 | 0 | 0 | 2 | 100 | 2 | 100 | 2 | 100 | |
| Meningitis/death | 3 | 50 | 6 | 100 | 0 | 0 | 5 | 83 | 6 | 100 | 6 | 100 | |
| Pooled‡ | All | 7 | 14 | 23 | 50 | 2 | 4 | 18 | 39 | 37 | 80 | 46 | 100 |
| Meningitis | 0 | 0 | 1 | 50 | 0 | 0 | 1 | 50 | 1 | 50 | 2 | 100 | |
| Death | 0 | 0 | 4 | 67 | 0 | 0 | 3 | 50 | 5 | 83 | 6 | 100 | |
| Meningitis/death | 0 | 0 | 5 | 71 | 0 | 0 | 4 | 57 | 6 | 86 | 7 | 100 | |
| Present†+ pooled‡ | All | 18 | 22 | 46 | 55 | 8 | 10 | 37 | 45 | 71 | 86 | 83 | 100 |
| Meningitis | 2 | 33 | 5 | 83 | 0 | 0 | 4 | 67 | 5 | 83 | 6 | 100 | |
| Death | 1 | 13 | 6 | 75 | 0 | 0 | 5 | 63 | 7 | 88 | 8 | 100 | |
| Meningitis/death | 3 | 23 | 11 | 85 | 0 | 0 | 9 | 69 | 12 | 92 | 13 | 100 | |
| Data . | IPD . | PPSV23 serotype . | PPSV23∗ serotype . | PCV15 serotype . | PCV20 serotype . | PCV21 serotype . | Total non-PCV13 serotypes . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | ||
| Present† | All | 11 | 30 | 23 | 62 | 6 | 16 | 19 | 51 | 34 | 92 | 37 | 100 |
| Meningitis | 2 | 50 | 4 | 100 | 0 | 0 | 3 | 75 | 4 | 100 | 4 | 100 | |
| Death | 1 | 50 | 2 | 100 | 0 | 0 | 2 | 100 | 2 | 100 | 2 | 100 | |
| Meningitis/death | 3 | 50 | 6 | 100 | 0 | 0 | 5 | 83 | 6 | 100 | 6 | 100 | |
| Pooled‡ | All | 7 | 14 | 23 | 50 | 2 | 4 | 18 | 39 | 37 | 80 | 46 | 100 |
| Meningitis | 0 | 0 | 1 | 50 | 0 | 0 | 1 | 50 | 1 | 50 | 2 | 100 | |
| Death | 0 | 0 | 4 | 67 | 0 | 0 | 3 | 50 | 5 | 83 | 6 | 100 | |
| Meningitis/death | 0 | 0 | 5 | 71 | 0 | 0 | 4 | 57 | 6 | 86 | 7 | 100 | |
| Present†+ pooled‡ | All | 18 | 22 | 46 | 55 | 8 | 10 | 37 | 45 | 71 | 86 | 83 | 100 |
| Meningitis | 2 | 33 | 5 | 83 | 0 | 0 | 4 | 67 | 5 | 83 | 6 | 100 | |
| Death | 1 | 13 | 6 | 75 | 0 | 0 | 5 | 63 | 7 | 88 | 8 | 100 | |
| Meningitis/death | 3 | 23 | 11 | 85 | 0 | 0 | 9 | 69 | 12 | 92 | 13 | 100 | |
Each column is a different subgroup of the total. All non-PCV13 IPD, irrespective of period when these occurred.
15BC in 9 cases (24%); 15A, 22F, or 23B in 4 (11%) cases each; 06C or 12F in 3 (8%) each; 33F or 35B in 2 (5%) each; 7C, 10A, 16F, 23A, or 38 in 1 (3%) case each.
Between 1994 and 2002, IPD serotypes associated with death included 19F in 3; 6A, 18C, and 23F (all covered in PCV7); and 15C (non-PCV7/PCV13). Meningitis was associated with serotypes 23F in 2 (17%), 6A, 14, 18C, and 19F (all covered in PCV7). Between 2002 and 2018 meningitis was associated with serotypes 6A, 12F, 15A, and 15B, and death with serotype 12F (4/5 non-PCV13 serotypes).
HbSS vs HbSC
Overall, IPD was less frequent in children with HbSC compared with children with HbSS (1994-2018, incidence rate ratio HbSC vs HbSS: 0.5; 95% CI, 0.3-0.8; P = .004); this difference between HbSS and HbSC was most pronounced during the period 2000-2009 (incidence rate ratio, 0.2; 95 % CI, 0.1-0.7; P = .013; Table 2). Although there were no significant differences in serotype distribution between children with HbSS vs HbSC (P = .2207), no children with HbSC were infected with serotypes 19A or 19F, whereas 7 children with HbSS developed IPD with serotype 19A (all during the PCV7 era) and 7 with serotype 19F. The frequency of IPD occurring at an older age (5-9 years) was 25 (29%) in HbSS and 2 (12%) in HbSC (P = .2265).
Pneumococcal vaccinations
Pneumococcal vaccination data were available in 93 (89%) IPD episodes in SCD. Before 2002, for children aged ≥2 years, at least 1 dose of PPSV23 had been received in 53% (16/30). After 2002, at least 1 dose of PPSV23 had been received in 67% (18 of 27), and 84% (31/37) had been received at least 1 dose of either PCV7 or PCV13 before infection. Within 3 years of vaccination, indirect-cohort effect estimates of PPSV23 vaccination on non-PCV13 serotypes included in PPSV23, plus vaccine-related serotypes 15A/15C35 were 85.3% (95% CI, 30.0-96.9), P = .025, and 92.4% (95% CI, 40.8-99.0), P = .014, when adjusted for age and hydroxyurea use (refer to supplemental Table 6 for sensitivity analysis).
PPSV23, PCV15, PCV20, and PCV21 coverage
Potential coverage of non-PCV13 isolates by PPSV23, PCV15, PCV20, and PCV21 vaccines for this study and pooled data, are presented in Table 4 and supplemental Table 8.
Discussion
The present study assessed the IPD incidence rate over a quarter century in children with HbSS or HbSC aged <10 years, who were followed-up at the only pediatric hospital–based health care system providing specialized care for SCD, a cohort that accounted to ∼95% of the expected person-time of observation estimated from newborn screening results from the region examined. Compared with the period before the introduction of PCV7 in 2000, the overall IPD incidence rate in children aged 0 to 9 years with HbSS or HbSC during the PCV13 era decreased by 86%, pneumococcal meningitis by 91%, and death by 94%, in parallel to the general population. Nevertheless, after 2009, children with HbSS were 33 times more likely to develop IPD, 28 times more likely to have meningitis, and 95 times more likely to die from IPD compared with the reference population of Black or African American children. Indeed, overall incidence rates in children with HbSS/SC aged 0 to 9 years from 2010 to 2018 were ∼2.7 times higher than that of the general population before PCV7 licensure (Figure 1). Children with SCD and IPD were significantly older than those in the reference population, suggesting that risk does not diminish after the fifth birthday as significantly in children with SCD as it does in the general population.
The incidence rate ratios of IPD increased in children with HbSS over time, as previously observed.18 A possible explanation includes the continuing practice of obtaining blood cultures in all febrile children with SCD33,47 whereas blood culture use decreased in children in the general population after PCV licensure.48 Inadequate use of antibiotic prophylaxis12 may also explain increased incidence rate ratios over time. Before PCV7,13 children with SCD whose guardians reported taking penicillin prophylaxis before IPD were significantly more likely to be infected with penicillin-nonsusceptible isolates,13 and in general, IPD resistance to penicillin was significantly higher in patients with SCD compared with in the general population.13 In this study, after PCV7 introduction, no significant differences were noted in penicillin susceptibility. Another possible explanation may be differences in the relative invasive potential of non-PCV13 serotypes in children with SCD compared with in the reference population. Serotypes 15B and 15C were responsible for IPD in children who were older and were more frequent in children with HbSS after PCV7 licensure compared with in the reference population.
Among children with HbSS, IPD case fatality decreased from 14% before 2002 to 3% after 2002, when pneumococcal conjugate vaccines became widely available; the frequency of meningitis decreased from 16% to 8%. Notably, after 2002, 1 death and 2 of 3 cases of meningitis were due to non-PCV13 serotypes. Cases that occurred in children after bone marrow transplantation suggest the need for ongoing vigilance, even after curative therapy. In addition to prompt antibiotic administration, improved IPD case fatality and morbidity can be attributed to targeted vaccination after PCV7 licensure17; the disappearance of serotypes previously associated with lethal IPD in children with HbSS, in particular 19F; and ascertainment of additional cases of meningitis and death before 2002.13,17 Case fatality and morbidity from meningitis in the reference population did not follow these trends.
IPD frequency was lower in children with HbSC compared with those with HbSS,17,49 consistent with the delayed development of functional asplenia in children with HbSC.2,3
Previous PPSV23 effectiveness estimation in children with SCD ranged from 63%44 to 80%.13 The latter estimation was based on the assumption that the vaccine protective effect was limited to the first 3 years after vaccination, because the immune response 3 to 7 years after vaccination is poor.45 Within the same assumption, PPSV23 appeared effective against non-PCV13 serotypes included in PPSV23, plus vaccine-related serotypes 15A35 and 15C.35,36 Yet, approximately a third of children aged ≥2 years with IPD had not received a first dose of PPSV23, and over 80% of children aged >5 years had not received a second dose, representing an opportunity to improve coverage.
In children with SCD from the same region, an analysis of IPD that occurred during targeted vaccination, within the first 3 years after PCV7 licensure,17 showed that children with SCD were, overall, more likely to be vaccinated than children in the general population.17 Sixty-two percent of children with SCD in the first decade of life received ≥1 doses of PCV7, with a direct protective effect estimated between 81% and 84.5% in adjusted models.17 Two years after licensure, when herd immunity was not widespread, the crude direct protective effect was 88.5%.17 In this analysis, IPD rates in children with SCD aged ≥5 years were higher than those reported in PROPS II,10 and decreased significantly after PCV7 licensure. In contrast, prior studies have reported little change in IPD rates in children aged ≥5 years with SCD after PCV7 licensure.18,50 Reasons may include low IPD rate estimates before PCV7 licensure,50 or that initially after PCV7 licensure children with SCD who were older were not vaccinated, in contrast to data from this study. In this study, between 2010 and 2018, IPD incidence rates in both age groups, (eg, aged 0-4 years and 5-9 years), declined by 87% and 80%, respectively. Moreover, in both age groups, no IPD with PCV13 serotypes occurred after licensure of PCV13 although immunization with PCV13 was absent or incomplete among some children with SCD and IPD. These findings suggest that herd immunity in the general population contributed to the reduction of IPD incidence rate in SCD.17
Although this study is limited to 1 region in the United States, results may have implications for regions of Africa with high SCD prevalence.51 In a meta-analysis of children with pneumococcal meningitis or septicemia hospitalized in Africa, the odds ratio for HbSS in the population with pneumococcal sepsis or meningitis was 26 times higher compared with respective cohorts of children without infection.52,53 Although two-thirds of African children are reported to have received PCV13 vaccination,54 herd immunity appears diminished,55 and mortality in children with SCD remains high.56-58 Children with SCD identified by screening,59-61 including unvaccinated children who are older, should be offered pneumococcal vaccination. Hydroxyurea may also provide protection against IPD.62-65 In a recent study of the incidence of all bloodstream infections in children with SCD between 2010 and 2019 at our institution, we comparably found significantly lower odds of infection in those who were prescribed hydroxyurea.47
Expanded-coverage vaccines PCV15 and PCV20 were approved for children in 202216,66 and 2023,67,68 respectively. Assuming vaccine serotype crossprotection,35-37 based on pooled data and the 25-year review presented here, PCV15, PCV20, and PCV21/V116 (in development) could offer protection against IPD with non-PCV13 serotypes, in 4% to 16%, 39% to 51%, and 80% to 92%, respectively, in children with SCD and IPD. In addition, PCV20 and PCV21/V116 could provide protection against 57% to 83% or 86% to 100% of pneumococcal isolates with serotypes causing meningitis or death, respectively. Administration of PPV23 (after PCV vaccination, not to blunt the immune response)69 may offer some additional crossprotection against vaccine-related serotypes not provided by PCV15 or PCV20.35,37 Other pneumococcal vaccines are in development.70 Vaccine schedules could be amended or changed as more data become available.
A limitation of this study is that some children with SCD may not have been included if they received care in other, nonspecialized clinics or hospitals. Such children were previously found to be at a significantly increased risk of death from IPD.17 Other limitations include that administration of penicillin prophylaxis immediately before IPD,13 vaccination coverage of all children,17 protective effect of hydroxyurea on IPD, and acute chest syndrome/pneumonia as an outcome, were not examined. Because of the limited geographical area of IPD surveillance, as well as small numbers in some IPD outcomes, data may not be entirely generalizable. Lastly, this study did not include analysis of IPD in patients with SCD after the first decade of life, who remain at risk.13,18,49
In conclusion, IPD remains a persistent, life-threatening risk in children with SCD, thus underscoring the need for vaccines with broader serotype coverage and continued IPD surveillance to identify emerging targets for vaccines and other prophylactic strategies.
Acknowledgments
The authors thank Monica Farley for invaluable guidance, and the research administration staff at Morehouse School of Medicine and Children’s Healthcare of Atlanta. The authors thank Victor Lui and Alice Lui, and staff and students at Perimeter Pediatrics for encouragement, administrative, and clerical support; the Centers for Disease Control and Prevention (CDC) Emerging Infections Program, Streptococcal laboratory for serotype and susceptibility results; and the Georgia Sickle Cell Data Collection program of the CDC for providing newborn screening data.
This work was supported by grants from the National Institutes of Health (1P20MD003383-01 and 1T90HG004004-01), and by grant support from the Abraham J. & Phyllis Katz Foundation, United States.
Authorship
Contribution: T.V.A. was responsible for conception draft and review, methodology, resources, project supervision and administration, data curation, investigation, analysis, writing the original manuscript draft, and review and editing of the manuscript; M.A.M.Y. was responsible for conception review, methodology, project administration, data curation, investigation, analysis, writing the original manuscript draft, and review and editing of the manuscript; S.T. was responsible for conception review, methodology, project administration, data curation, investigation, analysis, writing the original manuscript draft, and review and editing of the manuscript; A.T. was responsible for conception review, methodology, project administration, data curation, investigation, analysis, writing the original manuscript draft, and review and editing of the manuscript; K.W.L. was responsible for conception review, methodology, project administration, data curation, investigation, analysis, writing the original manuscript draft, and review and editing of the manuscript; F.O. was responsible for investigation, supervision, resources, and project administration; P.A.L. was responsible for conception review, resources, investigation, writing the original manuscript draft, and review and editing of the manuscript; and I.Y. was responsible for conception review, investigation, writing the original manuscript draft, and review and editing of the manuscript.
Conflict-of-interest disclosure: I.Y. has received funding to her institution to conduct clinical research unrelated to this manuscript from the Gates Foundation, Centers for Disease Control and Prevention, National Institutes of Health, Moderna, and Pfizer; and consults for Merck and Sanofi-Pasteur. The remaining authors declare no competing financial interests.
Correspondence: Thomas Adamkiewicz, Pediatric Hematology; Perimeter Pediatrics; 3020 Mercer University Drive, Atlanta, GA, 30341; e-mail: tadamkiewicz@msm.edu.
References
Author notes
Data that support the findings of this study are available upon reasonable request from the corresponding author, Thomas V. Adamkiewicz, (tadamkiewicz@msm.edu).
The full-text version of this article contains a data supplement.