Key Points
Fifty-two percent of patients with iMCD treated with siltuximab with/without corticosteroids achieved response.
Corticosteroids alone are not effective in iMCD symptom management.
Abstract
Idiopathic multicentric Castleman disease (iMCD) is a rare hematologic disorder with an unknown etiology. Clinical presentation is heterogeneous, ranging from mild constitutional symptoms with lymphadenopathy to life-threatening multiorgan dysfunction. International, consensus treatment guidelines developed in 2018 relied upon a limited number of clinical trials and small case series; however, to our knowledge, real-world performance of these recommendations has not been subsequently studied. Siltuximab, a monoclonal antibody against interleukin 6 (IL6), is approved for the treatment of iMCD and recommended first-line, and tocilizumab, a monoclonal antibody directed against the IL6 receptor, is recommended when siltuximab is unavailable. Chemotherapy, rituximab, and immunomodulators are recommended as second- and third-line treatments based on limited evidence. Corticosteroid monotherapy is used by clinicians, although not recommended. Here, we draw upon the ACCELERATE Natural History Registry to inventory regimens and evaluate regimen response for 102 expert–confirmed iMCD cases. Siltuximab with/without (w/wo) corticosteroids was associated with a 52% response, whereas corticosteroid monotherapy was associated with a 3% response. Anti-IL6–directed therapy with siltuximab or tocilizumab demonstrated better response and more durability than was observed with rituximab w/wo corticosteroids. Cytotoxic chemotherapy was associated with a 52% response and was predominantly administered in patients characterized by thrombocytopenia, anasarca, fever, renal failure/reticulin fibrosis, and organomegaly. Our results provide evidence in support of current recommendations to administer anti-IL6 as first-line treatment, to administer cytotoxic chemotherapy in patients with severe refractory disease, and to limit corticosteroid monotherapy. Evidence remains limited for effective agents for patients who are refractory to anti-IL6–directed therapy. This trial was registered at www.clinicaltrials.gov as #NCT02817997.
Introduction
Idiopathic multicentric Castleman disease (iMCD) is a rare cytokine storm–driven inflammatory disorder.1 Diagnosis is challenging, because it is based on lymph node histopathology review, which has significant interpathologist discordance, and there is a heterogeneous clinical presentation that overlaps with closely related disorders.2,3 Etiology and pathogenesis are yet unknown; however, interleukin 6 (IL6) has been found to drive disease in some patients.4,5 Some patients experience an aggressive and rapid disease onset that requires urgent intervention. These patients often meet criteria for the thrombocytopenia, anasarca, fever/elevated C reactive protein (CRP), reticulin fibrosis/renal failure, and organomegaly (TAFRO) subtype.6 Other patients who do not meet TAFRO criteria tend to experience a milder disease course that sometimes includes thrombocytosis, hypergammaglobulinemia, and plasmacytosis.7 These patients are considered not otherwise specified (NOS) and a subset of these patients are sometimes referred to as the idiopathic plasmacytic lymphadenopathy subtype.
Treatment guidelines for iMCD were developed by an international expert panel in 2018 based on review of a limited number of clinical trials and small case series, and recommendations were stratified by disease severity.3 In both severe and mild/moderate disease, siltuximab, a monoclonal antibody directed against IL6 that is the only medication approved for the treatment of iMCD in the United States and Europe, is recommended first-line. This was based on evidence from its registrational phase 2 trial, which demonstrated a 34% response,8 together with data supporting its long-term safety.9 Adjunctive corticosteroids are recommended as needed.8 Tocilizumab, which has a similar mechanism of action but targets the IL6 receptor, is recommended as an alternative when siltuximab is not available.10 The addition of cytotoxic chemotherapy is recommended for patients with severe disease who progress on anti-IL6 therapy. Data are more limited for alternative treatments outside of IL6-directed therapy. For patients with mild/moderate disease who are not responding to IL6 blockade or do not exhibit cytokine-driven symptomatology, rituximab with/without (w/wo) immunomodulators is recommended as second-line treatment. Rituximab, a monoclonal antibody that depletes B cells, is highly effective for human herpesvirus 8–associated MCD but has never been studied in a clinical trial in iMCD. Corticosteroid monotherapy is not recommended because of limited data in support of its use, anecdotal experience from the expert panel, and historically high rates of complications.11 A number of immunomodulators are listed as possible second- and third-line treatments, but there are limited available data on use of these drugs to treat iMCD.
Considering that iMCD is a rare disease, diagnosed in ∼1000 to 1200 individuals in the United States annually,12 it is difficult to conduct additional clinical trials that might inform treatment. Consequently, real-world data or data collected from patients treated in clinical practice and not on treatment trials have become increasingly important for understanding the natural history of, and effective treatments for, a rare disease.13 Although clinical trials are the gold standard, real-world data can contribute to the understanding of treatment effectiveness using clearly defined response criteria.
Herein, we present comprehensive treatment data from a cohort of 102 patients with iMCD enrolled in the ACCELERATE Natural History Registry (NCT02817997) and provide a large-scale evaluation of treatment effectiveness in this vulnerable population.
Methods
Patient cohort
Patients self enrolled into the ACCELERATE Natural History Registry between October 2016 and August 2022, and eligibility was confirmed upon receipt of a reference pathology report.14 Comprehensive medical data from disease onset until time of analysis was collected from all treating institutions and abstracted into the study database. To confirm a diagnosis of iMCD, a panel of iMCD experts (4 clinicians and 3 hematopathologists) adjudicated each case, including central pathology review, resulting in a final cohort of 102 patients with iMCD (supplemental Figure 1). All patients provided informed consent, and the research was approved by the University of Pennsylvania Institutional Review Board.
Regimen and response definition
Regimens were standardized as ≥1 drugs or procedures (treatments) that were initiated within 2 weeks of the start of another drug or procedure. Treatments initiated >2 weeks after a previous treatment started a distinct, new regimen. This grouping strategy enabled systematic evaluation of treatments given together.
Response was defined based on the change in the proportion of abnormal clinical and laboratory abnormalities (elevated CRP, anemia, thrombocytopenia/thrombocytosis, hypoalbuminemia, renal dysfunction, hypergammaglobulinemia, constitutional symptoms, organomegaly, fluid accumulation, eruptive cherry hemangiomatosis or violaceous papules, and lymphocytic interstitial pneumonitis) after regimen initiation.2 To achieve a response, the proportion of symptoms present at regimen initiation had to decrease by at least 50% after regimen initiation, and a new regimen could not be initiated within 1 year. Nonresponse either did not ever meet 50% reduction in proportion of symptoms or met 50% reduction in symptoms but required a new regimen within 1 year.
Consistent with the primary end point used in the phase 2 trial of siltuximab,8 lymph node and symptomatic response (LNSR) in this study required at least a 50% decrease in the short axis measurement(s) of the enlarged lymph node(s) as well as at least a stable best clinical response (ie, no change in the proportion of clinical and laboratory abnormalities).
Disease severity was defined per the iMCD treatment guidelines.8 Specifically, severe disease required at least 2 of renal failure, fluid accumulation, severe anemia, pulmonary involvement, or hospitalization. Adverse events were coded and categorized per the Medical Dictionary for Regulatory Activities.
Statistical analyses
Siltuximab w/wo corticosteroids and tocilizumab w/wo corticosteroids were consolidated into anti-IL6 w/wo corticosteroids to compare the effect of anti-IL6 w/wo corticosteroids with rituximab w/wo corticosteroids on response. The effect was tested using a generalized linear mixed effects model with severity, age, and sex as covariates; the patient was included as the random intercept to account for the multiple regimens for some patients. The relationship between clinical subtype and severity was also tested by generalized linear mixed effects model with the patient as the random intercept. Cohen κ statistic was used to measure interrater reliability between response and LNSR. A linear mixed model was used to test for the effect of regimen on hemoglobin, albumin, and CRP at time of best response; the nearest pretreatment value was included as a covariate and the patient was included as the random intercept when the model required. Post-hoc comparison adjusted by the Tukey method was performed upon finding a global significance. A Cox proportional hazards model adjusted by age category (<35 years vs ≥35 years), sex, and clinical subtype was used to calculate the effect of treatment regimen on durability of response. The model was stratified by severity to account for different baseline risks and clustered by patient to account for repeated regimens. The Grambsch and Therneau method was used to test for proportional hazards. A likelihood ratio test was used to test the assumption that covariates act similarly on the baseline hazard function within each stratum.
Results
Cohort of 102 patients panel-confirmed to meet iMCD diagnosis
In total, 102 patients with iMCD were confirmed to have a diagnosis of iMCD by an expert panel of 7 clinicians and pathologists. Forty-four (43.1%) patients identified as female, and nearly two-thirds identified as White. The mean age (standard deviation) is 35.9 (16.4) years, and there are 19 (18.6%) pediatric patients. At the time of analysis, 8 (7.8%) patients with iMCD had died from their disease, and more than half of the patients (n = 61, 59.8%) had the TAFRO subtype. We found high consistency of diagnosis confirmation among patients with the TAFRO subtype; of the 73 patients who met TAFRO criteria and were reviewed by the panel, 60 (82.2%) were confirmed by the panel. Of note, there was considerable inconsistency with regards to confirming Castleman disease (CD) diagnoses among the full cohort of CD cases considered for this study. In fact, 127 of the 328 cases considered for this study were not confirmed for inclusion either because of missing data or because the expert panel determined that these cases were not clinicopathologically consistent with any subtype of CD. An additional 99 patients were determined to have a subtype of CD other than iMCD (supplemental Figure 1). Interestingly, of the 74 iMCD cases with paired data available on histopathological subtype from local sites and central panel review, only 36 (48.6%) cases were concordant and 38 (51.4%) were discordant. Patients demonstrated considerable clinical and laboratory abnormalities at the time of diagnosis irrespective of treatment status (Table 1).
Cohort characteristics at the time of diagnosis
| . | N = 102 . |
|---|---|
| Age at diagnosis, y | |
| Mean (SD) | 35.9 (16.4) |
| <18, n (%) | 19 (18.6) |
| Deceased, n (%) | 8 (7.8) |
| Sex∗ , n (%) | |
| Female | 44 (43.1) |
| Male | 58 (56.9) |
| Race∗ , n (%) | |
| American Indian/Alaska Native | 1 (1.0) |
| Asian | 14 (13.7) |
| Black/African American | 12 (11.8) |
| Native Hawaiian/Pacific Islander | 1 (1.0) |
| White | 66 (64.7) |
| Other/not stated | 8 (7.8) |
| Histopathological subtype, n (%) | |
| Hyaline vascular | 1 (1.0) |
| Hypervascular | 62 (63.9) |
| Mixed | 27 (27.8) |
| Plasmacytic | 7 (7.2) |
| Unknown | 5 |
| Time from diagnostic biopsy to pathologic diagnosis, d | |
| Median (interquartile range) | 4 (2-8) |
| Clinical subtype, n (%) | |
| TAFRO | 61 (59.8) |
| NOS | 41 (40.2) |
| Clinical symptoms, n (% of those assessed) | |
| Constitutional symptoms | 92 (91.1) |
| Organomegaly | 73 (79.3) |
| Cherry hemangioma/violaceous papules | 2 (2.6) |
| Lymphocytic interstitial pneumonitis | 0 |
| Fluid retention | 79 (84.0) |
| Laboratory features | |
| CRP, mg/L | 80.0 (22.0-180.0) |
| ESR, mm/h | 73.0 (43.0-107.0) |
| Platelets, 103/μL | 134.0 (64.0-275.8) |
| Hemoglobin, g/dL | 10.0 (8.4-11.6) |
| Albumin, g/dL | 2.7 (2.3-3.3) |
| Creatinine, mg/dL | 1.1 (0.9-1.7) |
| eGFR, mL/min per 1.73m2, n (%) | |
| 0-20 | 6 (9.5) |
| 20-40 | 9 (14.3) |
| 40-60 | 12 (19.0) |
| ≥60 | 36 (57.1) |
| Not documented | 39 |
| IgG, mg/dL | 1150 (780-1727) |
| Gammaglobulin, g/dL | 1.22 (0.9-1.8) |
| . | N = 102 . |
|---|---|
| Age at diagnosis, y | |
| Mean (SD) | 35.9 (16.4) |
| <18, n (%) | 19 (18.6) |
| Deceased, n (%) | 8 (7.8) |
| Sex∗ , n (%) | |
| Female | 44 (43.1) |
| Male | 58 (56.9) |
| Race∗ , n (%) | |
| American Indian/Alaska Native | 1 (1.0) |
| Asian | 14 (13.7) |
| Black/African American | 12 (11.8) |
| Native Hawaiian/Pacific Islander | 1 (1.0) |
| White | 66 (64.7) |
| Other/not stated | 8 (7.8) |
| Histopathological subtype, n (%) | |
| Hyaline vascular | 1 (1.0) |
| Hypervascular | 62 (63.9) |
| Mixed | 27 (27.8) |
| Plasmacytic | 7 (7.2) |
| Unknown | 5 |
| Time from diagnostic biopsy to pathologic diagnosis, d | |
| Median (interquartile range) | 4 (2-8) |
| Clinical subtype, n (%) | |
| TAFRO | 61 (59.8) |
| NOS | 41 (40.2) |
| Clinical symptoms, n (% of those assessed) | |
| Constitutional symptoms | 92 (91.1) |
| Organomegaly | 73 (79.3) |
| Cherry hemangioma/violaceous papules | 2 (2.6) |
| Lymphocytic interstitial pneumonitis | 0 |
| Fluid retention | 79 (84.0) |
| Laboratory features | |
| CRP, mg/L | 80.0 (22.0-180.0) |
| ESR, mm/h | 73.0 (43.0-107.0) |
| Platelets, 103/μL | 134.0 (64.0-275.8) |
| Hemoglobin, g/dL | 10.0 (8.4-11.6) |
| Albumin, g/dL | 2.7 (2.3-3.3) |
| Creatinine, mg/dL | 1.1 (0.9-1.7) |
| eGFR, mL/min per 1.73m2, n (%) | |
| 0-20 | 6 (9.5) |
| 20-40 | 9 (14.3) |
| 40-60 | 12 (19.0) |
| ≥60 | 36 (57.1) |
| Not documented | 39 |
| IgG, mg/dL | 1150 (780-1727) |
| Gammaglobulin, g/dL | 1.22 (0.9-1.8) |
Data represent closest information to the date of diagnosis within 90 days before, through 15 days after, diagnosis date; laboratory data presented in median (interquartile range) unless otherwise stated.
eGFR, estimated glomerular filtration rate; ESR, erythrocyte sedimentation rate; IgG, immunoglobulin G; SD, standard deviation.
Patient reported.
High degree of variability in the treatments administered in iMCD
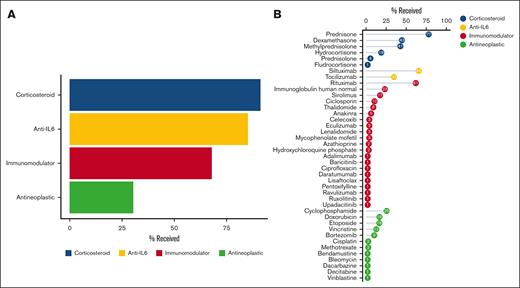
First, we set out to establish an inventory of iMCD treatments and regimens. Drugs were categorized as corticosteroid, antineoplastic, anti-IL6–directed therapy, or other immunomodulator. We found that across the cohort of 102 patients, 93 patients (91.2%) received at least 1 corticosteroid, 87 (85.3%) received anti-IL6–directed therapy, 69 (67.6%) received at least 1 other immunomodulator, and 31 (30.4%) received at least 1 antineoplastic agent (Figure 1A). In total, 41 unique drugs were administered to this cohort, including siltuximab and tocilizumab, 12 antineoplastic agents, 6 corticosteroids, and 21 immunomodulators. Figure 1B displays the proportion of patients who ever received each drug as part of any regimen. After prednisone, which was administered to 77.5% (79/102) of patients, siltuximab was administered to 64.7% (66/102) as part of various regimens. Among procedures, we identified 4 used for iMCD treatment, including plasmapheresis/plasma exchange (n = 6), radiation therapy (n = 3), splenectomy (n = 3), and thymus excision (n = 2).
Many treatments across several treatment categories are used in the treatment of iMCD. (A) Patients with iMCD receive a variety of treatments, including corticosteroids (91%), immunomodulators (68%), antineoplastic agents (30%), and anti-IL6–directed therapy (85%). (B) Forty-one unique drugs have been administered across a cohort of 102 patients with iMCD, and siltuximab, the first-line recommended therapy, has been administered to 65% of this cohort as part of various regimens.
Many treatments across several treatment categories are used in the treatment of iMCD. (A) Patients with iMCD receive a variety of treatments, including corticosteroids (91%), immunomodulators (68%), antineoplastic agents (30%), and anti-IL6–directed therapy (85%). (B) Forty-one unique drugs have been administered across a cohort of 102 patients with iMCD, and siltuximab, the first-line recommended therapy, has been administered to 65% of this cohort as part of various regimens.
We examined the adverse drug reaction profiles of the most commonly administered targeted treatments: siltuximab, tocilizumab, and rituximab. Musculoskeletal and connective tissue disorder events occurred most frequently among rituximab-associated events (24.1%, 20/83), skin and subcutaneous tissue disorders occurred most frequently among siltuximab-associated events (20.0%, 18/90), and gastrointestinal disorders occurred most frequently among tocilizumab-associated events (23.1%, 6/26) (supplemental Table 1). Rigors was the most frequently observed adverse event with rituximab (n = 8 occurrences), rash (n = 9 occurrences) with siltuximab, and anaphylactic reaction (n =4 occurrences) with tocilizumab (supplemental Table 2).
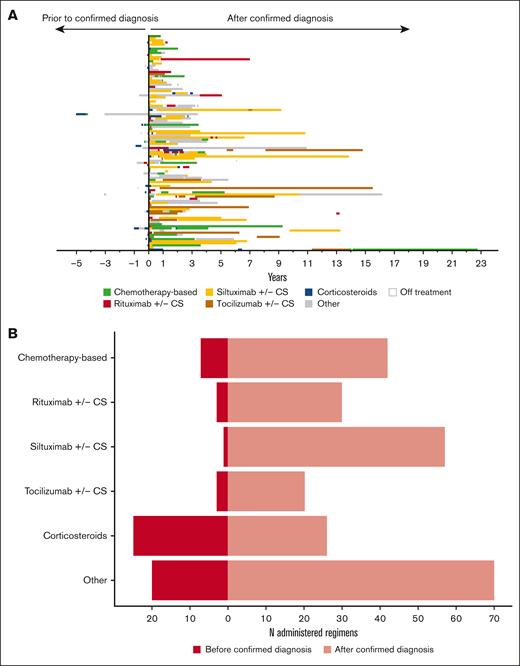
Next, we cataloged the regimens that represent combinations of these drugs and procedures. A total of 304 regimens were administered and 110 of them were unique combinations of drugs and procedures (Figure 2A; supplemental Table 3). We categorized these 110 combinations into 13 regimen categories (supplemental Table 4). Two of the 102 patients received no medical treatment after their diagnostic lymph node excision. Siltuximab w/wo corticosteroids was the most frequently administered regimen; 51 (50.0%) patients received this regimen at least once. Corticosteroid monotherapy was also frequently administered, with 45 (44.1%) patients receiving at least 1 corticosteroid monotherapy regimen. We examined the timing of regimen initiation with the hypothesis that many of the corticosteroid regimens were administered before iMCD diagnosis was confirmed (Figure 2B). Indeed, we found that 49.0% (25/51) of the corticosteroid regimens were administered after symptomatic presentation but before confirmed diagnosis. Overall, these data demonstrate the wide variety of treatments administered to patients with iMCD.
Treatment regimen administration in iMCD is highly variable and more generalized regimens are often administered before confirmed diagnosis. (A) Thirteen different regimen categories were identified and administered among this cohort. A total of 304 regimens were administered among the 102 patients with iMCD. Fifty-one (50%) patients received siltuximab w/wo corticosteroids at least once throughout their treatment course. The plot is sequentially ordered with the earliest enrollees at the bottom and the most recent enrollees at the top. Regimens administered before confirmed diagnosis are represented to the left of the vertical bar, and regimens administered on, or after, diagnosis are represented to the right of the vertical bar. (B) Given variability in presentation and the time until accurate diagnosis, some regimens are administered before confirmed diagnosis. In this cohort, 49% of the corticosteroid regimens were administered before confirmed diagnosis, whereas only 1.7% of the siltuximab w/wo corticosteroids regimens were administered before confirmed diagnosis. In this figure, regimens defined as immunomodulator(s) w/wo corticosteroids, anti-IL6 therapy + rituximab w/wo other treatments, anti-IL6 therapy + immunomodulator(s) w/wo corticosteroids, anti-IL6 therapy + procedure w/wo corticosteroids, procedure + drug therapy, procedure, and no medical treatment have been combined into an “Other” category. CS, corticosteroids.
Treatment regimen administration in iMCD is highly variable and more generalized regimens are often administered before confirmed diagnosis. (A) Thirteen different regimen categories were identified and administered among this cohort. A total of 304 regimens were administered among the 102 patients with iMCD. Fifty-one (50%) patients received siltuximab w/wo corticosteroids at least once throughout their treatment course. The plot is sequentially ordered with the earliest enrollees at the bottom and the most recent enrollees at the top. Regimens administered before confirmed diagnosis are represented to the left of the vertical bar, and regimens administered on, or after, diagnosis are represented to the right of the vertical bar. (B) Given variability in presentation and the time until accurate diagnosis, some regimens are administered before confirmed diagnosis. In this cohort, 49% of the corticosteroid regimens were administered before confirmed diagnosis, whereas only 1.7% of the siltuximab w/wo corticosteroids regimens were administered before confirmed diagnosis. In this figure, regimens defined as immunomodulator(s) w/wo corticosteroids, anti-IL6 therapy + rituximab w/wo other treatments, anti-IL6 therapy + immunomodulator(s) w/wo corticosteroids, anti-IL6 therapy + procedure w/wo corticosteroids, procedure + drug therapy, procedure, and no medical treatment have been combined into an “Other” category. CS, corticosteroids.
Response metrics support current treatment guidelines
Next, we sought to evaluate regimen effectiveness. Of particular interest was the evaluation of regimen categories defined in the 2018 iMCD treatment guidelines, including anti-IL6 w/wo corticosteroids, which comprised siltuximab w/wo corticosteroids and tocilizumab w/wo corticosteroids; rituximab w/wo corticosteroids; and chemotherapy-based regimens. In addition, we evaluated the performance of corticosteroid monotherapy, which we found to be frequently administered.
We found that 50.0% (29/58) of patients treated with anti-IL6 w/wo corticosteroids ever achieved response. Specifically, 52.3% (22/42) of patients treated with siltuximab w/wo corticosteroids and 44.4% (8/18) treated with tocilizumab w/wo corticosteroids ever achieved response. Moreover, 26.9% (7/26) treated with rituximab w/wo corticosteroids, 52.0% (13/25) treated with chemotherapy-based regimens, and 2.8% (1/36) treated with corticosteroid monotherapy ever achieved response (Table 2). Given that rituximab w/wo corticosteroids is recommended as an alternative first-line treatment to anti-IL6 w/wo corticosteroids in specific cases, we tested for a differential effect between these regimens. Controlling for severity (ꞵ = −0.97; P = .12), age at regimen initiation (ꞵ = −0.10; P = .70), and sex (ꞵ = –0.23; P = .67), we found that rituximab w/wo corticosteroids is associated with a 1.18-fold lower log-odds or a 69.3% decrease in the odds of response compared with anti-IL6 w/wo corticosteroids (ꞵ = –1.18; 95% confidence interval [CI], –2.29 to –0.06; P = .038). This finding supports the recommendation to first use anti-IL6–directed therapy; however, the 26.9% response to rituximab w/wo corticosteroids is evidence that its use is reasonable in mild/moderate cases when anti-IL6 directed therapy is ineffective.
Response by regimen category
| . | Patients ever achieved a response∗ . | Patients with evaluable regimen, n . | |
|---|---|---|---|
| Yes, n (%) . | No, n (%) . | ||
| Anti-IL6 w/wo corticosteroids† | 29 (50.0) | 29 (50.0) | 58 |
| Siltuximab w/wo corticosteroids | 22 (52.4) | 20 (47.6) | 42 |
| Tocilizumab w/wo corticosteroids | 8 (44.4) | 10 (55.6) | 18 |
| Rituximab w/wo corticosteroids | 7 (26.9) | 19 (73.1) | 26 |
| Chemotherapy-based regimen | 13 (52.0) | 12 (48.0) | 25 |
| Immunomodulator w/wo corticosteroids | 4 (19.0) | 17 (81.0) | 21 |
| Anti-IL6 + rituximab w/wo other | 5 (41.7) | 7 (58.3) | 12 |
| Anti-IL6 + immunomodulator(s) w/wo corticosteroids | 6 (60.0) | 4 (40.0) | 10 |
| Anti-IL6 + procedure w/wo corticosteroids | 0 | 1 (100) | 1 |
| Rituximab + immunomodulator(s) w/wo corticosteroids | 1 (12.5) | 7 (87.5) | 8 |
| Corticosteroids | 1 (2.8) | 35 (97.2) | 36 |
| Procedure + drug therapy | 1 (25.0) | 3 (75.0) | 4 |
| Procedure | 0 | 2 (100) | 2 |
| No medical treatment | 0 | 2 (100) | 2 |
| . | Patients ever achieved a response∗ . | Patients with evaluable regimen, n . | |
|---|---|---|---|
| Yes, n (%) . | No, n (%) . | ||
| Anti-IL6 w/wo corticosteroids† | 29 (50.0) | 29 (50.0) | 58 |
| Siltuximab w/wo corticosteroids | 22 (52.4) | 20 (47.6) | 42 |
| Tocilizumab w/wo corticosteroids | 8 (44.4) | 10 (55.6) | 18 |
| Rituximab w/wo corticosteroids | 7 (26.9) | 19 (73.1) | 26 |
| Chemotherapy-based regimen | 13 (52.0) | 12 (48.0) | 25 |
| Immunomodulator w/wo corticosteroids | 4 (19.0) | 17 (81.0) | 21 |
| Anti-IL6 + rituximab w/wo other | 5 (41.7) | 7 (58.3) | 12 |
| Anti-IL6 + immunomodulator(s) w/wo corticosteroids | 6 (60.0) | 4 (40.0) | 10 |
| Anti-IL6 + procedure w/wo corticosteroids | 0 | 1 (100) | 1 |
| Rituximab + immunomodulator(s) w/wo corticosteroids | 1 (12.5) | 7 (87.5) | 8 |
| Corticosteroids | 1 (2.8) | 35 (97.2) | 36 |
| Procedure + drug therapy | 1 (25.0) | 3 (75.0) | 4 |
| Procedure | 0 | 2 (100) | 2 |
| No medical treatment | 0 | 2 (100) | 2 |
Patients with >1 instance of the same regimen category are considered to have achieved response if response was achieved at least 1 time.
Includes patients ever treated with either siltuximab w/wo corticosteroids and/or tocilizumab w/wo corticosteroids. Best response among those regimens is included; therefore, the number of patients who are evaluable for each siltuximab w/wo corticosteroids and tocilizumab w/wo corticosteroids may not sum to the number of patients who are evaluable for anti-IL6 w/wo corticosteroids
Next, we examined the timing of treatment with anti-IL6 w/wo corticosteroids and whether there was a difference in the effectiveness of anti-IL6 w/wo corticosteroids between patients who received it as a first-line therapy (with the exception of corticosteroid monotherapy) or as a subsequent therapeutic approach. Among patients who were diagnosed after the approval of siltuximab for the treatment for iMCD (22 April 2014) and received anti-IL6 w/wo corticosteroids, the median (interquartile range) time to treatment with anti-IL6 w/wo corticosteroids was 22 (0, 70) days and the mean (standard deviation) was 113.4 (224.6) days. We then looked at the effectiveness of patients treated early vs later in their treatment course. Among the 58 patients who had an evaluable anti-IL6 w/wo corticosteroids regimen, 33 (56.9%) received it as first-line therapy and 25 (43.1%) received it subsequent to another therapeutic approach. We found that there was a 48.5% (16/33) response among patients who received anti-IL6 w/wo corticosteroids as first-line therapy and a 52.0% (13/25) response among patients who received ant-IL6 w/wo corticosteroids as a subsequent approach. There was no statistical difference (X = 0; P = 1).
Because chemotherapy-based regimens are defined by the inclusion of multiple different antineoplastic/cytotoxic agents and may contain other agents including anti-IL6–directed therapy, immunomodulators, or corticosteroids, we also further interrogated these regimens to identify trends among those that elicited a response. Among the 13 patients who ever achieved a response to a chemotherapy-based regimen, there were 24 chemotherapy-based regimens administered. Fifteen (62.5%) resulted in response and 9 (38.5%) did not. Comparatively, among the 12 patients who never achieved a response to a chemotherapy-based regimen, there were 16 chemotherapy-based regimens administered (supplemental Table 5). To investigate the heterogeneity of chemotherapy-based regimens, we categorized the inclusion of each antineoplastic agent among regimens that achieved response compared with those that did not achieve response. We did not identify a trend that would suggest superiority of a specific regimen, but the sample size was likely underpowered to detect significant differences (supplemental Table 6).
To evaluate the use of alternatives with unknown efficacy, we examined response to immunomodulator regimens. Among the immunomodulator regimens, we found 17 unique combinations, the most frequent of which was sirolimus w/wo corticosteroids (n = 7). Across all immunomodulator w/wo corticosteroid regimens, we observed a relatively low response. Four (19%) patients with an evaluable regimen achieved at least 1 response (supplemental Figure 2).
Lastly, we performed a secondary analysis to investigate LNSR, which includes radiologic response and closely matches the primary end point in the phase 2 trial.8 Applying that definition, we found comparable response across regimen categories (supplemental Table 7), and among regimens for which there were corresponding response data, we found substantial agreement (κ = 0.64; P = 4.0 × 10–14). The strong concordance of results between our definition of response and LNSR strengthens our findings.
Characterization of response during severe disease and by clinical subtype
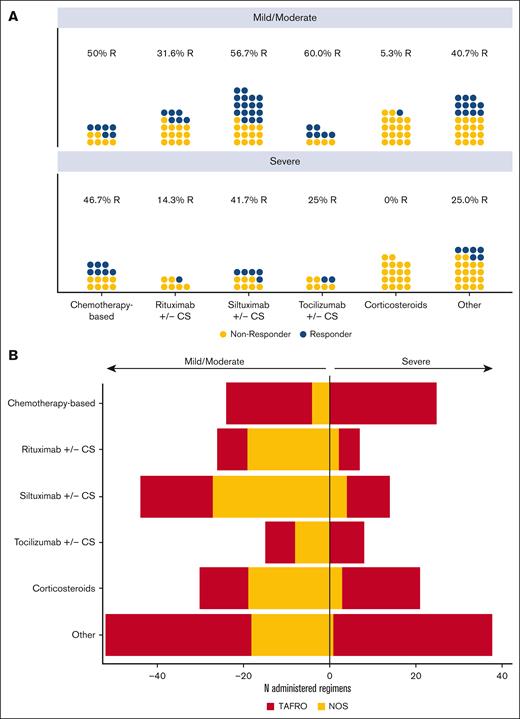
Because treatment recommendations are stratified by disease severity, we characterized response by disease severity at the time of regimen initiation (Figure 3A). First, we examined the relationship between severity and regimen received and after accounting for multiple regimens within a given patient, we found no significant relationship (X = 7.6; P = .18). Within each regimen category, there was a larger number of patients who had ever initiated a regimen during mild/moderate compared with those ever initiated during severe disease. Notably, we observed a substantial proportion of patients who achieved a response to siltuximab w/wo corticosteroids each during mild/moderate (56.7%, 17/30) and severe (41.7%, 5/12) disease. There appeared to be a lower response to tocilizumab w/wo corticosteroids during severe disease compared with mild/moderate (25.0% [2/8] vs 60.0% [6/10]), but the number of observations was low and a statistical comparison to evaluate the difference in response between mild/moderate and severe disease within each regimen category was not performed because of small number of observations that do not allow for covariate adjustment.
Regimen response by severity, and relationship between severity and clinical subtype. (A) Best response by regimen category stratified by disease severity at the start of the regimen. Each dot represents a given patient within a regimen category and severity status colored by best response (responder status indicated by blue, and non-responder status indicated by gold). Within each regimen category, there was a higher number of regimens initiated in mild/moderate compared with severe disease. A comparable proportion of patients achieved a response to siltuximab w/wo corticosteroids during both mild/moderate (57%) and severe (42%) disease. Corticosteroids alone was associated with response in 1 patient during mild/moderate disease only. (B) Severe disease was strongly associated with TAFRO status (ꞵ = 3.14; 95% CI, 2.00-4.27; P < .001). The majority (91.2%) of regimens initiated in severe disease occurred in patients with TAFRO subtype, but regimens initiated in mild/moderate disease occurred equally among patients with TAFRO (50.3%) and NOS (49.7%) subtypes.
Regimen response by severity, and relationship between severity and clinical subtype. (A) Best response by regimen category stratified by disease severity at the start of the regimen. Each dot represents a given patient within a regimen category and severity status colored by best response (responder status indicated by blue, and non-responder status indicated by gold). Within each regimen category, there was a higher number of regimens initiated in mild/moderate compared with severe disease. A comparable proportion of patients achieved a response to siltuximab w/wo corticosteroids during both mild/moderate (57%) and severe (42%) disease. Corticosteroids alone was associated with response in 1 patient during mild/moderate disease only. (B) Severe disease was strongly associated with TAFRO status (ꞵ = 3.14; 95% CI, 2.00-4.27; P < .001). The majority (91.2%) of regimens initiated in severe disease occurred in patients with TAFRO subtype, but regimens initiated in mild/moderate disease occurred equally among patients with TAFRO (50.3%) and NOS (49.7%) subtypes.
Given the high proportion of patients that met TAFRO criteria in our cohort (59.8%, 61/102) and that patients with the TAFRO subtype typically demonstrate severe symptoms, we also investigated the relationship between severity and clinical subtype. First, we found a strong association between severity and TAFRO status (ꞵ = 3.14; 95% CI, 2.00-4.27; P < .001). The odds of severe disease occurring in a patient with the TAFRO subtype is approximately 23 times the odds of severe disease occurring in a patient with NOS subtype. Among regimens initiated in severe disease, 91.2% (103/113) occurred in patient with the TAFRO subtype, whereas regimens initiated in mild/moderate disease equally represent patients who met TAFRO criteria (50.3%, 96/191) and those with NOS (49.7%, 95/191) (Figure 3B). Response proportions by TAFRO and NOS subtypes were similar to those observed in mild/moderate and severe disease (supplemental Figure 3). Of note, patients with TAFRO subtype received the majority of chemotherapy-based regimens, which resulted in a 47.8% response. These data support the recommendation to initiate patients in all stages of disease on anti-IL6–directed therapy and substantiate chemotherapy as an option in severe disease/TAFRO subtype.
Substantial improvement in objective laboratory parameters notable in siltuximab w/wo corticosteroids
As a quantitative assessment of regimen performance, we examined 3 reliable markers of disease activity (hemoglobin, albumin, and CRP) at the time of regimen initiation and time of best response within regimen categories of interest. For each regimen category, mean hemoglobin, albumin, and CRP levels were abnormal (<12.0 g/dL, <3.5 g/dL, and >10 mg/L, respectively) at regimen initiation (Figure 4). When controlling for parameter levels before treatment initiation, we found that siltuximab w/wo corticosteroids resulted in a substantial and statistically significant increase in hemoglobin compared with both rituximab w/wo corticosteroids (P = .034) and corticosteroid monotherapy (P < .001). Chemotherapy-based regimens (P = .0198) and tocilizumab w/wo corticosteroids (P = .0448) also each demonstrated a significant increase compared with corticosteroids. Likewise, chemotherapy-based regimens (P < .001), siltuximab w/wo corticosteroids (P < .001) and tocilizumab w/wo corticosteroids (P = .0232) each resulted in a substantial and statistically significant increase in albumin compared with corticosteroid monotherapy. Corticosteroid monotherapy was the only treatment regimen that did not result in raising mean hemoglobin or albumin levels to the normal range. Siltuximab w/wo corticosteroids was the only regimen to result in a clinically substantial improvement in CRP (within normal limits), although interpretation of CRP data is restricted because the smaller number of CRP measurements available prevented detection of differences between regimens. These findings demonstrate additional support for the current treatment recommendations and for limiting the use of corticosteroid monotherapy.
Laboratory parameters indicate that some regimen categories outperform others. Mean and standard error of (A) hemoglobin (red) and albumin (blue) and (B) CRP at the initiation of a given regimen category (closest value within ± 7 days) and at the time of best response (closest value within ± 7 days). Anti-IL6–directed therapies show the most dramatic improvements in laboratory parameters, whereas corticosteroids show limited improvement. Slope between time points shown; available data points contributing to plots provided below plots. Only statistically significant results are marked, and statistical significance is defined by the number of asterisks: ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, and ∗∗∗∗P < .00001.
Laboratory parameters indicate that some regimen categories outperform others. Mean and standard error of (A) hemoglobin (red) and albumin (blue) and (B) CRP at the initiation of a given regimen category (closest value within ± 7 days) and at the time of best response (closest value within ± 7 days). Anti-IL6–directed therapies show the most dramatic improvements in laboratory parameters, whereas corticosteroids show limited improvement. Slope between time points shown; available data points contributing to plots provided below plots. Only statistically significant results are marked, and statistical significance is defined by the number of asterisks: ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, and ∗∗∗∗P < .00001.
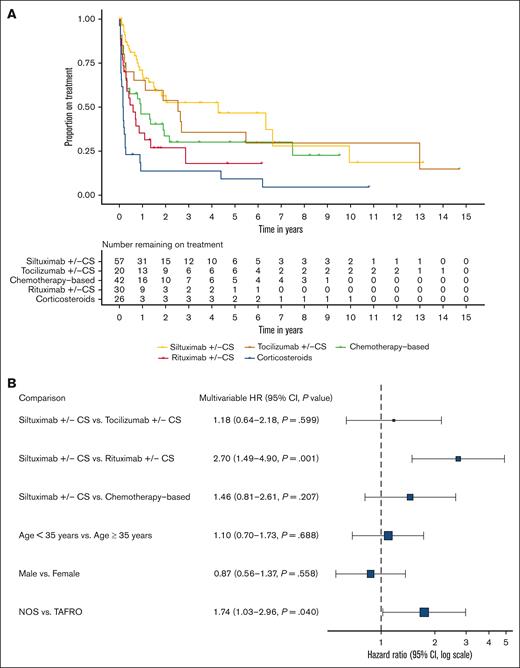
Time-to-event analysis highlights successful durability of siltuximab w/wo corticosteroids
Lastly, as an assessment of regimen durability, we analyzed time to event (disease progression or start of new regimen) for regimens initiated after confirmed diagnosis. Median time-to-event for siltuximab w/wo corticosteroids was 1566 days (95% CI, 546-no upper limit), tocilizumab w/wo corticosteroids was 924 (95% CI, 233-no upper limit), chemotherapy-based regimens was 338 (95% CI, 120-2734), rituximab w/wo corticosteroids was 214 (95% CI, 119-no upper limit), and corticosteroid monotherapy was 56.5 (95% CI, 27-98) (Figure 5A). We compared siltuximab w/wo corticosteroids, tocilizumab w/wo corticosteroids, rituximab w/wo corticosteroids, and chemotherapy-based regimens and controlled for age, sex, and clinical subtype in a Cox proportional hazards model stratified by severity. Siltuximab w/wo corticosteroids demonstrated durability over rituximab w/wo corticosteroids (hazard ratio, 2.70; 95% CI, 1.49-4.90; P = .001) (Figure 5B). Regimens administered to patients with NOS subtype also demonstrated durability over those administered to patients with TAFRO subtype (hazard ratio, 1.74; 95% CI, 1.03-2.96; P = .04), which may be because of the fact that patients with TAFRO subtype typically experience a more intense flare-like disease. These strong and consistent results highlight that first-line therapy is able to induce a durable response.
Time-to-event analysis highlights the durability of anti-IL6–directed therapies. (A) Survival curve showing time to event by regimen category. Event is defined as disease progression or start of new regimen. (B) Results from a Cox proportional hazards model comparing siltuximab w/wo corticosteroids, tocilizumab w/wo corticosteroids, rituximab w/wo corticosteroids, and chemotherapy-based regimens, stratified by severity and controlled for age, sex, and clinical subtype. Siltuximab w/wo corticosteroids demonstrated stronger durability over rituximab w/wo corticosteroids (hazard ratio, 2.72; 95% CI, 1.50-4.91; P = .001).
Time-to-event analysis highlights the durability of anti-IL6–directed therapies. (A) Survival curve showing time to event by regimen category. Event is defined as disease progression or start of new regimen. (B) Results from a Cox proportional hazards model comparing siltuximab w/wo corticosteroids, tocilizumab w/wo corticosteroids, rituximab w/wo corticosteroids, and chemotherapy-based regimens, stratified by severity and controlled for age, sex, and clinical subtype. Siltuximab w/wo corticosteroids demonstrated stronger durability over rituximab w/wo corticosteroids (hazard ratio, 2.72; 95% CI, 1.50-4.91; P = .001).
Discussion
Although treatment guidelines for iMCD were developed in 2018, to our knowledge, this is the first systematic assessment of the treatments included in those guidelines. Given the frequency of off-label prescribing for iMCD and limited active clinical trials underway, real-world data collected and abstracted as part of the ACCELERATE Natural History Registry provide an ideal source of information for evaluating treatment outcomes in iMCD. Increasingly, rare disease researchers are leveraging real-world data to provide valuable clinical insights when clinical trials are not able to be performed. A recent study on immune-mediated thrombotic thrombocytopenic purpura (iTTP) used real-world data to report the current clinical treatment practice and to assess the benefits and risks of caplacizumab, an approved treatment for iTTP, outside of a clinical trial setting.15 Similar to iMCD, in which most patients with severe disease were excluded from the only phase 2 clinical trial, limited data are available on the outcomes of patients with iTTP who are severely ill, and this real-world data report found concordance between real-world data and clinical trial results.
Our evaluation of treatment patterns in 102 confirmed patients with iMCD identified 41 unique drugs that have been used in the treatment of iMCD. Our finding that 85% of patients with iMCD were treated with siltuximab or tocilizumab conflicts with a recent epidemiologic study that reported treatment with IL6-directed therapy in <10% of patients with iMCD, based on insurance claims data.12 This discrepancy might be explained by the fact that this study looked at claims data between 2006 and 2020 and could reflect a gradual adoption of IL6-directed therapy. Alternatively, considering that patients self-enroll into ACCELERATE, it is possible that this represents a bias toward enrollment of patients more likely to be treated with recommended treatment. It is also possible that our strict adjudication process resulted in a cohort of patients more likely to have iMCD than those identified by insurance claims data, which could have included a large number of patients with unicentric CD or other diseases that could not be removed from the analysis.
Beyond anti-IL6–directed therapies, there is no consensus for optimal second-line therapy. Sirolimus, a mammalian target of rapamycin inhibitor, identified as a potential iMCD treatment, has been administered to 17% of our cohort. It has previously been found to induce a clinically beneficial response in a small number of patients, and a clinical trial is underway to further evaluate its efficacy (NCT03933904).16-18 Here, we found evidence of response in a small number of patients. Interestingly, other immunomodulators recommended in the 2018 treatment guidelines, including cyclosporine A, anakinra, and thalidomide, which was recently reported along with cyclophosphamide and prednisone to be an effective treatment in a small phase 2 trial,19 were only used in a small percentage of patients in this cohort. Bortezomib has also been reported along with thalidomide and dexamethasone to be an effective treatment approach from a single-center phase 2 trial in patients with relapsed/refractory iMCD3,20; however, no patients in our cohort received that regimen.
Given the challenges in assessing treatment response to individual drugs administered concurrently, we defined regimens per the timing of administration, and developed a response criteria well suited to real-world data.14 These data reveal a higher response to siltuximab w/wo corticosteroids (52%) than was observed in the phase 2 clinical trial (34%).8 Because our response definition differed from the phase 2 study, we also applied a response criteria that corresponded with the definition used in the trial and showed concordance for all regimen categories. This supports defining response using clinical metrics, which are more aligned with patient-reported challenges. This also suggests that the difference in response observed in these real-world data compared with those of the phase 2 trial is less likely to be due to the difference in response variables. In fact, retrospective review of patients enrolled in the phase 2 trial suggests that some patients may not have had iMCD and that patients who met more criteria had a greater likelihood of response.2 Patients who did not satisfy the iMCD clinical criteria (n = 16) had a 0% response, potentially diluting the signal of efficacy among confirmed iMCD patients. Given that each case herein was rigorously reviewed, this cohort is highly likely to represent iMCD and the response to siltuximab was similar to that in patients in the phase 2 study who retrospectively met criteria.2
Our study reports on regimens administered during both mild/moderate and severe disease. The phase 2 siltuximab clinical trial excluded patients with severe disease and therefore siltuximab effectiveness in patients with severe disease was largely unknown and unreported. We found comparable response during both mild/moderate and severe disease. Our results also demonstrate that use of anti-IL6 w/wo corticosteroids is associated with a higher response than rituximab w/wo corticosteroids after controlling for severity, supporting the current international guideline recommendations. Notably, we showed that the vast majority of regimens that were initiated during severe disease occurred in patients with TAFRO subtype, and stratification of response by clinical subtype was similar to that observed during stratification by severity. A recent study on a large cohort of patients with TAFRO subtype reported no significant differences in response to tocilizumab or rituximab between TAFRO and NOS.21 Although patients with TAFRO subtype and those with NOS subtype demonstrate distinct clinical symptomology, it is not yet known whether this is because of different disease mechanisms.
We found improvement in objective laboratory metrics after the initiation of appropriate therapy. Clinical improvement of hemoglobin, albumin, and CRP was seen in most regimen categories; however, none improved to clinically significant levels on corticosteroid monotherapy, which was associated with a 3% response. CRP, hemoglobin, albumin, and performance status have been combined into a “CHAP” score and proposed as a marker of disease activity.22 Hemoglobin was previously identified in a model of laboratory parameters (along with CRP, fibrinogen, and immunoglobulin G) predictive of response to siltuximab but has not been validated.23
There are several limitations to this study. First, to address the inherent limitations to real-world data, we created systematic rules to define a regimen and response as well as rigorous criteria to ensure that each patient’s diagnosis of iMCD was confirmed by central review of extensive clinical, histopathologic, and radiologic data. Real-world data are at risk for missingness, bias because of lack of randomization, lack of objectively defined and systematically evaluated response, etc. Here, response is based on the change in the proportion of abnormal clinical and laboratory criteria after a treatment regimen is initiated, which enables determination of trends in improvement even when data are missing for a specific criterion. Nevertheless, comparative data need to be interpreted with caution given heterogeneity. Second, variability in regimens limited interpretation in some cases. Chemotherapy-based regimens were highly variable and sometimes included anti-IL6–directed agents or other immunomodulators but always included a cytotoxic agent. Third, limited sample size for some newly identified and potentially promising treatment approaches precluded statistical investigation of response. Although there were a high number of unique regimens, certain regimens of interest that have been recently identified, such as combination of thalidomide, cyclophosphamide, and prednisone, were not present in this data set.19 Likewise, JAK inhibition has been recently identified as a promising possible therapeutic target in iMCD and has been shown to have clinical benefit in some patients,24-26 but our data included too few patients treated with JAK inhibitors to assess. A larger sample size would have improved our ability to detect differences between regimens. Lastly, C-X-C motif chemokine ligand 13 (CXCL13) has been recently identified as an early indicator of response to siltuximab and is under investigation as a possible treatment target, but no drugs targeting CXCL13 or its receptor, CXCR5, are approved in humans thus precluding clinical investigation.27 One of the most pressing needs for patients with iMCD is the identification of a consensus second-line therapy for patients who are anti-IL6 refractory, and this study was not powered to make such comparisons. However, the rigor with which our cohort was reviewed and selected likely improved the accuracy of our results. Notably, our sample was biased toward the TAFRO clinical subtype and 65% of our cohort was White, which may not be consistent with the population of iMCD. Despite these limitations, we assembled a large, expert-confirmed cohort of patients with iMCD and obtained extensive clinical and treatment data.
Our study of 102 confirmed patients with iMCD demonstrates support for the current treatment guidelines. We found a 50% response to anti-IL6 w/wo corticosteroids and showed that objective laboratory metrics and time-to-event data support the use of anti-IL6–directed regimens and limiting corticosteroid monotherapy. These results also demonstrate that additional agents are needed for patients with refractory disease, who have few options and are at risk of death because of progression.
Acknowledgments
The authors thank the Castleman Disease Collaborative Network scientific advisory board for their support. Notably, the authors thank Thomas Uldrick, Elaine Jaffe, Amy Chadburn, Angela Dispenzieri, and Ariela Noy for their collaborations and scientific discussions. The authors also thank former ACCELERATE Registry team members, including Amy Liu, Erin Napier, Faizaan Ahkter, Jasira Ziglar, Johnson Khor, Eric Haljasmaa, Katherine Floess, Mark-Avery Tamakloe, Victoria Powers, Alexander Gorzewski, Freda R. Coren, and Reece Williams, as well as former contributors, including Shawnee Bernstein, Nathan Hersh, Gerard Hoeltzel, and Jeremy Zuckerberg. Importantly, the authors thank patients with iMCD and their families for their participation in the ACCELERATE Registry and contribution to this research. The ACCELERATE Natural History Registry has received funding from Janssen Pharmaceuticals (2016-2018), EUSA Pharma LLC (United States), which has merged with Recordati Rare Diseases Inc (2018 to present), and the US Food and Drug Administration (R01FD007632) (2022 to present). D.C.F has also received funding relevant to this project from the NIH National Heart, Lung, & Blood Institute (R01HL141408).
Authorship
Contribution: S.K.P. conceptualized the study, performed formal analysis, prepared the original manuscript draft and reviewed and edited the manuscript, and was responsible for data visualization; M.S.L. supervised the study, and reviewed and edited the manuscript; G.S. and J.D.B. supervised the study, and reviewed and edited the manuscript; M.S.B., S.S., N.M, C.L., and B.A. performed investigation, and reviewed and edited the manuscript; D.A., M.J.L., and A.B. supervised the study, and reviewed and edited the manuscript; H.L. supervised the study, developed the methodology, and reviewed and edited the manuscript; C.C. and F.v.R. conceptualized and supervised the study, and reviewed and edited the manuscript; and D.C.F. conceptualized and supervised, acquired funding, and reviewed and edited the manuscript.
Conflict-of-interest disclosure: D.C.F. has received research funding for the ACCELERATE Registry and consulting fees from EUSA Pharma; has received research study drug, with no associated research funding, for the clinical trial of sirolimus from Pfizer (NCT03933904); and has 2 provisional patents pending related to the diagnosis and treatment of iMCD, including 1 related to CXCL13 as a biomarker in iMCD. G.S. has received speaker’s bureau fees from Takeda, Janssen Pharmaceuticals, Foundation Medicine, and EUSA Pharma. J.D.B. has received consulting fees from EUSA Pharma. F.v.R. has received consulting fees from EUSA Pharma, GlaxoSmithKline, Karyopharm, and Takeda; and has received research funding from Janssen Pharmaceuticals and Bristol Myers Squibb. C.C has received consulting fees from EUSA Pharma. The remaining authors report no competing financial interests.
Correspondence: David C. Fajgenbaum, Department of Medicine, Center for Cytokine Storm Treatment & Laboratory, Perelman School of Medicine, University of Pennsylvania, 3535 Market St, Suite 700, Philadelphia, PA 19104; e-mail: davidfa@pennmedicine.upenn.edu.
References
Author notes
Data are available upon reasonable request from accelerate@pennmedicine.upenn.edu.
The full-text version of this article contains a data supplement.