TO THE EDITOR:
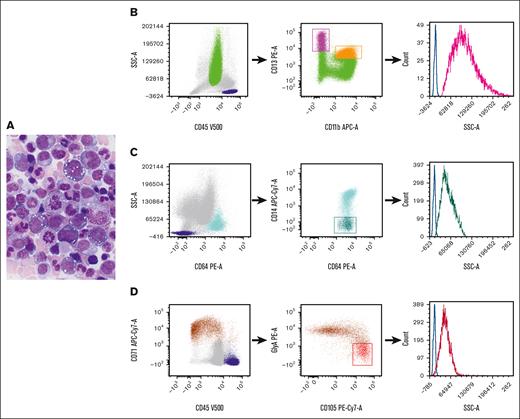
VEXAS (Vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic) syndrome is a systemic autoinflammatory disease caused by somatic mutations in the UBA1 gene and presents with adult-onset, long-term refractory inflammatory symptoms, associated with macrocytic anemia that may progress to pancytopenia, progressive bone marrow (BM) failure, myelodysplastic syndrome, and plasma cell neoplasm.1-3UBA1 mutations are detected in hematopoietic stem and progenitor cells and peripheral-blood myeloid cells but not in mature lymphocytes or fibroblasts.2 The characteristic morphologic features in this syndrome are cytoplasmic vacuoles in myeloid and erythroid precursors.2,4-6Figure 1A depicts a marrow from a patient with VEXAS syndrome, demonstrating cytoplasmic vacuoles in erythroid and myeloid precursors. Detection of a pathogenic UBA1 mutation is required for the diagnosis of VEXAS syndrome. Immunophenotypic alterations detected via flow cytometry have been described,7 but, to our knowledge, there are no specific markers for the identification of this disorder in the BM.
Representative morphology in aspirate smear and flow cytometric gating strategy. (A) Wright-Giemsa–stained BM aspirate smear from a patient with VEXAS syndrome, demonstrating cytoplasmic vacuoles in myeloid and erythroid precursors. (B-D). Flow cytometric gating strategy for (B) neutrophil, (C) monocyte, and (D) erythroid precursors. (B) Dot plots of neutrophils selected based on their CD45 expression and SSC properties (green). The CD11b and CD13 expression on neutrophils allows for the delineation of neutrophil maturation stages, ranging from the least mature (high CD13/low CD11b) (magenta) to the most mature ones (high CD13/high CD11b) (orange).8 (C) The identification of monocytes (light blue) based on their intense CD64 expression and SSC properties, and the selection of immature monocytes based on their poor expression of CD14 (dark green). (D) The selection of nucleated red blood cells (brown) based on their intense CD71 expression and poor or no expression of CD45. Early red cell precursors are identified by their high CD105 and relatively low glycophorin A expression (red). The histograms on the right graphs depict the SSC intensity of precursors (neutrophil precursors, magenta; monocyte precursors, dark green; and early red cell precursors, red). Lymphocytes are selected based on CD45 and SSC properties (dark blue) and the SSC intensity of CD4+ cells (identified based on their coexpression of CD3 and CD4; not shown) serves as internal controls (blue lines). APC-Cy, allophycocyanin; GlyA, glycophorin A; PE, phycoerythrin.
Representative morphology in aspirate smear and flow cytometric gating strategy. (A) Wright-Giemsa–stained BM aspirate smear from a patient with VEXAS syndrome, demonstrating cytoplasmic vacuoles in myeloid and erythroid precursors. (B-D). Flow cytometric gating strategy for (B) neutrophil, (C) monocyte, and (D) erythroid precursors. (B) Dot plots of neutrophils selected based on their CD45 expression and SSC properties (green). The CD11b and CD13 expression on neutrophils allows for the delineation of neutrophil maturation stages, ranging from the least mature (high CD13/low CD11b) (magenta) to the most mature ones (high CD13/high CD11b) (orange).8 (C) The identification of monocytes (light blue) based on their intense CD64 expression and SSC properties, and the selection of immature monocytes based on their poor expression of CD14 (dark green). (D) The selection of nucleated red blood cells (brown) based on their intense CD71 expression and poor or no expression of CD45. Early red cell precursors are identified by their high CD105 and relatively low glycophorin A expression (red). The histograms on the right graphs depict the SSC intensity of precursors (neutrophil precursors, magenta; monocyte precursors, dark green; and early red cell precursors, red). Lymphocytes are selected based on CD45 and SSC properties (dark blue) and the SSC intensity of CD4+ cells (identified based on their coexpression of CD3 and CD4; not shown) serves as internal controls (blue lines). APC-Cy, allophycocyanin; GlyA, glycophorin A; PE, phycoerythrin.
Flow cytometry is a useful technology for the diagnosis of lymphohematopoietic diseases. Light scattering, together with fluorescence signals, is routinely used for the characterization of hemopoietic and lymphoid cells. The intensity of the light scattered at different angles from the light excitation point provides information regarding cellular and physical properties. Forward and side scatters (SSCs) are the 2 flow cytometric nonfluorescent light measurements that are informative of cell size and internal complexity, respectively. SSC intensity increases with increase in the quantity of cytoplasmic granules9 and also reflects the presence of vacuole-like structures within the cytoplasm of neutrophils producing reactive oxygen species.10 Here, we report a simple flow cytometric analysis strategy to recognize likely vacuolated neutrophil, monocytic, and erythroid precursors in the BMs of patients with VEXAS syndrome through SSC intensity measurements.
We studied 27 patients with VEXAS syndrome with confirmed UBA1 mutations. Patients were all male, with a median age of 68 years. Concurrent myelodysplastic syndrome was diagnosed in 8 of these patients. Cytoplasmic vacuoles in marrow cell precursors were observed in 26 patients. One patient received an autologous stem cell transplant, and his marrow cells after HSCT showed no vacuoles. A group of 106 randomly selected patients without VEXAS syndrome served as controls. Their diagnoses included aplastic anemia (n = 34), myelodysplastic syndrome with wild-type UBA1 gene (MDS UBA1-WT) (n = 32), non-VEXAS syndrome marrows with cytoplasmic vacuoles in precursors (n = 13), and other conditions, possibly associated with stressed marrow (n = 27). Detailed information on the control cases is shown in supplemental Table 1. All patients were enrolled in institutional review board–approved National Institutes of Health protocols.
Sample handling and analysis are described in supplemental Methods. The data in each sample were analyzed as follows: neutrophil, monocyte, and erythroid precursors were selected using the gating strategy depicted in Figure 1B-D. CD4+ T cells that served as internal controls were identified as a subset of CD3+ lymphocytes using a standard gating strategy. The light intensity of the SSC for neutrophil, monocyte, and erythroid precursors was expressed as the SSC ratio, which was calculated by dividing the SSC geometric mean intensity of the population of interest by that of CD4+ T lymphocytes from the same sample. Student t test was used to compare differences among groups, and 2-sided P values < 0.05 were considered statistically significant.
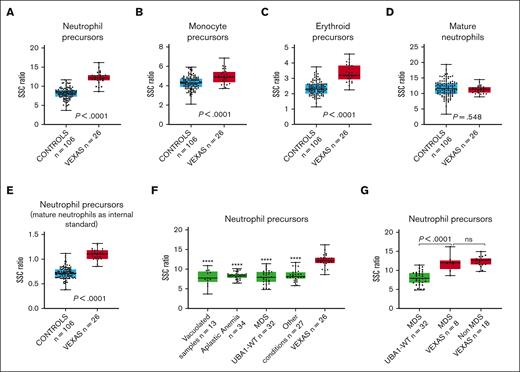
Cytoplasmic vacuoles in VEXAS syndrome are predominantly found in myeloid and erythroid precursors.2,11 SSC intensity, measured using flow cytometry, reflects the internal complexity of cells, and it would be anticipated that the presence of vacuoles increases the intensity of the SSC light. This was confirmed through our results. Our analysis demonstrated a significantly higher SSC ratio for neutrophil, monocytic, and erythroid precursors (Figure 2A-C). The difference was most striking among neutrophil precursors and less prominent for monocyte precursors, probably because of the frequent cytoplasmic vacuolization observed in monocytes in many conditions. In contrast, there was no difference in SSC ratios when the measurements were made using mature neutrophils instead of precursors (Figure 2D). Based on the receiver operating curve for the entire VEXAS syndrome and control cohort (area under the curve = 0.97; supplemental Figure 1), using a SSC ratio cutoff for neutrophil precursors of 11 (sensitivity = 0.85; specificity = 0.98), the positive predictive value for detecting VEXAS syndrome cases was 91.7%, whereas the negative predictive value was 96.3%. However, these values would be affected by disease prevalence, which is known to be low.12 In addition, we calculated the SSC intensity of neutrophil precursors using mature neutrophils as internal controls. This precursor-to-mature neutrophil SSC ratio also demonstrated a significantly higher value in VEXAS syndrome marrows (Figure 2E), but mature neutrophils may not be ideal controls, because hypogranularity in these cells may result in decreased SSC intensity.
Box plots displaying the SSC ratios of marrow cells in patients with VEXAS syndrome and in controls. SSC ratios of (A) neutrophil precursors, (B) monocyte precursors, (C) erythroid precursors, and (D) mature neutrophils in VEXAS syndrome marrows (n = 26) compared with those of the entire control group (n = 106), using SSC intensity of CD4+ T cells as an internal control. (E) SSC ratio of neutrophil precursors in marrow samples of patients with VEXAS syndrome (n = 26) compared with that of the entire control group (n = 106), using SSC intensity of mature neutrophil instead of CD4+ lymphocytes as an internal control. (F) SSC ratio of neutrophil precursors in marrow samples of patients with VEXAS syndrome compared with that of subgroups of marrows in the control cohort, including non-VEXAS syndrome marrows with vacuolated cells, marrow samples of patients with aplastic anemia, marrow samples of patients with MDS UBA1-WT, and marrow sample of patients with other conditions (stressed marrows). (G) SSC ratio of neutrophil precursors in marrow samples of patients with VEXAS syndrome with MDS compared with marrows from those with MDS UBA1-WT (P < .0001) and non-MDS VEXAS syndrome (P = .55). In each graph, the boxes show median and lower and upper quartiles, and the whiskers show the full range of the data. Individual data (black dots) are superimposed on the box plots. ∗∗∗∗P < .0001 when SSC ratio of neutrophil precursors in each subgroup is compared with those of marrow samples of patients with VEXAS syndrome.
Box plots displaying the SSC ratios of marrow cells in patients with VEXAS syndrome and in controls. SSC ratios of (A) neutrophil precursors, (B) monocyte precursors, (C) erythroid precursors, and (D) mature neutrophils in VEXAS syndrome marrows (n = 26) compared with those of the entire control group (n = 106), using SSC intensity of CD4+ T cells as an internal control. (E) SSC ratio of neutrophil precursors in marrow samples of patients with VEXAS syndrome (n = 26) compared with that of the entire control group (n = 106), using SSC intensity of mature neutrophil instead of CD4+ lymphocytes as an internal control. (F) SSC ratio of neutrophil precursors in marrow samples of patients with VEXAS syndrome compared with that of subgroups of marrows in the control cohort, including non-VEXAS syndrome marrows with vacuolated cells, marrow samples of patients with aplastic anemia, marrow samples of patients with MDS UBA1-WT, and marrow sample of patients with other conditions (stressed marrows). (G) SSC ratio of neutrophil precursors in marrow samples of patients with VEXAS syndrome with MDS compared with marrows from those with MDS UBA1-WT (P < .0001) and non-MDS VEXAS syndrome (P = .55). In each graph, the boxes show median and lower and upper quartiles, and the whiskers show the full range of the data. Individual data (black dots) are superimposed on the box plots. ∗∗∗∗P < .0001 when SSC ratio of neutrophil precursors in each subgroup is compared with those of marrow samples of patients with VEXAS syndrome.
Cytoplasmic vacuoles in myeloid and erythroid precursors can be found in conditions other than VEXAS syndrome.13-17 In this study, we found significantly higher SSC ratios for neutrophil precursors in VEXAS syndrome cases when compared with non-VEXAS syndrome marrows containing precursor cells with cytoplasmic vacuoles (Figure 2F). Patients with VEXAS syndrome usually present with characteristic macrocytic anemia and may develop progressive pancytopenia and BM failure.1-3 When compared with marrows of patients with aplastic anemia, the SSC ratio in neutrophil precursors was significantly higher in VEXAS syndrome cases (Figure 2F). A similar finding was observed when VEXAS syndrome marrow samples were compared with samples derived from other conditions (Figure 2F).
BM hypercellularity with dysplasia is frequently present in VEXAS syndrome,11 and these findings often overlap with those seen in non-VEXAS syndrome (UBA1-WT) MDS.1,18 We compared the VEXAS syndrome marrows with those of with patients with MDS UBA1-WT. The neutrophil precursors in the marrows from patients with VEXAS syndrome showed significantly higher SSC ratios than those in marrows from patients with MDS UBA1-WT (Figure 2F). Similar findings were observed when only MDS VEXAS syndrome cases were compared with MDS UBA1-WT cases (Figure 2G). There was no significant difference in the SSC ratio of neutrophil precursors when non-MDS cases were compared with MDS cases within the VEXAS syndrome cohort (Figure 2G). Vacuolization may be found in myeloid cells in MDS UBA1-WT19,20 However, in the 3 cases of MDS UBA1-WT marrow with vacuolization, the SSC intensity in neutrophil precursors was lower than that in the VEXAS syndrome cases (data not shown), suggesting that the vacuoles in the MDS UBA1-WT cases may be less numerous or smaller than those in VEXAS syndrome cases.4
Interestingly, the SSC intensity of marrow precursors in a patient with VEXAS syndrome who underwent transplantation because of a concurrent plasma cell neoplasm and did not show vacuoles in the marrow cells was much lower than that in VEXAS syndrome cases with vacuolization and comparable with that of the control group (supplemental Figure 2), further suggesting that the higher SSC in neutrophil and erythroid precursors in patients with VEXAS syndrome is, indeed, due to vacuolization.
Cytoplasmic vacuoles are not specific to VEXAS syndrome, given that they have been reported in other disorders.13-17 However, the presence of a high number of significantly vacuolated myeloid precursors has been associated with VEXAS syndrome with an excellent sensitivity and specificity.4,21 Thus, in the appropriate clinical and laboratory context, the detection of high SSC intensity signals from myeloid or erythroid precursors should raise high suspicion for VEXAS syndrome, a diagnosis that would then have to be confirmed via molecular testing. The ability to quantify cellular vacuolization may potentially be helpful in assessing disease burden in patients with VEXAS syndrome. Additionally, the identification of vacuolated cells via flow cytometry could also facilitate the sorting of mutated cells, which may be useful for functional and biochemical studies in this disease.
Acknowledgments: This work was supported by grants from the Intramural Research Program of the National Institutes of Health (NIH) and the NIH Clinical Center.
Contribution: Y.D. and R.C.B. designed the study, performed analysis, and wrote the manuscript; E.M.G., B.A.P., D.B.B., P.C.G., M.A.F., and N.S.Y. provided clinical care to patients; Y.D., A.E.D.-F., K.R.C., and R.C.B. provided pathologic interpretation and recommendations; and E.M.G., B.A.P., D.B.B., P.C.G., M.A.F., and N.S.Y. edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Raul C. Braylan, Department of Laboratory Medicine, Clinical Center, National Institutes of Health, 10 Center Dr, Bethesda, MD 20892; e-mail: raul.braylan@nih.gov.
References
Author notes
Original data are available on request from the corresponding author, Raul C. Braylan (raul.braylan@nih.gov).
The full-text version of this article contains a data supplement.