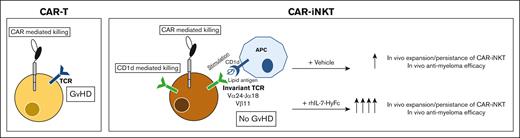
Key Points
Human BCMA CAR-iNKTs show in vivo efficacy in a myeloma xenograft model that can be enhanced by a long-acting IL-7, rhIL-7-hyFc.
CAR-iNKTs may have lower CRS risk than CAR-Ts.
Abstract
Multiple myeloma (MM), a malignancy of mature plasma cells, remains incurable. B-cell maturation antigen (BCMA) is the lead protein target for chimeric antigen receptor (CAR) therapy because of its high expression in most MM, with limited expression in other cell types, resulting in favorable on-target, off tumor toxicity. The response rate to autologous BCMA CAR-T therapy is high; however, it is not curative and is associated with risks of cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome. Outcomes in patients treated with BCMA CAR-T cells (CAR-Ts) may improve with allogeneic CAR T-cell therapy, which offer higher cell fitness and reduced time to treatment. However, to prevent the risk of graft-versus-host disease (GVHD), allogenic BCMA CAR-Ts require genetic deletion of the T-cell receptor (TCR), which has potential for unexpected functional or phenotype changes. Invariant natural killer T cells (iNKTs) have an invariant TCR that does not cause GVHD and, as a result, can be used in an allogeneic setting without the need for TCR gene editing. We demonstrate significant anti-myeloma activity of BCMA CAR-iNKTs in a xenograft mouse model of myeloma. We found that a long-acting interleukin-7 (IL-7), rhIL-7-hyFc, significantly prolonged survival and reduced tumor burden in BCMA CAR-iNKT–treated mice in both primary and re-challenge settings. Furthermore, in CRS in vitro assays, CAR-iNKTs induced less IL-6 than CAR-Ts, suggesting a reduced likelihood of CAR-iNKT therapy to induce CRS in patients. These data suggest that BCMA CAR-iNKTs are potentially a safer, effective alternative to BCMA CAR-Ts and that BCMA CAR-iNKT efficacy is further potentiated with rhIL-7-hyFc.
Introduction
Multiple myeloma (MM), a malignancy of mature plasma cells, is the second most common blood cancer, and although expanded treatment options have improved patient outcomes,1 it remains incurable. B-cell maturation antigen (BCMA) is the lead immunotherapy target in MM because of high expression on myeloma cells and limited expression on non-tumor cells (restricted to plasma and late B cells). Although BCMA chimeric antigen receptor (CAR) T-cell response rates are high, it comes with risk of cytokine release syndrome (CRS) and neurotoxicity, and the duration of response is limited.2-7 The reasons for the lack of durable response by BCMA CAR-T-cell therapy are likely because of the immunosuppressive immune environment in MM and/or reduced cell fitness of heavily pretreated lymphocytes for CAR T-cell generation and, therefore, activity/persistence of autologous CAR-T cells (CAR-Ts). One strategy to improve durability is to treat with allogenic BCMA CAR-T-cell therapy. The advantages of allogenic CAR-Ts are improved cell fitness, streamlined logistics, reduced time to treatment because of “off-the-shelf” production, and lower cost. However, allogenic CAR-Ts require genetic deletion of the endogenous T-cell receptor (TCR) to prevent graft-versus-host disease (GVHD). An alternative promising strategy for allogeneic CAR-Ts is to use non-αβ T cells, including natural killer (NK), γδ T cells, or invariant natural killer T cells (iNKTs), which do not require genetic deletion of their TCR to prevent GVHD, alleviating concerns of potential negative impacts of gene editing on the CAR-modified cellular product.
Unlike T cells, iNKTs express an invariant TCRα (Vα24-Jα18) chain and thus, will not cause GVHD and, in mouse models, can even mitigate GVHD.8,9 Although they make up <1% of peripheral blood mononuclear cells (PBMCs), iNKTs can be extensively expanded by stimulation with CD1d-expressing antigen-presenting cells in the presence of the α-galactosyl ceramide glycolipid (αgc). When engineered to express a CAR (CAR-iNKT), they demonstrate CAR-mediated antitumor cytotoxicity.10-15 Furthermore, the iNKT TCR can induce direct killing of CD1d-expressing tumor cells. Their robust expansion, lack of GVHD potential, combined with both TCR- and CAR-mediated killing make iNKTs an attractive off-the-shelf alternative cellular therapy to CAR-T-cell therapy.
iNKTs express multiple cytokine receptors, including interleukin-7 (IL-7),11,12 which is integral to the development and function of both NKT and T cells.16 A recombinant human interlukin-7 (IL-7) with an extended half-life (rhIL-7-hyFc; efineptakin α) significantly increased expansion of T cells in patients with cancer and healthy volunteers.17,18 Our group demonstrated that mice with tumor engraftment treated with CAR-Ts plus rhIL-7-hyFc had dramatic expansion of CAR-Ts and significantly better tumor control than CAR-T–treated mice.19 These studies provided rational for an ongoing clinical trial combining rhIL-7-hyFc and CD19 CAR-Ts in patients with relapsed/refractory large B-cell lymphoma (NCT05075603). Here, we show anti-myeloma efficacy of BCMA CAR-iNKTs in an NSG/MM.1S myeloma model. We show enhanced expansion and persistence of BCMA CAR-iNKTs in mice with tumor engraftment that were also treated with rhIL-7-hyFc, which led to significant reduction of tumor burden and prolonged survival. In a model of CRS, through side-by-side comparisons of CAR-Ts, CAR-iNKTs, and negative control CAR memory-like (ML) NK cells, we found lower levels of secreted IL-6 by CAR-iNKT and CAR-ML NK cells than that by CAR-Ts, suggesting the possibility for reduced CRS potential of CAR-iNKTs.
Methods
CAR constructs
The CD1920 and BCMA (clone J22.xi) single-chain variable fragment sequences (patent WO2014068079A1) were synthesized (Genescripts) and cloned into PLV (Vector Builder) or PELNS (kindly provided by Carl June, University of Pennsylvania) vectors.
Generation of CAR-iNKTs, CAR-Ts and CAR-ML NK cells
iNKTs, T cells, NK cells, and monocytes were isolated using leftover PBMC product from deidentified platelet donors (Barnes Jewish Hospital) or Leukopacks (Miltenyi) and sorted using anti-Vα24Jα18 (iNKTs), PAN-T, NK, or classical monocyte magnetic beads on an autoMACS (Miltenyi). Negative fraction cells were irradiated (40 Gy) and incubated with 100 ng/mL αgc (Enzo) for 1 hour. Two protocols were used to generate CAR-iNKTs with similar results. Protocol 1: iNKTs were co-cultured with irradiated PBMCs (10:1 ratio) and αgc on days 0, 5, or 6 and day ±18 of iNKT culture in RPMI 1640 medium with 200 U/mL human IL-2 (Peprotech), 10% fetal bovine serum, Glutamax (Gibco), 50 mM β-mercaptoethanol (Gibco), 1% penicillin/streptomycin, 1% sodium pyruvate, and 2% N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (all from Corning). iNKTs were transduced (day +13) with lentivirus in the presence of 6 μg/mL polybrene (Sigma) and centrifuged for 1.5 hours at 1000g. Protocol 2: iNKTs were cocultured with irradiated PBMCs and αgc on days 0, 6, and ±18, transduced on day +1 with 6 μg/mL polybrene, and maintained in Optimizer media, serum replacement (Thermo Fisher), and 200 U/mL human IL-2. CAR-Ts and CAR-ML NKs were generated as previously described.20,21
Lentivirus production
Lentivirus was produced as previously described.21
CRS and killing assays
Monocytes were matured for 5 days into immature DCs (iDCs) by culturing in RPMI, 10% human serum type AB (Gemini Bio), 2000 U/mL IL-4, and 2000 U/mL granulocyte-macrophage colony–stimulating factor (GM-CSF; Peprotech). Effector cells were cocultured with targets at a range of effector-to-target (E:T) ratios in presence of iDCs for 48 hours. Luciferin (150 μg/μL) was added to plates and imaged (AMI Imager; Spectral Instruments) to measure photon flux. Medium from lowest E:T ratio that induced 100% killing (most often 0.5:1 (25 000 effectors, 50 000 targets, and 5000 iDCs) was used to measure cytokines via enzyme-linked immunosorbent assay (ELISA; R&D systems) or Luminex assays (Millipore-Sigma) Killing assays were performed as above but without iDCs.
Flow cytometry
Antibodies: CD3 (UCHT1; BD Horizon), CD4 (RPA-T4; BD Pharmingen), CD34 Pool (Beckman Coulter), anti-Jα18-vα24 (6B11; Miltenyi), and 7AAD (BD Pharmingen). Samples were run on an Attune Cytometer and analyzed using FlowJo version 10 (TreeStar).
Animal model and in vivo efficacy
Animal protocols were compliant with Washington University School of Medicine Animal Studies Committee regulations. NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice, 6 to 10 weeks old, were injected with 500 000 MM.1S-CG cells and treated with CAR iNKTs (IV into tail veins). rhIL-7-hyFc (10 mg/kg; NeoImmuneTech, Inc) was injected via subcutaneous injection on days +1 and +14 after CAR-iNKT administration. Bioluminescent imaging (BLI) was performed as previously described.22 Tumor burden was described using mean and standard deviation at each time point. Between-group differences and over-time changes were assessed using a linear mixed model incorporating random intercept and slope; time was centered at the median of measurement days. Logarithmic transformation was performed to tumor burden to satisfy the assumptions of normality distribution and homogeneity of variance. All tests were 2-sided; significance was set at P = .05. Analyses were performed using SAS 9.4 (SAS Institutes). Significant differences in survival were determined using Mantel-Cox analysis.
Results
BCMA CAR-iNKT demonstrates anti-myeloma activity in vitro and in vivo
To assess the efficacy of BCMA CAR-iNKT, we first used the same CAR construct that showed anti-myeloma activity in our BCMA CAR T-cell experiments (supplemental Figure 1).21 This construct is driven by the EF-1α promoter and is composed of the single-chain variable fragment, a CD8 hinge, a CD28 transmembrane domain, CD28 and 4-1BB costimulatory domains, and the CD3ζ signaling domain. A P2A self-cleaving peptide followed by a truncated human CD34 protein enables detection and purification of CAR-expressing cells.
To generate BCMA CAR-iNKTs, we co-cultured iNKT with irradiated PBMCs in the presence of αgc and transduced these cells with lentivirus encoding the BCMA CAR construct. Flow cytometry was used to confirm iNKT purity, quantify CAR+ cells before and after sorting (when applicable), and assess CD4 distribution (Figure 1B). BCMA CAR-Ts were generated from T cells isolated from the same healthy donor. BCMA-expressing MM.1S or OPM2 (modified to express a click beetle red luciferase–green fluorescent protein fusion protein; MM.1S-CG; OPM2-CG) target cells were co-cultured with BCMA CAR-Ts or BCMA CAR-iNKTs at various E:T ratios; 48 hours later, killing was assessed by using BLI. Both BCMA CAR-Ts and BCMA CAR-iNKTs efficiently killed MM.1S-CG and OPM2-CG tumor cells, whereas nontransduced (NTD) effectors cells did not (Figure 1C).
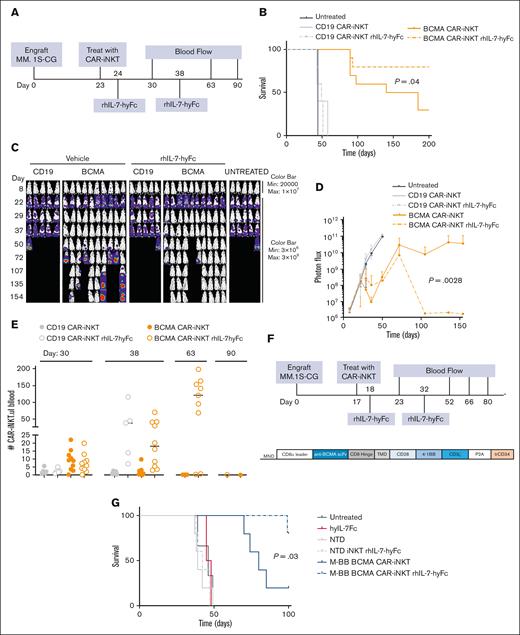
BCMA CAR-iNKTs demonstrate anti-myeloma activity in vitro and in vivo. (A) Constructs. Third-generation CARs comprised the single-chain variable fragment (scFv), a CD8 hinge, a CD28 transmembrane domain, CD28 and 4-1BB intracellular domains, and a CD3ζ chain. The extracellular domain of human CD34 protein (trCD34) after a P2A peptide was incorporated into the construct to enable detection of CAR+ T cells and purification of CAR+ cells for use in functional assays. (B) Flow cytometry showing iNKT purity, CAR+ cell levels, and CD4 distribution. Transduction efficiency before cell sorting was 10% BCMA CAR-iNKTs and 15% CD19 CAR-iNKTs. Data shown are the cells used in panels D to G. (C) BLI-based killing assay of MM.1S-CG and OPM2-CG targets by BCMA CAR-Ts and BCMA CAR-iNKTs 48 hours after coculture. (D) In vivo schema. (E) Kaplan-Meier survival analysis. (F) Normalized BLI images of mice. The asterisk denotes a mouse treated with BCMA CAR-iNKTs (10 × 106) that had low tumor burden but was euthanized because of suffering from a likely neck injury/ataxia. (G) Quantitation of tumor burden over time using BLI. (H) Flow cytometry of mouse blood was used to quantitate the absolute number of CAR-iNKTs per μL blood on day 24 (day 6 after BCMA CAR-iNKT administration) and day 31 after MM.1S-CG engraftment (day 13 after BCMA CAR-iNKT treatment). (I) Kaplan-Meier survival curve of a repeat experiment with mice treated with 2 × 106 BCMA CAR-iNKTs or controls. (J) Quantitation of tumor burden over time using BLI. P values for BLI were comparisons of either CD19 CAR-iNKTs or NTD iNKTs with BCMA CAR-iNKTs. TMD, transmembrane domain.
BCMA CAR-iNKTs demonstrate anti-myeloma activity in vitro and in vivo. (A) Constructs. Third-generation CARs comprised the single-chain variable fragment (scFv), a CD8 hinge, a CD28 transmembrane domain, CD28 and 4-1BB intracellular domains, and a CD3ζ chain. The extracellular domain of human CD34 protein (trCD34) after a P2A peptide was incorporated into the construct to enable detection of CAR+ T cells and purification of CAR+ cells for use in functional assays. (B) Flow cytometry showing iNKT purity, CAR+ cell levels, and CD4 distribution. Transduction efficiency before cell sorting was 10% BCMA CAR-iNKTs and 15% CD19 CAR-iNKTs. Data shown are the cells used in panels D to G. (C) BLI-based killing assay of MM.1S-CG and OPM2-CG targets by BCMA CAR-Ts and BCMA CAR-iNKTs 48 hours after coculture. (D) In vivo schema. (E) Kaplan-Meier survival analysis. (F) Normalized BLI images of mice. The asterisk denotes a mouse treated with BCMA CAR-iNKTs (10 × 106) that had low tumor burden but was euthanized because of suffering from a likely neck injury/ataxia. (G) Quantitation of tumor burden over time using BLI. (H) Flow cytometry of mouse blood was used to quantitate the absolute number of CAR-iNKTs per μL blood on day 24 (day 6 after BCMA CAR-iNKT administration) and day 31 after MM.1S-CG engraftment (day 13 after BCMA CAR-iNKT treatment). (I) Kaplan-Meier survival curve of a repeat experiment with mice treated with 2 × 106 BCMA CAR-iNKTs or controls. (J) Quantitation of tumor burden over time using BLI. P values for BLI were comparisons of either CD19 CAR-iNKTs or NTD iNKTs with BCMA CAR-iNKTs. TMD, transmembrane domain.
Next, we sought to test in vivo efficacy of BCMA CAR-iNKTs. To this end, NSG mice were injected i.v. with 0.5 × 106 MM.1S-CG cells, and 18 days later (BLI signal of 3 × 108), they were treated with 10 × 106 BCMA CAR-iNKTs, 2 × 106 BCMA CAR-iNKTs, or control CD19 CAR -iNKTs (Figure 1D). We chose the 10 × 106 dose to match published reports12,14 and the 2 × 106 dose to match our BCMA CAR-T-cell studies (supplemental Figure 1).21 There was a significant survival benefit and reduction of tumor burden assessed based on longitudinal BLI in mice treated with either dose of BCMA CAR-iNKTs compared with that in untreated or CD19 CAR-iNKT–treated negative controls (Figure 1E-G). CAR-iNKT expansion was evaluated by measuring CAR-iNKTs in the peripheral blood using flow cytometry. We found low but detectable levels of circulating CAR-iNKTs at day +6 after CAR treatment, with a return to low/undetectable levels by days 13 and 28 after CAR treatment (Figure 1H, not shown). A repeat experiment using cells from a separate healthy donor at the 2 × 106 dose showed similar results (Figure 1I-J). Together, these data demonstrate the antimyeloma activity of BCMA CAR-iNKTs.
rhIL-7-hyFc enhanced expansion, persistence, and tumor efficacy of BCMA CAR-iNKTs
Although we saw significant antitumor efficacy of BCMA CAR-iNKTs, we observed transient, low levels of circulating CAR-iNKTs after injection. We predicted that improved expansion of BCMA CAR -iNKTs would lead to better in vivo tumor cytotoxicity. Our group previously showed that administration of a long-acting IL-7, rhIL-7-hyFc, increased the persistence and expansion of CAR-Ts, leading to higher antitumor efficacy.23 Because iNKTs also express the IL-7 receptor (supplemental Figure 2),11,12 we hypothesized that CAR-iNKTs would also show enhanced expansion/persistence and efficacy when combined with rhIL-7-hyFc. To test this, we injected NSG mice with 0.5 × 106 MM.1S-CG (day 0), and when tumor burden was established (BLI signal of 108; day 23), we treated mice with 10 × 106 BCMA CAR-iNKTs plus rhIL-7-hyFc or vehicle control (Figure 2A). BCMA CAR-iNKT (+ vehicle)–treated mice had significantly increased survival compared with CD19 CAR-iNKT negative controls (P < .001), and reduced tumor burden, compared with untreated or CD19 CAR-iNKT negative controls; however, durability of the response was limited (Figure 2B-D). BCMA CAR-iNKT + rhIL-7-hyFc–treated mice had significantly longer survival than BCMA CAR-iNKT–treated mice (P = .04) that had not reached median survival by day 200 and had significantly reduced tumor burden (P = .028) compared with BCMA CAR-iNKT + vehicle–treated mice (Figure 2D). Of the 10 BCMA CAR-iNKT + rhIL-7-hyFc–treated mice, 7 had no detectable tumor on day 200 (Figure 2C). These data demonstrate that mice treated with BCMA CAR-iNKT + rhIL-7-hyFc had improved anti-tumor activity and survival in a mouse model of myeloma.
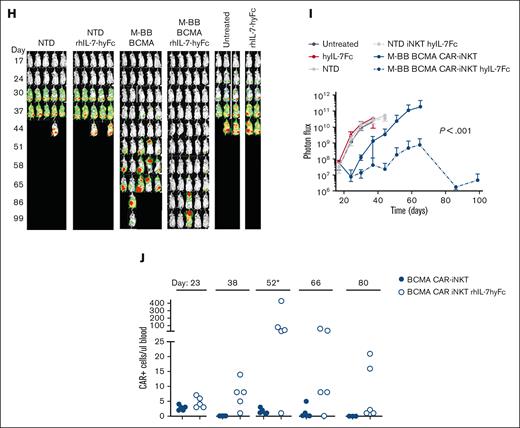
Anti-tumor efficacy of BCMA CAR-iNKTs is enhanced with rhIL-7-hyFc. (A) In vivo schema. Actively growing BCMA CAR-Ts, CD19 CAR-Ts, BCMA CAR-iNKTs and CD19 CAR-iNKTs were used in this experiment. rhIL-7-hyFc (10 mg/kg; NeoImmuneTech, Inc) was injected subcutaneously on days +1 and +14 after CAR-iNKT administration. (B) Kaplan-Meier survival curve. (C) Normalized longitudinal BLI images. (D) Quantitation of photon flux. (E) Absolute number of CAR-iNKTs per μL blood. Because the BCMA CAR-iNKTs were 100% CD4+ (not shown), we initially used CD45, CD3, CD4, and CD34 to track CAR+ iNKTs in vivo. On day 64, the panel was expanded and confirmed that all CD4+ cells were Vα24-Jα18+. (F) In vivo schema. Previously expanded and cryopreserved NTD and M-BB BCMA CAR-iNKTs were used in this experiment. Cells were thawed the day before injection and placed in the incubator overnight, counted, and injected into mice with MM1S-CG engraftment. (G) Kaplan-Meier survival analysis. (H) Longitudinal normalized BLI images. (I) Quantitation of BLI signal. (J) Absolute number of CAR-iNKTs per μL blood (CD45, CD3, Vα24-Jα18, and CD34). On day 52, total CD45+ cells are shown because of a technical issue.
Anti-tumor efficacy of BCMA CAR-iNKTs is enhanced with rhIL-7-hyFc. (A) In vivo schema. Actively growing BCMA CAR-Ts, CD19 CAR-Ts, BCMA CAR-iNKTs and CD19 CAR-iNKTs were used in this experiment. rhIL-7-hyFc (10 mg/kg; NeoImmuneTech, Inc) was injected subcutaneously on days +1 and +14 after CAR-iNKT administration. (B) Kaplan-Meier survival curve. (C) Normalized longitudinal BLI images. (D) Quantitation of photon flux. (E) Absolute number of CAR-iNKTs per μL blood. Because the BCMA CAR-iNKTs were 100% CD4+ (not shown), we initially used CD45, CD3, CD4, and CD34 to track CAR+ iNKTs in vivo. On day 64, the panel was expanded and confirmed that all CD4+ cells were Vα24-Jα18+. (F) In vivo schema. Previously expanded and cryopreserved NTD and M-BB BCMA CAR-iNKTs were used in this experiment. Cells were thawed the day before injection and placed in the incubator overnight, counted, and injected into mice with MM1S-CG engraftment. (G) Kaplan-Meier survival analysis. (H) Longitudinal normalized BLI images. (I) Quantitation of BLI signal. (J) Absolute number of CAR-iNKTs per μL blood (CD45, CD3, Vα24-Jα18, and CD34). On day 52, total CD45+ cells are shown because of a technical issue.
To test our hypothesis that the improved efficacy of BCMA CAR-iNKT by rhIL-7-hyFc was due to increased expansion and survival of CAR-iNKTs, mice were subjected to bleeding longitudinally, and flow cytometry was used to quantitate CAR-Ts (Figure 2E). One week after injection of CAR-iNKTs (day 30), similar levels of CAR+ cells were found in BCMA CAR-iNKT – and BCMA CAR-iNKT + rhIL-7-hyFc–treated mice. In vehicle-treated mice, we detected very few BCMA CAR+ cells at the second time point, and undetectable levels at the third time point. In contrast, rhIL-7-hyFc treatment induced expansion of BCMA CAR-iNKTs to a higher degree on day 38 that was even more pronounced on day 63. These data suggest that BCMA CAR-iNKTs expanded early and then contracted, whereas rhIL-7-hyFc treatment prolonged and amplified expansion of BCMA CAR-iNKTs in vivo.
In addition, we tested whether rhIL-7-hyFc enhanced the efficacy of a second-generation BCMA CAR cells with a 4-1BB costimulatory domain and driven by the MPSV LTR, NCR deleted, and d/587 PBS(MND) promoter 24(M-BB BCMA; Figure 2F), which are used clinically for MM.5,25,26 NSG mice were engrafted with 0.5 × 106 MM.1S-CG, and 17 days later (BLI signal of 3 × 107), mice were treated with 10 × 106 M-BB BCMA CAR-iNKTs + rhIL-7-hyFc or vehicle. Mice treated with M-BB BCMA CAR-iNKTs (+ vehicle) had significantly longer survival than mice treated with NTD iNKT (+ vehicle) (P = .002; Figure 2G). Survival was significantly improved in mice treated with M-BB BCMA CAR-iNKTs + rhIL-7-hyFc (median survival: M-BB BCMA CAR-iNKT, 80 days; M-BB BCMA CAR-iNKT + rhIL-7-hyFc, not reached; P = .03). We also observed significant reduction of tumor burden in mice treated with M-BB BCMA CAR-iNKTs + rhIL-7-hyFc compared with mice treated with M-BB BCMA CAR-iNKTs (P < .001; Figure 2H-I). When given alone, hIL-7-hyFc had no direct anti-tumor activity, confirming that the agent’s activity was directed at iNKTs (Figure 2B; data not shown; multiple experiments).
We tracked exogenously administered effector cells in vivo and observed a small expansion of M-BB BCMA CAR-iNKTs early (day +6 CAR) that was reduced a week later, similar to that in our prior experiment (Figure 2E). Mice treated with M-BB BCMA CAR-iNKT + rhIL-7-hyFc showed higher expansion and persistence of CAR-iNKTs on days 52, 66, and 80 after MM.1S-CG injection (Figure 2J). Our negative control cells were NTD, so we additionally tracked the expansion of total iNKTs, which showed similar trends (supplemental Figure 3). In addition to the expansion of CAR+ cells in both experiments, we observed expansion of negative control iNKTs in rhIL-7-hyFc–treated mice (Figure 2E, J; supplemental Figure 3). Together, these data demonstrate enhanced anti-myeloma activity by rhIL-7-hyFc due to increased expansion and persistence of BCMA CAR-iNKTs with 2 different BCMA CAR constructs.
rhIL-7-hyFc enhances re-expansion and anti-myeloma efficacy of BCMA CAR-iNKTs in vivo
To evaluate the duration of BCMA CAR-iNKT function, we subjected the 7 living, tumor-free mice that were treated with BCMA CAR-iNKTs + rhIL-7-hyFc (in the experiment described earlier in Figure 2C) to re-challenge with 0.5 × 106 MM.1S-CG cells. To test our hypothesis that rhIL-7-hyFc would improve the efficacy of BCMA CAR-iNKTs in the tumor challenge setting, mice were treated with vehicle (n = 3) or a second course of rhIL-7-hyFc (n = 4). As a control, 5 NSG mice were injected with 5 × 105 MM.1S-CG cells and received no treatment. Vehicle-treated, re-challenged mice showed a modest reduction of tumor burden (Figure 3A-B) and a trend toward survival benefit compared with NSG controls (Figure 3C). These results suggest some persistence of functional long-lived BCMA CAR-iNKTs in vivo at the time of re-challenge in mice treated with BCMA CAR-iNKTs + rhIL-7-hyFc. Moreover, mice treated with BCMA CAR-INKT + rhIL-7-hyFc and then treated again with rhIL-7-hyFc at the time of tumor re-challenge had a significant survival benefit (compared with control NSG treated mice; P = .006; Figure 3A-C) and improved tumor control compared with vehicle-treated mice.
rhIL-7-hyFc enhances re-expansion and anti-myeloma efficacy of BCMA CAR-iNKTs in vivo. (A) The 7 rhIL-7-hyFc–treated tumor-free mice at the end of the experiment shown in Figure 2C (BCMA+ rh-IL7-hyFc) were subjected to re-challenge with MM.1S-CG cells and treated with vehicle or again with hIL-7-hyFc (10 mg/kg subcutaneously, the day of MM.1S-CG infusion and, again, 1 week later). Normalized BLI images of MM.1S-CG re-challenged mice. (B) Quantitation of BLI signal in each mouse. (C) Kaplan-Meier survival analysis. (D) Absolute numbers of BCMA CAR-iNKTs per μL blood (CD45 CD3 Vα24-Jα18 CD34) in re-challenged mice.
rhIL-7-hyFc enhances re-expansion and anti-myeloma efficacy of BCMA CAR-iNKTs in vivo. (A) The 7 rhIL-7-hyFc–treated tumor-free mice at the end of the experiment shown in Figure 2C (BCMA+ rh-IL7-hyFc) were subjected to re-challenge with MM.1S-CG cells and treated with vehicle or again with hIL-7-hyFc (10 mg/kg subcutaneously, the day of MM.1S-CG infusion and, again, 1 week later). Normalized BLI images of MM.1S-CG re-challenged mice. (B) Quantitation of BLI signal in each mouse. (C) Kaplan-Meier survival analysis. (D) Absolute numbers of BCMA CAR-iNKTs per μL blood (CD45 CD3 Vα24-Jα18 CD34) in re-challenged mice.
To test our hypothesis that the enhanced cytotoxicity of re-challenge MM.1S-CG tumor cells was due to the expansion of BCMA CAR-iNKTs by rhIL-7-hyFc, we quantitated circulating BCMA CAR-iNKTs. Vehicle-treated mice showed few BCMA CAR-iNKTs, consistent with the more limited anti-tumor response during re-challenge (Figure 3D). Although the kinetics were variable, 3 of 4 mice had dramatic re-expansion of BCMA CAR-iNKTs in the blood after re-treatment with rhIL-7-hyFc (Figure 3D). These data suggest that BCMA CAR-iNKTs can persist long term in vivo, retain anti-tumor function, and that rhIL-7-hyFc can re-expand long-lived BCMA CAR-iNKTs and improve outcomes in the setting of tumor re-challenge.
CAR-iNKTs may have lower CRS potential compared with CAR-Ts
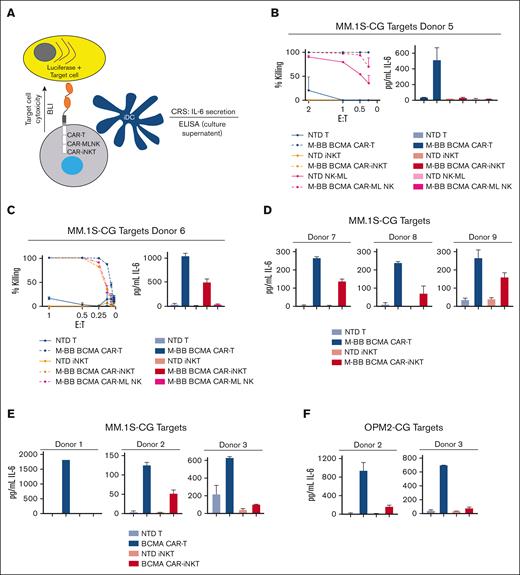
A major safety concern of CAR-Tcell therapy is severe (grade 3-4) CRS and neurotoxicity, which occurs in 15% and 18% of patients treated with BCMA CAR-Ts, respectively.27 It is currently unknown whether BCMA CAR-iNKTs will induce CRS in patients with myeloma, so we tested this using an in vitro model developed in our laboratory (Figure 4A). To assess CRS, BLI killing assays are performed by co-culturing effector and target cells but in the presence of monocytes that have been differentiated into iDCs. In the presence of tumor cytotoxic CRS−inducing effector cells, iDCs secrete IL-6. Quantitation of IL-6 levels in the media is used as a measure of potential CRS.
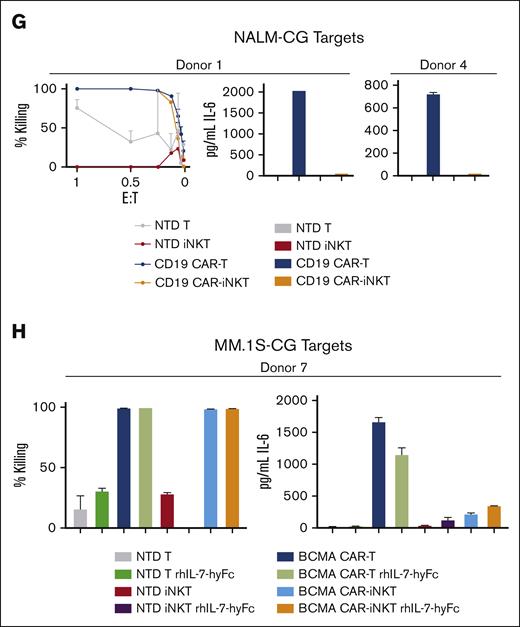
CAR-iNKTs and CAR-ML NK cells have lower CRS potential compared with CAR-Ts. (A) Schema of CRS assay. (B) Killing by M-BB BCMA CAR-Ts, CAR-iNKTs, and CAR-ML NK cells using MM.1S-CG target cells (left); levels of secreted IL-6 (right). Secreted IL-6 levels shown are from cultures at the lowest E:T ratio at which killing was 100% by all effector cell types (most often 0.5:1). All CRS killing and IL-6 assays were performed in duplicate. (C) Similar CRS killing as in panel B but in an independent experiment using cells from a separate donor. Killing (left) and secreted IL-6 levels (right). (D) Three separate CRS experiments comparing M-BB BCMA CAR-Ts and M-BB BCMA CAR-iNKTs and MM.1S target cells. (E) CRS assays comparing BCMA CAR-Ts (EF-1α-BCMA-41BB) and CAR-iNKTs using MM.1S-CG targets or (F) OPM2-CG target cells. (G) Killing (left) and secreted IL-6 (right) using CD19 CAR-Ts, CD19 CAR-iNKTs (EF-1α-CD19-CD28 4-1BB), and NALM-CG target cells, which endogenously express CD19. (H) (Left) Killing efficiency at the 0.5:1 E:T ratio was similar for all BCMA CAR samples. Minimal effect of rhIL-7-hyFc on IL-6 levels in CRS assays. ELISA, enzyme-linked immunosorbent assay.
CAR-iNKTs and CAR-ML NK cells have lower CRS potential compared with CAR-Ts. (A) Schema of CRS assay. (B) Killing by M-BB BCMA CAR-Ts, CAR-iNKTs, and CAR-ML NK cells using MM.1S-CG target cells (left); levels of secreted IL-6 (right). Secreted IL-6 levels shown are from cultures at the lowest E:T ratio at which killing was 100% by all effector cell types (most often 0.5:1). All CRS killing and IL-6 assays were performed in duplicate. (C) Similar CRS killing as in panel B but in an independent experiment using cells from a separate donor. Killing (left) and secreted IL-6 levels (right). (D) Three separate CRS experiments comparing M-BB BCMA CAR-Ts and M-BB BCMA CAR-iNKTs and MM.1S target cells. (E) CRS assays comparing BCMA CAR-Ts (EF-1α-BCMA-41BB) and CAR-iNKTs using MM.1S-CG targets or (F) OPM2-CG target cells. (G) Killing (left) and secreted IL-6 (right) using CD19 CAR-Ts, CD19 CAR-iNKTs (EF-1α-CD19-CD28 4-1BB), and NALM-CG target cells, which endogenously express CD19. (H) (Left) Killing efficiency at the 0.5:1 E:T ratio was similar for all BCMA CAR samples. Minimal effect of rhIL-7-hyFc on IL-6 levels in CRS assays. ELISA, enzyme-linked immunosorbent assay.
NK cell therapies do not cause CRS,28 shown in multiple clinical trials with ML-NK cells,29-31 and a trial using cord blood–derived CD19 CAR-NK cells.32 We, therefore, used CAR-ML NK cells that specifically kill CAR-antigen–positive targets33 as a key clinical negative control for CRS in vitro experiments. Because CAR-ML NK cells have optimal CAR expression and signaling using the MND promoter with a 4-1BB costimulatory domain,33 we used the M-BB BCMA CAR for these studies. We isolated monocytes, T cells, iNKTs, and ML NK cells from the same donor to remove donor-to-donor variability within each experiment and performed assays to directly compare the CRS potential of M-BB-BCMA CAR-Ts, M-BB-BCMA CAR-iNKTs, and M-BB-BCMA CAR-ML NK cells using MM.1S-CG target cells. We found efficient killing by all 3 effector cell types and highest secreted IL-6 induced by M-BB BCMA CAR-Ts compared with M-BB BCMA CAR-iNKTs and M-BB BCMA CAR-ML NK cells in 2 separate experiments (Figure 4B-C). To extend these results, we directly compared the CRS potential of M-BB BCMA CAR-Ts and MND BCMA CAR-iNKTs isolated from 3 other matched donors (Figure 4D) and observed similar trends, that is, the CAR-Ts induced higher IL-6 secretion than CAR-iNKTs.
In addition to testing the M-BB BCMA CAR, we assessed the original BCMA CAR construct (EF-1α BCMA-CD28-41BB) by comparing BCMA CAR-Ts and BCMA CAR-iNKTs in CRS assays using MM.1S-CG targets from 3 separate donors (matched; Figure 4E) or OPM2-CG targets (2 matched donors; Figure 4F). BCMA CAR-Ts induced higher levels of IL-6 compared with BCMA CAR-iNKTs. To confirm that these results were not specific to BCMA, we ran CRS assays using CD19 CAR-Ts and CD19 CAR-iNKTs (EF-1α-CD19-CD28 4-1BB) and NALM-CG (CD19+) targets and, again, found that CAR-Ts induced higher IL-6 secretion than CAR-iNKTs (Figure 4G). Furthermore, we measured levels of secreted GM-CSF, also implicated in CRS,34 and found mostly lower levels induced by CAR-iNKTs compared with that by CAR-Ts, albeit with some variability (supplemental Figure 5). Although there was some variability of absolute IL-6 levels across donors, together, these data suggest lower CRS potential of CAR iNKTs than that of CAR-Ts, assessed using 2 separate BCMA CAR designs and a CD19 CAR.
To determine whether rhIL-7-hyFc affected IL-6 levels in our CRS assays, we added 1000 ng/mL rhIL-7-hyFc or vehicle into CRS assays using BCMA CAR-T and BCMA CAR-iNKT effectors and MM.1S-CG targets. We found that rhIL-7-hyFc did not negatively affect cytotoxicity or IL-6 levels (Figure 4H).
INF-γ, TNF, and IL-2 secreted by BCMA CAR-Ts compared with BCMA CAR-iNKTs and BCMA CAR-ML NK cells
Next, we compared effector cytokine secretion induced by BCMA CAR-Ts, CAR-iNKTs, and CAR-ML NK cells (all donor matched; M-BB BCMA CAR). To this end, we harvested media from killing assays shown in Figure 4 and quantitated secreted interferon γ (INF-γ), tumor necrosis factor (TNF), and IL-2. M-BB BCMA CAR-Ts secreted the highest levels of all 3 cytokines (donor 5) and highest IL-2 levels (donor 6). M-BB BCMA CAR-Ts and CAR-iNKTs secreted similar levels of TNF and INF-γ (donor 6), whereas M-BB BCMA CAR-ML NK cells showed the lowest levels of secreted cytokines (Figure 5A-B). To expand these results, we compared levels of effector cytokine secretion by BCMA CAR-Ts and BCMA CAR-iNKTs in our CRS assay using effector cells generated from 2 other separate, matched donors and either MM.1S-CG or OPM2-CG target cells. Although total cytokine levels showed some variability, in most studies shown, BCMA CAR-Ts induced higher levels of all 3 cytokines compared with BCMA CAR-iNKTs (Figure 5C). We also saw no major changes of effector cytokines by rhIL-7-hyFc in these short-term assays (Figure 5D).
High effector cytokine secretion by BCMA CAR-Ts compared with that by BCMA CAR-iNKTs and BCMA CAR-ML NK cells. (A) Media from CRS assays shown in Figure 4A was used to measure INF-γ, TNF, and IL-2 effector cytokines. Direct comparison of M-BB BCMA CAR-Ts, CAR-ML NK cells, and CAR-iNKTs (1:1 E:T ratio). (B) A similar experiment shown in panel A from separate donor (0.5:1 E:T ratio) and from the experiment shown in Figure 4B. (C) Effector cytokines secretion in CRS assays from 2 separate donors and either MM.1S-CG or OPM2-CG target cells. Media obtained from the CRS assay shown in Figure 4D-E. (D) rhIL-7-hyFc (1000 ng/mL) was added to standard CRS assays, and effector cytokine levels were measured.
High effector cytokine secretion by BCMA CAR-Ts compared with that by BCMA CAR-iNKTs and BCMA CAR-ML NK cells. (A) Media from CRS assays shown in Figure 4A was used to measure INF-γ, TNF, and IL-2 effector cytokines. Direct comparison of M-BB BCMA CAR-Ts, CAR-ML NK cells, and CAR-iNKTs (1:1 E:T ratio). (B) A similar experiment shown in panel A from separate donor (0.5:1 E:T ratio) and from the experiment shown in Figure 4B. (C) Effector cytokines secretion in CRS assays from 2 separate donors and either MM.1S-CG or OPM2-CG target cells. Media obtained from the CRS assay shown in Figure 4D-E. (D) rhIL-7-hyFc (1000 ng/mL) was added to standard CRS assays, and effector cytokine levels were measured.
The mechanisms of CRS are not completely defined. We hypothesized that the difference in effector cytokine levels may partly be responsible for the differences in IL-6 production in our CRS assay. Because INF-γ is implicated in CRS,35 we tested whether adding recombinant INF-γ to CRS assays would lead to increased IL-6. We found no increase in IL-6 when INF-γ was added, suggesting exogenous INF-γ alone was not sufficient to induce IL-6 secretion by iDCs in this assay (supplemental Figure 4). We ran similar assays testing addition of TNF or IL-2, and found no significant increase of IL-6 by either CAR-Ts or CAR-iNKTs nor any effects on killing efficiency (supplemental Figure 4). These results suggest that the overall cytokine milieu and/or direct cell-to-cell interactions both likely contribute to mechanisms of CRS.
Discussion
Here, we demonstrate anti-myeloma activity of BCMA CAR-iNKTs, a natural off-the-shelf cell source, in a xenograft mouse model of myeloma. We found that mice treated with BCMA CAR-iNKTs + rhIL-7-hyFc had enhanced expansion and persistence of BCMA CAR-iNKTs compared with mice treated with BCMA CAR-iNKTs alone, leading to superior tumor control. We also found that CAR iNKTs may have a lower risk of CRS compared with CAR-Ts.
We first demonstrated anti-myeloma activity of BCMA CAR-iNKTs using 2 treatment doses. We used 2 × 106 per mouse to match typical preclinical doses of CAR-Ts21 and 10 × 106 per mouse to match the few published CAR-iNKT studies.12,14 At the 2 × 106 dose, we observed anti-myeloma efficacy; however, it was inferior to 2 × 106 CAR-Ts (Figures 1-2; supplement Figure 1), suggesting that in our model, the potency of CAR-iNKTs was inferior. Because MM1S-CG cells are not CD1d+, our studies specifically assessed CAR-dependent anti-myeloma activity but not the contribution of the invariant TCR, which we, and others, have shown to mediate direct killing of CD1d+ tumor targets (supplemental Figure 6).14 These studies demonstrate that the CD1d-TCR axis is functional in CAR iNKTs, and although CD1d is not universally expressed on human myeloma cells,36 in human patients BCMA CAR-iNKTs are predicted to kill heterogenous tumor populations expressing BCMA and/or CD1d proteins. That, combined with the ability of iNKTs to promote antitumor activity of T cells and NK cells37 and studies showing higher efficacy of CAR-iNKTs in allogeneic settings,38 predicts the efficacy of CAR-iNKT activity in human patients.
To enhance CAR-iNKT efficacy, we tested our prediction that long-acting IL-7 would increase the expansion/persistence of CAR-iNKT and, therefore, tumor control. We saw a modest expansion of BCMA CAR-iNKTs with or without rhIL-7-hyFc that contracted by 2 weeks. In contrast, rhIL-7-hyFc induced a second, delayed expansion of BCMA CAR-iNKTs, responsible for the longer-term tumor control. The resultant persisting BCMA CAR-iNKTs better controlled MM1S-CG tumor in a re-challenge setting, when mice were treated with rhIL-7-hyFc again. Donor variability is a known property of iNKTs,8 affecting in vitro expansion and immunophenotype. CD4 distribution on iNKTs is typically a mix of CD4− and CD4+ cells, or a mostly CD4+ cell population. The immunophenotype of the CAR-iNKTs that responded to rhIL-7-hyFc in vivo was 100% CD4+; 63% CD62L+ (Figure 2A-E), and 100% CD4+ and 62% CD62L+ (Figure 2F-J). CD4+ and/or CD62L+ CAR- iNKTs are reported to have better in vivo persistence, cytolytic activity, and higher IL-7R+ expression12,39 and may explain why we observed a response to rhIL-7-hyFc. Future experiments aimed at characterizing the immunophenotype and cytokine secretion of CAR-iNKTs before and after rhIL-7-HyFc will clarify mechanisms of response of iNKT subsets to IL-7 as will studies testing the effects in immunophenotypic-sorted iNKT subsets. Prior studies showed higher efficacy of CAR-iNKTs in allogeneic mouse models compared with that in autologous models because of CAR-iNKT interactions with host dendritic cells (DCs) and CD8 T cells contributing to sustained tumor control.38 rhIL-7-hyFc, in this setting, would enhance CAR-iNKT expansion/persistence and also expand host T cells, which may potentiate these indirect effects of CAR-iNKTs on the host immune system.
Although iNKT do not cause GVHD, similar to other allogeneic products (eg, CAR-Tand stem cell transplantation), methods to prevent rejection of CAR-iNKTs by the host immune system, such as lymphodepletion with fludarabine and cyclophosphamide, are essential. The first clinical trial testing allogeneic CD19–specific CAR-iNKTs (NCT03774654) uses fludarabine/cyclophosphamide conditioning plus short interfering RNA–mediated reduction of β2M (HLA class I) and CD74 (HLA class II) to prevent rejection. Treatment with IL-7 potentially adds a layer of complexity to allogeneic CAR-iNKTs. The NSG model represents aspects of the lymphodepletion setting in which competition from normal lymphocytes would be minimal. However, because IL-7 accelerates immune reconstitution, this could increase host rejection of CAR iNKTs and may lower IL-7–mediated expansion of CAR-iNKTs via competition for IL-7. A prior study by our group23 showed that rhIL-7-hyFc enhanced CAR-T cell expansion in immunodeficient and immunocompetent models, but expansion levels were tempered in the immunocompetent model, presumably because of competition for rhIL-7-hyFc by endogenous T cells. Testing CAR-iNKTs + rhIL-7-hyFc in immunocompetent tumor models with lymphodepletion will be useful for clarifying protocols moving forward.
Our CRS model consistently demonstrated highest IL-6 induced by CAR-Ts40 and lowest/no IL-6 secretion by NK cell–derived CAR cells, known clinically to not cause CRS in humans.28-31 We recognize further studies are needed to assess the correlation of our model to clinical CRS. We found that CAR-iNKTs induced intermediate IL-6 levels that were consistently lower than those induced by CAR-Ts, suggestive of the possibility for reduced incidence and/or severity of CRS by CAR-iNKTs. Importantly, rhIL-7-hyFc had no major effect on IL-6, GM-CSF, or other cytokines tested in our CRS model (Figure 4). In an ongoing trial, GD2 CAR iNKTs has so far not caused CRS,10 and in another study (NCT03774654), grade 1 CRS occurred in 1 of 5 patients.41 Together, we demonstrate anti-myeloma activity of BCMA-targeted CAR-iNKTs that was enhanced by rhIL-7-hyFc, suggesting rationale for the development of BCMA CAR-iNKTs combined with rhIL-7-hyFc for the treatment of MM.
Acknowledgments
The authors thank Carl June (University of Pennsylvania) who generously provided us with the third-generation CAR construct.
This work was supported by a generous gift from the Riney Initiative for Blood Cancer Research, in addition to other funding sources: National Institutes of Health (NIH)/National Cancer Institute (NCI): R35 CA210084 NCI Outstanding Investigator Award (J.F.D); NIH: P50 CA171963 (J.F.D. and T.A.F.); NIH/NCI: U54 CA199092 (J.F.D); NIH: R01CA205239 (T.A.F.); NIH: P50 CA211466 (M.P.R.); NCI P30 CA091842 (Siteman Cancer Center Small Animal Cancer Imaging shared resource and Siteman Cancer Flow Cytometry Core), and the International Myeloma Society and the Paula and Roger Riney Foundation Translational Research Grant (J.O.).
Authorship
Contribution: J.F.D. conceptualized the project; J.O. and J.F.D. supervised the project and wrote the manuscript; J.O., M.L.C., J.K.R., J.N., S.G., L.S.G., E.S., G.J.H., A.C., P.N.A., M.B.-E., AZ, and M.P.R. designed and performed experiments, and analyzed data; F.G. performed the statistical analyses; T.A.F. provided expertise and analyzed data; and B.H.L. and D.C. provided technical advice and critical reagents.
Conflict-of-interest disclosure: B.H.L. and D.C. are full-time employees of NeoImmuneTech Inc J.F.D. is cofounder and equity owner in Magenta and Wugen. J.O. received royalties from Wugen and NeoImmuneTech. M.L.C. is employed by Wugen; declares Wugen stock; and received royalties from Wugen; and declares royalties from NeoImmuneTech. A.C. is a former employee of and declares stock in Wugen. T.A.F. is listed as an inventor on patent applications licensed to Wugen by Washington University that might result in future royalties; has consulting and equity interest in Wugen; serves on the scientific advisory board of Wugen, Affimed, Indapta, and Orca Bio; and receives research funding from Wugen, HCW Biologics, and Affimed. Washington University receives royalties from Wugen and NeoImmuneTech. The remaining authors declare no competing financial interests.
Correspondence: John DiPersio, Washington University School of Medicine, Saint Louis, MO 63110; e-mail: jdipersi@wustl.edu; and Julie O’Neal, Washington University School of Medicine, Saint Louis, MO 63110; e-mail: joneal@wustl.edu.
References
Author notes
Data are available on request from the corresponding authors, John F. DiPersio (jdipersi@wustl.edu) and Julie O’Neal (joneal@wustl.edu).
The full-text version of this article contains a data supplement.