TO THE EDITOR:
Vaccine-induced immune thrombotic thrombocytopenia (VITT) is a rare adverse effect of coronavirus disease 2019 (COVID-19) adenoviral vector vaccines. Most cases of VITT have been linked to the ChAdOx1 nCoV-19 (Vaxzevria AstraZeneca) vaccine.1-3 However, similar clinical and laboratory events occur in patients after vaccination with Ad26.COV2.S (Johnson & Johnson/Janssen).4 VITT is characterized by moderate to severe thrombocytopenia with arterial and/or venous thrombosis, often occurring in unusual locations.1-3 VITT involves immunoglobulin G (IgG) antibodies binding to platelet factor 4 (PF4/CXCL4) causing intense platelet activation.1,2,5 The binding site of VITT antibodies after AstraZeneca vaccination has been localized within the heparin-binding region on PF4, an epitope restricted to 8 amino acids.5 However, the binding site of VITT antibodies on PF4 after the Johnson & Johnson/Janssen vaccine is unknown. In this study, we investigated the binding characteristics of anti-PF4 antibodies in 3 patients with VITT after they received the Johnson & Johnson/Janssen vaccine.
Platelet activation, binding response, and epitope determination were measured as previously described.5-13 All 3 patients received the Johnson & Johnson/Janssen vaccine and did not receive heparin therapy before the onset of symptoms. Patient 1 was a 28-year-old woman who developed headaches and diplopia 12 days after vaccination. Imaging confirmed right internal jugular, right transverse, and superior sagittal sinus thrombosis. She presented with moderate thrombocytopenia (platelet count 71 × 109/L). Patient 2 was a 39-year-old woman who developed headaches 7 days after vaccination and had COVID-19 105 days before being vaccinated. Two days after the onset of the headaches, she developed lower extremity weakness. Then, after a fall on day 12, she was found to have thrombocytopenia (platelet count 36 × 109/L). Imaging studies confirmed cerebral venous sinus thrombosis associated with small cerebral bleeds and a pulmonary embolus. Patient 3 was a 61-year-old woman who developed extensive portal vein thrombosis and was found to have severe thrombocytopenia (platelet count 19 × 109/L) after being vaccinated.
All 3 patients with VITT were positive for antibodies against PF4-polyanion complexes enzyme-linked immunosorbent assay (ELISA) optical density at 405 nm was 2.41 for patient 1, 1.21 for patient 2, and 2.75 for patient 3). Serum from patients 1 and 3 demonstrated platelet activation in the serotonin release assay (SRA; 14C-serotonin release ≥20%) without the addition of heparin. When tested with therapeutic concentrations of heparin, there was no evidence of heparin dependence (supplemental Figure 1A). In the PF4 SRA, patients 1 and 3 demonstrated strong, dose-dependent PF4-mediated platelet activation (supplemental Figure 1B). Patient 2 was tested by using the P-selectin expression assay (PEA), and platelet activation was induced with the addition of 10 μg/mL PF4 (supplemental Figure 1C). Complete inhibition of platelet activation was achieved with the addition of the FcγRIIa-blocking monoclonal antibody IV.3 for all 3 patients.10 This indicates that the VITT antibodies developed after vaccination with the Johnson & Johnson/Janssen vaccine caused platelet activation similar to antibodies formed after AstraZeneca vaccination, and they required PF4 for platelet activation mediated by the engagement of FcγRIIa. In contrast to previously published data on patients with VITT, 2 of the 3 VITT patients in this study showed positive platelet activation in the standard SRA.5 This suggests that within the pool of polyclonal antibodies in the patients’ sera, there may also be heparin-dependent antibodies in addition to typical VITT antibodies.
The binding response and dissociation rates of anti-PF4 antibodies in the samples from the 3 patients with VITT were also tested using biolayer interferometry (BLI). Binding responses are a measure of the abundance of antigen-specific antibodies present in a given sample, and along with dissociation rates, provide information on the strength of the immune response and the avidity of the poly- and/oligo-clonal antibodies.14 The binding response was measured as the average wavelength shift (nm) ± standard deviation and was found to be 2.25 ± 1.6 nm with immobilized PF4 (supplemental Figure 2A) and 1.84 ± 1.2 nm with PF4/heparin (supplemental Figure 2B). The dissociation rates were found to be 4.42 × 10−3 ± 0.004 s−1 for PF4 and 3.62 × 10−3 ± 0.004 s−1 for PF4/heparin, similar to what was previously described with VITT caused by the AstraZeneca vaccine (Table 1).5
Clinical and laboratory findings of patients with VITT as a result of vaccination with Ad26.COV2.S (Johnson & Johnson/Janssen) compared with patients with VITT who were vaccinated with ChAdOx1 nCoV-19 (AstraZeneca)
| . | Patient 1 . | Patient 2 . | Patient 3 . | VITT∗ (n = 5) . |
|---|---|---|---|---|
| Age mean (range) | 28 | 39 | 61 | 44 (35-72) |
| Sex | F | F | F | 3M/2F |
| Platelet count × 109/L | 71 | 36 | 19 | — |
| Thrombosis | Y | Y | Y | Y |
| Site of thrombosis | Right internal jugular vein, right transverse, cerebral venous, and superior sagittal sinuses | Cerebral venous sinus | Portal vein | — |
| Anti-PF4 ELISA (OD405 nm >0.4) mean (range) | 2.41 | 1.21 | 2.75 | 2.71 (0.76-3.35) |
| Standard SRA (14C-serotonin ≥20%) | Positive | — | Positive | Negative 0/5 |
| PF4 SRA or PEA (14C-serotonin ≥20%) | Positive | Positive | Positive | Positive (5/5) |
| No. of amino acids in VITT binding site (n/8) | 8/8 | 5/8 | 6/8 | 4/8 (mean) |
| Binding response to PF4, nm (mean ± SD [range]) | 2.30 | 0.64 | 3.81 | 1.82 ± 0.88 (0.60-3.07) |
| Binding response to PF4/heparin, nm (mean ± SD [range]) | 1.93 | 0.62 | 2.97 | 1.24 ± 0.70 (0.43-2.18) |
| . | Patient 1 . | Patient 2 . | Patient 3 . | VITT∗ (n = 5) . |
|---|---|---|---|---|
| Age mean (range) | 28 | 39 | 61 | 44 (35-72) |
| Sex | F | F | F | 3M/2F |
| Platelet count × 109/L | 71 | 36 | 19 | — |
| Thrombosis | Y | Y | Y | Y |
| Site of thrombosis | Right internal jugular vein, right transverse, cerebral venous, and superior sagittal sinuses | Cerebral venous sinus | Portal vein | — |
| Anti-PF4 ELISA (OD405 nm >0.4) mean (range) | 2.41 | 1.21 | 2.75 | 2.71 (0.76-3.35) |
| Standard SRA (14C-serotonin ≥20%) | Positive | — | Positive | Negative 0/5 |
| PF4 SRA or PEA (14C-serotonin ≥20%) | Positive | Positive | Positive | Positive (5/5) |
| No. of amino acids in VITT binding site (n/8) | 8/8 | 5/8 | 6/8 | 4/8 (mean) |
| Binding response to PF4, nm (mean ± SD [range]) | 2.30 | 0.64 | 3.81 | 1.82 ± 0.88 (0.60-3.07) |
| Binding response to PF4/heparin, nm (mean ± SD [range]) | 1.93 | 0.62 | 2.97 | 1.24 ± 0.70 (0.43-2.18) |
None of the 3 patients received heparin therapy before onset of symptoms.
ELISA, enzyme-linked immunosorbent assay; F, female; M, male; OD, optical density; SD, standard deviation; Y, yes.
Data from Huynh et al.5
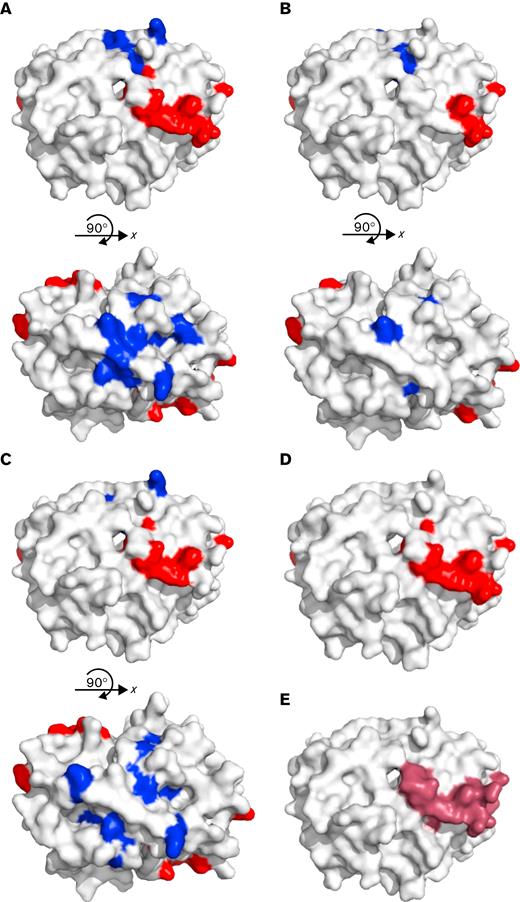
Alanine scanning mutagenesis was used to identify specific amino acid targets of VITT antibodies on PF4.12 We defined a critical binding amino acid as one that caused a >50% reduction in binding when mutated, that is not structurally integral to the tetramer of PF4, and is exposed on the surface of the tetramer in solution.9,15 The anti-PF4 antibodies of patient 1 bound 8 amino acids of PF4 with reduced binding by >50% when mutated, and all 8 amino acids have been previously described as the binding site of VITT antibodies after AstraZeneca vaccination (R22, H23, E28, K46, N47, K50, K62, and K66, Table 1).5 Patient 1 anti-PF4 antibodies also showed another 8 surface amino acids on PF4 that were critical for binding: L8, Q9, C12, K14, A32, G33, C52, and L55 (Figure 1A). For patient 2, the antibody binding site had 5 of the 8 amino acids implicated in VITT (R22, H23, K50, K62, and K66). An additional 3 surface amino acids were found on PF4 (L8, L11, and A32; Figure 1B) that were distinct from the heparin-binding region with a <50% reduction in binding. Patient 3 anti-PF4 antibodies bound 6 amino acids with a >50% reduction in binding similar to those found in VITT resulting from the AstraZeneca vaccine (H23, E29, K46, N47, K50, and K62) and an additional 5 surface amino acids on PF4 (C12, L11, K14, G33, and Q40; Figure 1C) with a >50% reduction in binding.
Alanine scanning mutagenesis was used to map epitopes of amino acids on PF4 that are critical for binding antibodies from patients 1, 2, and 3. (A) Patient 1, (B) patient 2, and (C) patient 3 showed that there is a binding region (red) that aligns with (D) the previously described VITT binding region for ChAdOx1 nCoV-19 (AstraZeneca) vaccination (red, R22, H23, E28, K46, N47, K50, K62, K66) and within (E) the heparin-binding region on PF4 (purple). All 3 patients also have additional amino acids that are important in binding that correspond to the PF4 binding site of typical HIT (blue). However, patient 2 only had 3 additional amino acids outside the typical VITT binding site. Antibody epitopes usually comprise 5 to 8 amino acids; therefore, although important binding amino acids were found in the typical HIT site, there are not enough amino acids to constitute a full epitope. Because patient antibodies are polyclonal, it is possible that the pool of antibodies that binds outside the typical VITT site has either a lower titer or a weaker affinity that cannot be differentiated in the assay. Images are modified from Protein Data Bank file 1RHP.
Alanine scanning mutagenesis was used to map epitopes of amino acids on PF4 that are critical for binding antibodies from patients 1, 2, and 3. (A) Patient 1, (B) patient 2, and (C) patient 3 showed that there is a binding region (red) that aligns with (D) the previously described VITT binding region for ChAdOx1 nCoV-19 (AstraZeneca) vaccination (red, R22, H23, E28, K46, N47, K50, K62, K66) and within (E) the heparin-binding region on PF4 (purple). All 3 patients also have additional amino acids that are important in binding that correspond to the PF4 binding site of typical HIT (blue). However, patient 2 only had 3 additional amino acids outside the typical VITT binding site. Antibody epitopes usually comprise 5 to 8 amino acids; therefore, although important binding amino acids were found in the typical HIT site, there are not enough amino acids to constitute a full epitope. Because patient antibodies are polyclonal, it is possible that the pool of antibodies that binds outside the typical VITT site has either a lower titer or a weaker affinity that cannot be differentiated in the assay. Images are modified from Protein Data Bank file 1RHP.
In all 3 patients, the majority of amino acids found to be important in the anti-PF4 antibody binding were in the heparin-binding site of PF4 (Figure 1D) and included a subset of the same 8 amino acids previously described in typical VITT anti-PF4 antibodies after AstraZeneca vaccination (Figure 1E).5 Patients 1 and 3 also had additional amino acids on PF4 where binding was disrupted corresponding to another site on PF4 outside the heparin-binding region. These additional amino acids were previously indicated to be important in the binding site of heparin-dependent anti-PF4/heparin antibodies in patients with typical heparin-induced thrombocytopenia (HIT), and model HIT pathogenic monoclonal antibodies.12,15-18 Therefore, patients 1 and 3 may have antibodies resembling the typical VITT binding site, and may also have another epitope that is similar to the binding site on PF4 of typical HIT antibodies. Further investigation is warranted to determine whether a similar phenomenon is found in patients who develop VITT after AstraZeneca vaccination.
Patient 2 also had 3 additional amino acids that showed reduced binding outside the heparin-binding region similar to the second site for patients 1 and 3. However, epitopes typically comprise 5 to 8 amino acids19,20 and therefore, patient 2 may or may not have an additional epitope, but the antibodies against the heparin-binding region have a higher titer or affinity and supersede the other antibodies in the assay. VITT has been linked to the 2 COVID-19 vaccines that use adenoviruses as their vector. The mechanism by which these vaccines cause development of anti-PF4 antibodies remains unknown. However, a previous study has shown that adenoviruses can bind platelets and trigger thrombocytopenia in mice.21 Alternatively, the adenovirus used in the AstraZeneca vaccine has a negative charge that could allow it to bind to the positively charged PF4 and set off a reaction similar to that of heparin in HIT.22
In this study, we document that after Johnson & Johnson/Janssen COVID-19 vaccination, patients can have a rare adverse reaction, clinically similar to the reaction after the AstraZeneca vaccination. The antibody reactivity based on platelet activation and BLI studies is within the range previously reported, and the target of these antibodies consists of the same 8 amino acids as described with VITT antibodies that developed after AstraZeneca vaccination.5 Therefore, VITT after vaccination with the Johnson & Johnson/Janssen Ad26.COV2.S vaccine is similar to VITT after the AstraZeneca ChAdOx1 nCoV-19 vaccine.
Acknowledgments: The authors acknowledge the Centre for Microbial Chemical Biology Core Facility and Tracey Campbell and Susan McCusker at McMaster University for their support and assistance in this study.
This work was supported by a grant from the Canadian Institutes of Health Research (CIHR 452655) (I.N.) and the Cardeza Foundation for Hematologic Research.
Contribution: A.H. created the epitope maps by using alanine scanning mutagenesis and analyzed the data; R.C. performed the BLI experiments; J.W.S. performed the standard SRA and PF4-SRA; J.V.M. performed PEA experiments and interpreted the data; M.D. performed some of the studies; J.G.K. and D.M.A. designed the research; S.E.M. interpreted the data; M.I.-I. provided clinical information and samples and interpreted the data; I.N. designed the research and analyzed and interpreted the data; and all authors helped write the manuscript and reviewed and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ishac Nazy, McMaster University, 1280 Main St W, HSC-3H53, Hamilton, ON, Canada L8S 4K1; e-mail: nazyi@mcmaster.ca.
References
Author notes
The datasets generated and/or analyzed during this study are available on reasonable request from the corresponding author, Ishac Nazy (nazyi@mcmaster.ca).
The full-text version of this article contains a data supplement.