TO THE EDITOR:
Follicular lymphoma (FL) is an indolent malignancy of mature B cells that are arrested at the germinal center (GC) stage.1,2 In physiological GC reactions, B cells undergo clonal expansion and B-cell receptor (BCR) affinity maturation towards antigen. B cells participating in a GC reaction commute between GC light zone (LZ) and dark zone (DZ).3 In the LZ, B cells become activated via antigens presented on follicular dendritic cells and interactions with follicular T helper cells. DZ B cells rapidly proliferate and diversify their BCR genes by somatic hypermutation (SHM). LZ/DZ transitions are accompanied by synchronized changes in the gene expression.4,5 These transcriptional GC programs are desynchronized in FL, but the gene expression profile of FL cells globally resembles LZ cells more than DZ cells.5,6
Like healthy B cells, FL cells depend on the expression of a functional BCR. Arrest at the GC stage and constitutive expression of activation-induced cytidine deaminase (AICDA) maintain continuous SHM, resulting in extensive intraclonal variation of the clonally rearranged FL BCR genes.7 Intriguingly, the majority of FL have acquired at diagnosis ≥1 N-linked glycosylation (NliG) motifs within the Fab fragment of their BCR.8,9 Glycosylation of these motifs renders FL cells susceptible to BCR-mediated stimulation through microenvironmental or bacterial lectins.10,11 Theoretically, Fab glycosylation bestows FL cells with a selective survival and growth advantage that explains the high prevalence and clonal dominance of FL cells with such motifs.12,13 However, the immediate effects of BCR Fab glycosylation on FL cells are difficult to examine experimentally because primary FL cells cannot be propagated and manipulated in vitro.
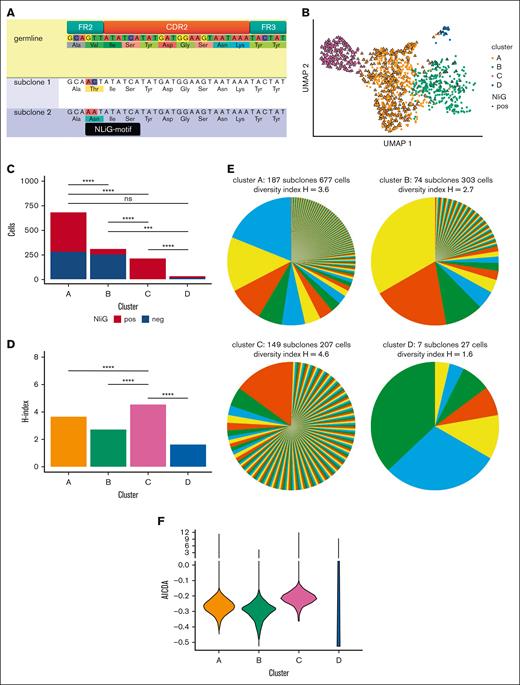
Here, we directly compare FL cells expressing a clonal BCR with or without an NliG motif. Clonal B cells were isolated from a left cervical lymph node biopsy specimen before any treatment. Sample acquisition was conducted according to the Declaration of Helsinki and based on the applicable biobank regulations of Leiden University Medical Center and the Ethical Committee of Leiden University Medical Center. The diagnosis of FL grade 2 was confirmed by immunohistopathology, with detection of an IGH::BCL2 translocation using fluorescence in situ hybridization. Whole exome sequencing demonstrated recurrent FL-associated mutations in CREBBP, BCL2, and MEF2B (supplemental Table 1). Combined single-cell 5′ gene expression profiling and BCR VDJ/VJ sequencing of sorted CD19+CD10+ cells (supplemental Figure 1A) using the 10x Genomics platform yielded 1214 cells with high-quality transcriptomes. All cells expressed a clonal immunoglobulin M (IgM) using IGHV3-30, IGHD3-10, and IGHJ6 for the heavy chain and IGKV1-17 and IGKJ4 for the light chain. With a median number of 27 (range, 24-31) acquired mutations in IGHV and IGKV combined, 55% of cells carried a GTT to AAT mutation of codon 55 of the BCR heavy chain. This tandem mutation created an Asn-Ile-Ser NliG motif at a canonical Fab glycosylation position in FL on the FR2-CDR2 boundary (Figure 1A; supplemental Figure 1B; supplemental Table 2).8 Seurat gene expression clustering resulted in 4 clusters (Figure 1B). Clusters B and C were dominated by NliG motif–negative or NliG motif–positive FL cells, respectively (Figures 1B-C). Cluster C comprised a majority of single cells with unique BCR sequences, reflected by a significantly higher diversity index of 4.6 (Figures 1D-E; Hutcheson's test, P < .0001).14 In contrast, clusters A and B were predominantly populated by several larger subclones, with lower diversity indices of 3.6 and 2.7, respectively (Figures 1D-E). These findings demonstrate a higher rate of ongoing SHM in FL cells of cluster C than that of the other clusters.
Expression of BCR with NliG-motif affects gene expression and subclonal diversity. (A) Partial heavy chain sequences of germ line, NliG-motif–negative subclone 1, and NliG motif–positive subclone 2. Nucleotide and amino acid substitutions in subclones 1 and 2 compared with those in the germ line are highlighted. The NliG motif is annotated in black. (B) Uniform manifold approximation and projection (UMAP) dimensionality reduction and clustering of single FL cells based on the transcriptome profiles. (C) Distribution of NliG motif–negative cells and NliG motif–positive cells per gene expression cluster. (D) Shannon-Weaver indices of diversity were calculated and compared according to Hutchenson's method and corrected for multiple testing. (E) BCR subclonal composition per gene expression cluster. Shannon-Weaver index of diversity (H) is included in the graph titles. Pie colors are randomly picked and are not associated with gene expression clusters. (F) Mean AICDA gene expression between gene expression clusters. pos, positive; neg, negative; ns, not significant.
Expression of BCR with NliG-motif affects gene expression and subclonal diversity. (A) Partial heavy chain sequences of germ line, NliG-motif–negative subclone 1, and NliG motif–positive subclone 2. Nucleotide and amino acid substitutions in subclones 1 and 2 compared with those in the germ line are highlighted. The NliG motif is annotated in black. (B) Uniform manifold approximation and projection (UMAP) dimensionality reduction and clustering of single FL cells based on the transcriptome profiles. (C) Distribution of NliG motif–negative cells and NliG motif–positive cells per gene expression cluster. (D) Shannon-Weaver indices of diversity were calculated and compared according to Hutchenson's method and corrected for multiple testing. (E) BCR subclonal composition per gene expression cluster. Shannon-Weaver index of diversity (H) is included in the graph titles. Pie colors are randomly picked and are not associated with gene expression clusters. (F) Mean AICDA gene expression between gene expression clusters. pos, positive; neg, negative; ns, not significant.
A total of 767 genes were differentially expressed between NliG motif–negative and NliG motif–positive FL cells (supplemental Figures 1C-E and 2; supplemental Table 3). As suggested by their higher SHM rate, cluster C FL cells expressed AICDA at a higher level than cells of the low-diversity cluster B (Figure 1F). To identify gene expression profiles associated with acquisition of the BCR NliG motif, we ranked all genes based on their variance in expression between NliG motif–positive and NliG motif–negative cells (supplemental Figure 3A). Unsupervised clustering based on the 58 genes with the highest variance was consistent with Seurat clusters and NliG motif status (Figure 2A; supplemental Figures 3B-D). To identify candidate cellular processes associated with BCR glycosylation status, we performed gene set enrichment analyses of defined and potentially relevant pathways in hallmark, reactome, go biological processes, and immune signature gene sets (supplemental Figure 4; supplemental Table 4). In cells from Seurat clusters A and B, that is, cells with a globally similar gene expression profile but equal distribution of absence and presence of the NliG motif, differentially higher gene expression between NliG motif–positive and NliG motif–negative cells was correlated with the gene set distinguishing DZ cells from naïve cells, whereas differentially lower gene expression was correlated with the gene set defining the difference between LZ and naïve cells (supplemental Figure 4B; supplemental Table 5). Based on this indirect comparison and the fact that SHM primarily occurs in DZ cells,3 we hypothesized that acquisition of an NliG motif programs FL cells from a more LZ-like gene expression profile toward a DZ-associated transcriptional program. To find evidence to support this hypothesis, we restricted a published set of 348 upregulated and 488 downregulated genes in DZ cells compared with LZ cells, to those genes differentially expressed according to NliG status (supplemental Table 6).5,6,15,16 Among the 41 DZ-associated and differentially expressed genes between NliG motif–positive and NliG motif–negative FL cells, 36 (86%) were expressed at significantly higher levels in NliG motif–positive cells, including the DZ master transcription factor FOXO1 and the DZ-instructing chemokine receptor CXCR4 (paired Student t test, P < .0001; Figure 2B; supplemental Figure 5A). In contrast, 47 of the 60 (78%) LZ-associated and differentially expressed genes were expressed more strongly in NliG motif–negative FL cells, with the strongest difference in the LZ marker CD83 (paired Student t test, P = .0002; Figure 2C; supplemental Figure 5B). In conclusion, NliG motif–negative cells were much more likely to express significantly higher levels of LZ genes, and NliG motif–positive cells upregulated DZ genes (Fisher's exact test, P < .0001). Together, these results indicate that BCR signaling by N-linked glycans drive polarization of FL toward a DZ phenotype, including higher expression of AICDA, a higher rate of SHM, and consecutively enhanced subclonal evolution. In contrast, FL cells lacking an NliG motif appear to have a closer resemblance to healthy LZ B cells.
NliG-motif drives FL cells from light to dark zone type GC B cells. (A) Most variable genes between NliG motif–positive and NliG motif–negative cells (58 genes) were used for unsupervised clustering. The top row depicts the Seurat cluster for each cell, and second row shows BCR NliG motif status. Vertical annotation (primary y-axis) depicts DZ (orange) and LZ (yellow) genes in healthy GC B cells (gene names in bold on secondary y-axis). (B-C). Average gene expression in the pseudobulk of NliG motif–positive and NliG motif–negative single cells. Genes were analyzed that were significantly differentially expressed in single cell data and were characteristic for DZ (B) and LZ (C) GC B cells. Sets of genes were obtained from data published by Victora et al5 (vict), Milpied et al6 (mil), Michaelsen et al15 (mich), or Tripodo et al16 (trip). pos, positive; neg, negative.
NliG-motif drives FL cells from light to dark zone type GC B cells. (A) Most variable genes between NliG motif–positive and NliG motif–negative cells (58 genes) were used for unsupervised clustering. The top row depicts the Seurat cluster for each cell, and second row shows BCR NliG motif status. Vertical annotation (primary y-axis) depicts DZ (orange) and LZ (yellow) genes in healthy GC B cells (gene names in bold on secondary y-axis). (B-C). Average gene expression in the pseudobulk of NliG motif–positive and NliG motif–negative single cells. Genes were analyzed that were significantly differentially expressed in single cell data and were characteristic for DZ (B) and LZ (C) GC B cells. Sets of genes were obtained from data published by Victora et al5 (vict), Milpied et al6 (mil), Michaelsen et al15 (mich), or Tripodo et al16 (trip). pos, positive; neg, negative.
Given the difficulty of studying the effects of acquisition of an NliG motif within the BCR in primary FL cells experimentally, our serendipitous finding of an untreated FL composed of clonal B cells with shared oncogenic mutations, yet containing a balanced proportion of cells whose BCR lacked or had acquired a Fab NliG motif, offers an unprecedented opportunity to investigate the effects of this BCR modification in a minimally confounded manner. By leveraging parallel 5′ gene expression profiling and BCR sequencing at the single-cell level, we demonstrate an NliG motif–induced shift from a LZ gene expression profile toward a DZ-associated profile. Despite the subtlety of differences in gene expression profiles between LZ and DZ cells,3 we were able to detect these changes with high significance, including marker genes used to delineate DZ and LZ B cells. The shift of the gene expression profile of FL cells toward a DZ program via the acquisition of an NliG motif in the antigen binding site of its BCR, facilitating consequent BCR-mediated stimulation by lectins, is in good agreement with current knowledge on normal GC dynamics. The observed changes offer a plausible explanation for the empirical observation of the selection of FL cells acquiring a glycosylated BCR and may facilitate novel approaches to interfere with the immunobiology of FL for therapeutic purposes.
Acknowledgments: The authors express their deepest gratitude to all patients who allowed them to use samples that were essential for this research. The authors thank the Leiden University Medical Center Flow Core Facility for excellent purification of lymphoma cells. Funding from the Dutch Cancer Society Koningin Wilhemina Fonds grant 13104 was used to conduct this research. M.A.N. and J.H.S.Y. were supported by Fondecyt 1230298 (Agencia Nacional de Investigación y Desarrollo, Chile).
Contribution: C.A.M.v.B., and H.V. designed the research and wrote the manuscript; C.A.M.v.B., E.Q., S.L.K., J.H.S.Y., M.T.K., and R.M. performed the research; C.A.M.v.B., H.V., M.G., and M.A.N. analyzed the data; P.M.J. performed the pathological reviewing; J.K. performed fluorescence in situ hybridization analysis; and S.M.K. contributed to analytical bioinformatics and statistics.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cornelis A. M. van Bergen, Department of Hematology, Leiden University Medical Center, Albinusdreef 2, 2333 ZA Leiden, The Netherlands; e-mail: c.a.m.van_bergen@lumc.nl.
References
Author notes
The single-cell gene expression and B-cell receptor data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE233953).
The whole exome sequencing data from the mutational analysis reported in this article have been deposited in the Sequence Read Archive (accession number PRJNA981686).
Data are available on request from the corresponding author, Cornelis A. M. van Bergen, (c.a.m.van_bergen@lumc.nl).
The full-text version of this article contains a data supplement.