TO THE EDITOR:
Vitamin C (Vc) deficiency is characterized by an array of mucocutaneous findings, including ecchymoses, petechiae, perifollicular hemorrhage, oral ulcerations, gingival hypertrophy, and corkscrew hairs. In addition, it can cause cardiovascular, pulmonary, and neuropsychiatric effects and may be fatal if left untreated.1,2 Also known as ascorbic acid, Vc acts as an essential cofactor to enzymes prolyl hydroxylase and lysyl hydroxylase, which crosslink propeptides to stabilize the structure of collagen. Scurvy, the clinical disease caused by Vc deficiency, is primarily seen in patients with restrictive diets, resource limitations, substance abuse, and malabsorption syndromes.2 Currently defined as Vc concentrations <11 μmol/L, many consider hypovitaminosis C to be represented by serum concentrations of <30 μmol/L.1
Patients with leukemia have long been recognized to have decreased serum Vc levels,3-5 and several studies have indicated decreased survival for individuals with malignancies who are Vc deficient.6 However, there is uncertainty whether decreased serum Vc in these patients is due to increased Vc use, decreased intake, altered Vc cellular internalization mechanisms, or a combination of these factors.4,7,8 The role that Vc plays in the oncogenic pathways leading to leukemia is an area of active research. Therapeutic benefits of Vc supplementation have been investigated but, because of study limitations, remain controversial among clinical oncologists.9,10
Herein, we identify 6 patients with acute myeloid leukemia (AML) and scurvy, diagnosed by the University of Pennsylvania Dermatology Consult Service. We postulate that because the mucocutaneous manifestations of scurvy appear similar to common sequelae of leukemia (eg, thrombocytopenia and chemotherapy-associated mucositis), signs of Vc deficiency may be inadvertently attributed to the malignancy itself or to therapeutic interventions, leading to diagnostic error. Recognition of Vc deficiency in this patient population represents an important practice gap.
In this series, we identified 4 female patients and 1 male patient with Vc deficiency (Table 1). One additional male patient (patient 6) was included in our series; he presented with findings typical of scurvy but had a Vc serum concentration of 26 μmol/L 1 week after initiating Vc supplementation. Of note, 5 patients were considered to have severe malnutrition, and half of them were on parenteral nutrition at the time of scurvy diagnosis. The parenteral nutrition formulation contained 10 mL of multivitamin injection–adult, which contains 200 mg of Vc. Patient 1 received oral supplements that contained at least 25 mg of Vc daily in addition to her hospital neutropenic diet.
Demographic characteristics, leukemia genetics, and therapeutic interventions of patients with low Vitamin C
| Patient no. . | Sex . | Age, y . | AML genetics∗ . | Recent or current chemotherapy treatment . | Treatment line . | Platelet count . | Vc level (μmol/L) . | Malnutrition present (yes/no)† . | Parental nutrition . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subtype . | Pathologic variants present . | Karyotype . | |||||||||
| 1 | F | 57 | AML-MRC | BRAF, NF1, NPM1, DNMT3A‡ | 46,XX,-18,+r[5]/46.XX[15] | Liposomal daunorubicin-cytarabine | Induction | 20 | 7 | Yes | No |
| 2 | M | 73 | M5 | DNMT3A, FLT3, NPM1, BRCA2‡, SMC1A‡ | 47,XY,+mar[2]/46,XY[18] | 7 + 3 and midostaurin | Induction | 4 | <5 | Yes | Yes |
| 3 | F | 66 | M5 | DNMT3A, IDH2, PTPN11, EZH2‡ | Normal | Decitabine and venetoclax | R/R | 24 | 8 | Yes | Yes |
| 4 | F | 60 | t-AML | IKZF1, BRAF‡, ETV6‡, RUNX1‡ | 47,XX,+8[20] | Liposomal daunorubicin-cytarabine | R/R | 10 | <5 | No | No |
| 5 | F | 65 | t-AML | NF1, TP3, CD79a‡ | Complex (monosomal) | Busulfan & cyclophosphamide | Conditioning for HSCT | 53 | 9 | Yes | Yes |
| 6 | M | 62 | t-AML | DNMT3A, JAK2, PLCG1‡ | 45,X,-Y,t(7;21;8)(q11.2;q22;q21.3)[19]/46,XY[1] | Azacitidine and venetoclax | R/R | 7 | 26 | Yes | No |
| Patient no. . | Sex . | Age, y . | AML genetics∗ . | Recent or current chemotherapy treatment . | Treatment line . | Platelet count . | Vc level (μmol/L) . | Malnutrition present (yes/no)† . | Parental nutrition . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subtype . | Pathologic variants present . | Karyotype . | |||||||||
| 1 | F | 57 | AML-MRC | BRAF, NF1, NPM1, DNMT3A‡ | 46,XX,-18,+r[5]/46.XX[15] | Liposomal daunorubicin-cytarabine | Induction | 20 | 7 | Yes | No |
| 2 | M | 73 | M5 | DNMT3A, FLT3, NPM1, BRCA2‡, SMC1A‡ | 47,XY,+mar[2]/46,XY[18] | 7 + 3 and midostaurin | Induction | 4 | <5 | Yes | Yes |
| 3 | F | 66 | M5 | DNMT3A, IDH2, PTPN11, EZH2‡ | Normal | Decitabine and venetoclax | R/R | 24 | 8 | Yes | Yes |
| 4 | F | 60 | t-AML | IKZF1, BRAF‡, ETV6‡, RUNX1‡ | 47,XX,+8[20] | Liposomal daunorubicin-cytarabine | R/R | 10 | <5 | No | No |
| 5 | F | 65 | t-AML | NF1, TP3, CD79a‡ | Complex (monosomal) | Busulfan & cyclophosphamide | Conditioning for HSCT | 53 | 9 | Yes | Yes |
| 6 | M | 62 | t-AML | DNMT3A, JAK2, PLCG1‡ | 45,X,-Y,t(7;21;8)(q11.2;q22;q21.3)[19]/46,XY[1] | Azacitidine and venetoclax | R/R | 7 | 26 | Yes | No |
AML-MRC, AML with myelodysplasia-related changes; F, female; HSCT, hematopoietic stem cell transplantation (both allo/auto); IDG2, isocitrate dehydrogenase 2; M, male; R/R, relapsed/refractory; 7 + 3, cytarabine and daunorubicin; t-AML, treatment-related AML.
At the time of AML diagnosis.
Severe protein calorie malnutrition, as established by a registered dietician.
Variant of unknown significance; however, the allele frequency was >15%.
All patients identified had AML: 3 patients had treatment-related AML, 2 had de novo AML, and 1 had AML that developed from prior myelodysplasia (see Table 1 for specific cytogenetic information). At the time of scurvy diagnosis, 3 patients were receiving cytarabine- and daunorubicin-based treatments, 2 were receiving hypomethylating agents plus venetoclax, and 1 was receiving conditioning chemotherapy for an upcoming hematopoietic stem cell transplantation. All patients had either petechiae or ecchymoses; 4 patients had mucosal ulceration or gingival bleeding, and 2 patients had the classic findings of corkscrew hairs with perifollicular hemorrhage (Figure 1). All patients had thrombocytopenia, although none were refractory to platelet transfusions (Table 1).
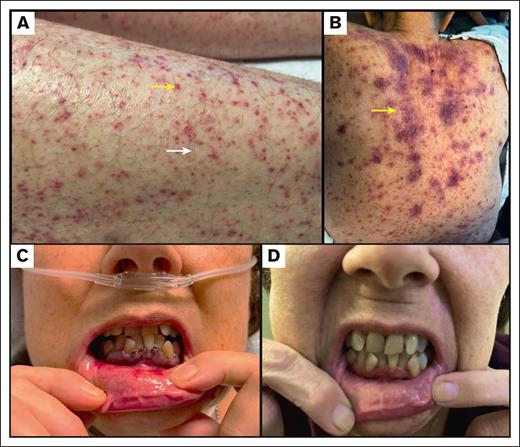
Cutaneous manifestations of scurvy. (A) Perifollicular hemorrhage (yellow arrow) and petechiae in patient 1; Corkscrew hair in patient 1 (white arrow). (B) Petechiae and ecchymoses (yellow arrow) in patient 2. (C) Mucosal ulceration and gingival bleeding in patient 4. (D) Resolution of mucosal ulceration and gingival bleeding in patient 4 after Vc supplementation.
Cutaneous manifestations of scurvy. (A) Perifollicular hemorrhage (yellow arrow) and petechiae in patient 1; Corkscrew hair in patient 1 (white arrow). (B) Petechiae and ecchymoses (yellow arrow) in patient 2. (C) Mucosal ulceration and gingival bleeding in patient 4. (D) Resolution of mucosal ulceration and gingival bleeding in patient 4 after Vc supplementation.
Upon establishing Vc deficiency, patients 1 and 2 received between 200 and 250 mg of ascorbic acid daily. Patients 3, 4, and 6 received 1 g of ascorbic acid daily for at least 1 week. The clinical status of patient 5 deteriorated because of idiopathic pulmonary syndrome, and she died shortly after receiving her Vc serum results. All patients who received Vc repletion had rapid improvement of their ecchymoses and petechiae. Three patients had complete resolution of their mucositis, 2 of whom had resolution before absolute neutrophil count recovery. One patient (patient 3) had mucositis improvement but continued oral ulceration because of acyclovir-resistant herpes simplex virus infection.
This case series highlights the importance of recognizing the signs and symptoms of Vc deficiency, especially because these clinical clues may be misattributed to a patient’s hematologic malignancy, chemotherapy, or stem cell therapy. For all patients presented, the Dermatology department was consulted because of concern for various alternative diagnoses. As such, we encourage oncologists and dermatologists to consider the diagnosis of scurvy when evaluating these patients and replete Vc to avoid associated morbidity.
It is notable that in our institutional experience, most cases of scurvy seen in patients with hematologic malignancy have been in patients with AML. We cannot rule out a referral bias or a high prevalence of AML in our patient population. There are multiple reports of Vc deficiency in patients with non-AML hematologic malignancies, although many studies simply show lower serum Vc levels in patients with leukemia as compared with those in healthy controls.3,4,7 A larger study of patients with leukemia is required to ascertain whether AML places patients at increased risk for the development of scurvy.
We initially assumed that our patients receiving parenteral nutrition or oral supplements were at low risk for the development of scurvy. However, it may be that the recommended dietary allowance should be higher for those with hematologic malignancy, as it is for woman who are pregnant or lactating and for smokers. To our knowledge, there is no evidence to indicate decreased Vc absorption or increased Vc excretion in hematologic malignancies.11,7 Patients receiving treatment for AML often experience significant fatigue and nausea, which lead to limited food consumption and reduced Vc intake. Furthermore, complications such as chemotherapy-induced mucositis or oral infections can cause odynophagia, which may limit ingestion of acidic fruits and vegetables in particular. Although dietary recommendations have changed for patients undergoing chemotherapy and stem cell therapy, many patients still adhere to diets that warn against rough-textured fruit, preprepared salads, and precut fruits during neutropenia.12
Various theories have been proposed regarding lower serum concentrations of Vc in patients with leukemia. Because leukocytes have high intracellular Vc concentrations, cancer-induced antioxidant depletion or chemotherapy-induced oxidative stress may lead to lower levels of Vc in patients with leukemia.7,13 It has also been established that at supraphysiologic levels, Vc can act as a pro-oxidant and selectively target malignant cells, thereby raising the question about a possible protective effect that treatment with Vc may have.11,14,15 Further work is needed to understand the role of Vc in leukemia, specifically AML, and whether there is a survival benefit of Vc supplementation in this population. However, multiple studies have shown improved quality of life in patients receiving Vc supplementation, noting few risks of therapy.9,16 Given that testing for Vc deficiency is relatively inexpensive and supplementation benign, we recommend that clinical oncologists and dermatologists maintain a low threshold for checking and repleting Vc levels in patients with AML and other hematologic malignancies.
Interestingly, a growing body of evidence shows that Vc has a unique modulatory effect on leukemic myeloid progenitor cells and may have a role in leukemogenesis.11,14,15,17,18 Vc induces apoptosis via the oxidation of reduced glutathione and activation of the Raf1 and extracellular signal-regulated kinase pathways in AML cells.14,15 As a cofactor for the ten-eleven translocation (TET) enzymes, Vc promotes DNA demethylation and dioxygenase activity, particularly notable because inactivating TET2 mutations are recurrent in AML.11,18 Although loss-of-function mutations in TET2 are common in AML, TET2 activity can also be decreased via competitive inhibition by oncometabolite 2-hydroxyglutarate, which accumulates in patients with AML-associated mutations in isocitrate dehydrogenase 1 or 2.19 It is theorized that either loss-of-function or reduced dioxygenase activity of TET2 may drive compensatory mechanisms to upregulate other TET enzyme activity and thereby increase Vc use.11,18,20 Surprisingly, there were no TET2 mutations identified in our cohort of patients with AML, but 4 patients had mutations in DNMT3A. Because DNMT3A and TET2 play opposing roles in epigenetic regulation, mutations in DNMT3A alter the activity of TET enzymes, with subsequent changes to Vc use.21 Future studies measuring TET activity may reveal whether epigenetic dysregulation contributes to decreased Vc levels and elucidate possible mechanisms by which Vc supplementation may enhance antitumor immune responses or synergize with hypomethylating agents to improve outcomes in patients with AML.11,22,23
Contribution: H.C.M., A.C., R.G.M., A.P., and M.R. designed the research; H.C.M. and R.D.S. analyzed and interpreted the data; H.C.M. wrote the manuscript; and all authors collected data and contributed to editing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Heather C. Milbar, Department of Dermatology, University of Pennsylvania, Perelman Center for Advanced Medicine, 3400 Civic Center Blvd, Philadelphia, PA 19104; e-mail: heather.milbar@pennmedicine.upenn.edu.
References
Author notes
Data are available on request from the corresponding author, Heather C. Milbar (heather.milbar@pennmedicine.upenn.edu).