Key Points
Cancer-predisposing GVs are present in patients with AML without suspicion of hereditary hematologic malignancy syndrome.
ATM nonsense mutations were found in 6.4% of patients of our cohort, warranting further studies to clarify their role in AML predisposition.
Abstract
Germ line predisposition in acute myeloid leukemia (AML) has gained attention in recent years because of a nonnegligible frequency and an impact on management of patients and their relatives. Risk alleles for AML development may be present in patients without a clinical suspicion of hereditary hematologic malignancy syndrome. In this study we investigated the presence of germ line variants (GVs) in 288 genes related to cancer predisposition in 47 patients with available paired, tumor-normal material, namely bone marrow stroma cells (n = 29), postremission bone marrow (n = 17), and saliva (n = 1). These patients correspond to 2 broad AML categories with heterogeneous genetic background (AML myelodysplasia related and AML defined by differentiation) and none of them had phenotypic abnormalities, previous history of cytopenia, or strong cancer aggregation. We found 11 pathogenic or likely pathogenic variants, 6 affecting genes related to autosomal dominant cancer predisposition syndromes (ATM, DDX41, and CHEK2) and 5 related to autosomal recessive bone marrow failure syndromes (FANCA, FANCM, SBDS, DNAJC21, and CSF3R). We did not find differences in clinical characteristics nor outcome between carriers of GVs vs noncarriers. Further studies in unselected AML cohorts are needed to determine GV incidence and penetrance and, in particular, to clarify the role of ATM nonsense mutations in AML predisposition.
Introduction
Germ line genetic predisposition is a well-known event in many solid tumors, although its role in hematologic malignancies was not fully appreciated until the widespread use of next-generation sequencing (NGS).1 Since then, efforts are being made to recognize the constitutional nature of cancer-related variants, owing to great impact on clinical management of patients with hematologic malignancies and their relatives.2 In acute myeloid leukemia (AML), low-penetrance variants along with late onset of the disease in some cases3 may be responsible for the delay in hereditary susceptibility recognition, and have led some experts to propose universal germ line testing strategies.4 However, study of constitutional variants requires germ line tissue without tumor contamination, a challenge in leukemic malignancies. For this purpose, skin fibroblasts are considered the gold standard,5 despite the requirement of a skin biopsy and long-lasting cultures. Bone marrow stromal cells (BMSCs) show the advantage of being a readily available material from routine bone marrow (BM) aspirations, which can be isolated by culture.6 This mesoderm-derived basic component of the BM niche does not share somatic alterations recurrently found in hematopoietic cells. Besides, if the variant allele fraction (VAF) of a particular mutation is >40% at diagnosis and remains stable in postremission BM (PRBM), a germ line origin can be suspected, although confirmation in a nonhematopoietic tissue or in other family members is necessary to avoid misinterpretation of variants involved in clonal hematopoiesis (CH), somatic copy number variants, or somatic loss of heterozygosity.5,7 More information about the suitability of potential germ line sources can promote research on AML hereditary risk alleles and therefore increase our knowledge about AML pathogenesis. Broad-sequencing techniques like whole-exome sequencing (WES) allow investigation of new candidate genes, being therefore preferred over targeted NGS panels in rapidly changing scenarios like germ line predisposition in hematopoietic malignancies.8
To date, we know that genetic lesions responsible for AML are highly diverse and interrelated in a complex manner.9,10 Diagnostic entities have been defined based on recurrent genetic alterations that correlate to a specific phenotype and prognosis. Nonetheless, recently published classifications11,12 share 2 broad AML entities that encompass a significant number of patients with heterogeneous mechanisms of disease. A deeper biological knowledge of “AML myelodysplasia related” (AML-MR) and “AML defined by differentiation” (AML-DD) may help to predict clinical behavior of individual cases and, therefore, the application of risk-adapted therapy.
In this study, we have explored the potential of WES to identify germ line variants (GVs) in patients diagnosed with AML-MR and AML-DD without a suspicion of hereditary hematologic malignancy syndrome (HHMS).
Methods
Patient inclusion and germ line sample collection
We selected all patients from our institution diagnosed of AML between 1998 and 2019 without any of the recurrent genetic abnormalities and myelodysplasia–related cytogenetic alterations that define an AML entity per the 2016 Revision of World Health Organization Classification of Myeloid Neoplasms and Acute Leukemia.13 Provisional entities were not considered, therefore our cohort includes patients with RUNX1 mutation. From the initial 114 identified patients, biobanked tumor DNA or cryopreserved BM was available for 74 patients. The final cohort consists of all patients from whom paired normal DNA could be obtained (n = 47), whether from BMSC cultures (n = 29), PRBM (n = 17), or saliva (n = 1) (supplemental Figure 1A). More information about germ line samples is detailed in supplemental Materials. Clinical data were obtained from clinical reports. All patients gave their informed consent for this study in accordance with the Declaration of Helsinki (institutional review board approval, HCB/2019/0971).
Genomic sequencing
WES was performed with ×150 coverage for tumor BM or peripheral blood samples and with ×30 coverage for germ line samples. Sequencing methods and bioinformatics analysis for variant calling are detailed in supplemental Methods.
GV identification and interpretation
We explored the genes related to myeloid neoplasm predisposition or cancer predisposition in general, as proposed by Yang et al,14 excluding DNMT3A, ASXL1, and TET2 (DAT) genes to avoid misinterpretation of somatic mutations constituent of CH in PRBM samples. GVs with a predicted high or moderate annotation impact affecting any of the 288 genes (see supplemental Methods) were selected. This rendered 1651 variants that were filtered based on the following criteria: germ line and tumor VAFs of >40%, GnomAD and ExAC mutation allele frequency of <0.01, and a combined annotation dependent depletion score of >15. Filtered GVs were classified per the 2015 American Society of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG-AMP) recommendations.15ATM variants were validated by Sanger sequencing.
Somatic variant identification and interpretation
We considered somatic variants with a predicted high or moderate annotation impact in 42 recurrently mutated genes (refer to supplemental Methods). Somatic variants were classified per Standards and Guidelines for Interpretation and Reporting of Sequence Variants in Cancer.16
Statistical analysis
Fisher exact test or χ2 test were used for comparisons in categorical variables, and Wilcoxon rank sum test for continuous variables. Overall survival was calculated using the Kaplan-Meier method; the log-rank test for univariate comparison. P values were 2-sided. Data were analyzed and plots performed with R software package version 4.0.4 (R core Team, R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
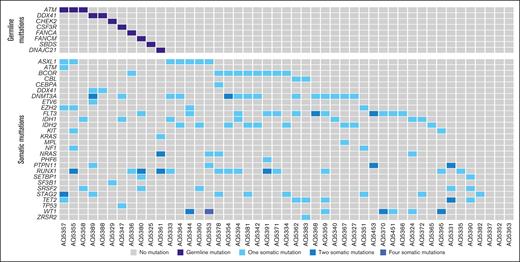
Patient baseline characteristics are outlined in Table 1 and somatic mutations are shown in Figure 1. Following the fifth edition of the World Health Organization classification of myeloid tumors,12 patient diagnosis consisted of AML-MR (n = 24), AML-DD (n = 22), and myeloid sarcoma (n = 1). Among patients with AML-MR, 5 had a previous diagnosis of myelodysplastic syndrome (MDS), 4 met the cytogenetic criteria that define this entity, and the remaining 14 patients were diagnosed owing to mutations affecting ASXL1, BCOR, EZH2, SF3B1, SRSF2, STAG2, U2AF1, or ZRSR2. Per the International Consensus Classification of Myeloid Neoplasms and Acute Leukemias,11 the number of AML-MR increases to 29 because of 5 patients with RUNX1 mutation. There were no differences in regard to leukocyte or BM blast counts.
Clinical characteristics of the entire cohort of patients (n = 47)
| . | n . | % . |
|---|---|---|
| Median age, y (range) | 55 (24-73) | |
| Sex (female/male) | 26/21 | 55/45 |
| Diagnosis (WHO 2022/ICC 2022) | ||
| AML myelodysplasia–related | 24/29 | 49/61 |
| Previous MDS | 5/5 | 11/11 |
| MDS-defining cytogenetics | 4/4 | 8/8 |
| MDS-defining mutations | 19/26 | 40/55 |
| AML-DD | 22/17 | 47/37 |
| With minimal differentiation | 3/1 | 6/2 |
| Without maturation | 8/7 | 17/15 |
| With maturation | 2/2 | 4/4 |
| Myelomonocytic | 3/2 | 6/4 |
| Monoblastic/monocytic | 6/5 | 13/11 |
| Myeloid sarcoma | 1/1 | 2/2 |
| Cytogenetics | ||
| MRC-defined intermediate risk | 43 | 96 |
| Normal karyotype | 32 | 71 |
| MRC-defined adverse risk | 2 | 4 |
| ELN 2022 defined genetic risk | ||
| Intermediate risk | 23 | 49 |
| Adverse risk | 24 | 51 |
| Somatically mutated genes | ||
| DNMT3A | 14 | 30 |
| RUNX1 | 13 | 28 |
| FLT3 (ITD/TKD) | 7/5 | 15/11 |
| IDH2 | 9 | 19 |
| IDH1 | 7 | 15 |
| Treatment received | ||
| Intensive induction chemotherapy | 44 | 94 |
| AlloHCT | 35 | 80 |
| Autologous HCT | 2 | 4 |
| Disease response after induction (n = 44) | ||
| Complete response | 37 | 88 |
| Refractory disease | 5 | 12 |
| . | n . | % . |
|---|---|---|
| Median age, y (range) | 55 (24-73) | |
| Sex (female/male) | 26/21 | 55/45 |
| Diagnosis (WHO 2022/ICC 2022) | ||
| AML myelodysplasia–related | 24/29 | 49/61 |
| Previous MDS | 5/5 | 11/11 |
| MDS-defining cytogenetics | 4/4 | 8/8 |
| MDS-defining mutations | 19/26 | 40/55 |
| AML-DD | 22/17 | 47/37 |
| With minimal differentiation | 3/1 | 6/2 |
| Without maturation | 8/7 | 17/15 |
| With maturation | 2/2 | 4/4 |
| Myelomonocytic | 3/2 | 6/4 |
| Monoblastic/monocytic | 6/5 | 13/11 |
| Myeloid sarcoma | 1/1 | 2/2 |
| Cytogenetics | ||
| MRC-defined intermediate risk | 43 | 96 |
| Normal karyotype | 32 | 71 |
| MRC-defined adverse risk | 2 | 4 |
| ELN 2022 defined genetic risk | ||
| Intermediate risk | 23 | 49 |
| Adverse risk | 24 | 51 |
| Somatically mutated genes | ||
| DNMT3A | 14 | 30 |
| RUNX1 | 13 | 28 |
| FLT3 (ITD/TKD) | 7/5 | 15/11 |
| IDH2 | 9 | 19 |
| IDH1 | 7 | 15 |
| Treatment received | ||
| Intensive induction chemotherapy | 44 | 94 |
| AlloHCT | 35 | 80 |
| Autologous HCT | 2 | 4 |
| Disease response after induction (n = 44) | ||
| Complete response | 37 | 88 |
| Refractory disease | 5 | 12 |
ELN 2022, European LeukemiaNet; ICC 2022, International Consensus Classification; ITD, internal tandem duplication; MRC, Medical Research Council cytogenetic category; TKD, tyrosine kinase domain; WHO 2022, World Health Organization classification.
All but 1 patient of the cohort belonged to the intermediate cytogenetics risk category from the Medical Research Council.17 Regarding the 2022 European LeukemiaNet recommendations,18 the cohort is distributed between intermediate- (n = 12) and adverse-risk categories (n = 35).
Remarkably, none of these patients had a clinical suspicion of HHMS, given the lack of characteristic phenotypic features, previous cytopenia, or a strong familial history of neoplasia. Only 3 patients should had been tested for GVs because of previous breast cancer (n = 2) or a concurrent cervical cancer (n = 1), following the 2022 European LeukemiaNet recommendations. Regarding genes that, based on current knowledge, should trigger germ line testing, there were 17 patients with mutations in RUNX1 (n = 13), DDX41 (n = 2; 1 with a concomitant ETV6 mutation), TP53 (n = 1), or CEBPA (n = 1). No mutations were found in GATA2 or ANKRD26.
Germ line samples characteristics
All PRBM samples corresponded to patients in morphological remission before allogeneic hematopoietic cell transplantation (alloHCT). On the contrary, disease status and blast percentage were diverse in patients from whom BMSCs were cultured, most of them with active disease (diagnosis, n = 22; relapse, n = 1) and 6 additional patients in remission. BMSC colonies grew in 35 of 42 cases (BMSC culture success rate of 83%) and these cells were proved to be CD45−, CD34−, and CD105+, as previously reported.6 Successful cultures showed a median time to 80% confluence of 23 days (range, 14-35 days). Our initial DNA-yield objective was 2 μg, which was achieved in 28 cases (80%). The amount of DNA in the remaining 6 samples was >0.5 μg (median, 1.8 μg; range, 0.8-1.9 μg), which was sufficient for sequencing procedures. Six samples had to be discarded because of a DNA integrity index of <3.4. Quality and quantity of PRBM DNA were not limiting conditions in any case (supplemental Figure 1A-E).
In 5 living patients that had received an alloHCT, we attempted to use saliva as germ line tissue but sex mismatch was detected in DNA quality assessment of 2 female recipient–male donor pairs because of detection of 2 bands in amelogenin gene amplification. This made us suspect a significant donor blood contamination in all samples, and this is the reason for discarding these samples. However, the 3 DNA samples obtained from BMSC grown after alloHCT showed a short tandem repeat microsatellite pattern concordant with the recipient (supplemental Figure 1F) without any contribution of donor genotype.
We assessed tumor contamination in BMSCs and PRBM, without finding any statistically significant differences between the estimates of the cross-sample fraction of reads (P = .8) (supplemental Figure 1). We also tracked the presence of mutated reads in PRBM samples, considering somatic mutations at diagnosis in 42 genes. In 29% (5 of 17) of samples some of the mutations found at diagnosis persisted at very low VAF, except in 2 cases affecting DNMT3A and TET2 (supplemental Figure 2).
Identification of GVs
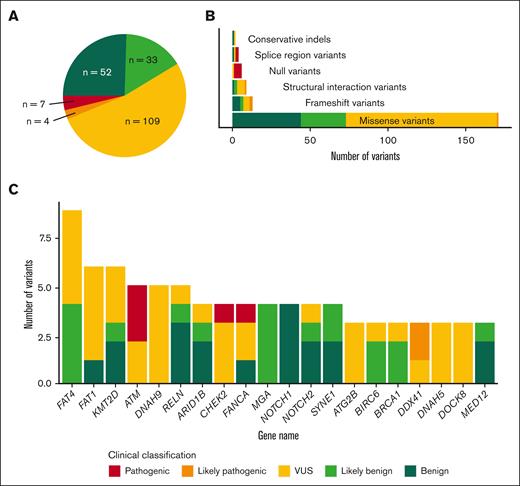
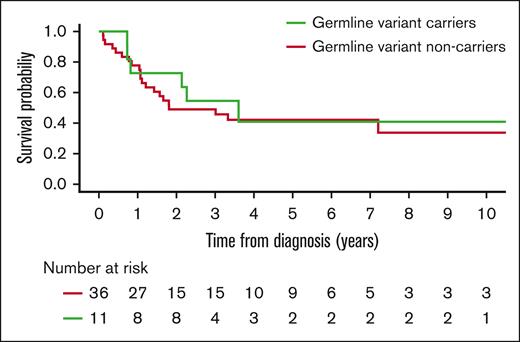
After the workflow detailed in “Methods,” we detected 205 GVs in 110 genes (supplemental Table 5), with all 47 patients having at least 1 GV. We categorized the variants based on ACMG/AMP criteria, which resulted in 11 pathogenic/likely pathogenic variants (P/LP), 109 variants of uncertain significance (VUS), and 85 benign/likely benign variants (Figure 2). Regarding P/LP variants, these affected genes related to DNA damage response pathway (ATM, CHEK2, FANCA, and FANCM), RNA splicing (DDX41), ribosomal biogenesis pathway (SBDS and DNAJC21), and cytokine signaling pathway (CSF3R). All these P/LP variants (Table 2) were found in 11 different patients, whose clinical characteristics are summarized in Table 3. Patients with GVs did not show any clinical differences, including age, and presented a comparable outcome to that of patients without GVs (Figure 3; supplemental Table 5).
Somatic mutations of recurrently mutated genes from patients with detected LP/P GVs.
Somatic mutations of recurrently mutated genes from patients with detected LP/P GVs.
Summary of the type and classification of germ line variants. (A) Absolute frequency of all detected GVs based on their clinical classification. (B) Absolute number of GVs classified by type of variant and colored per clinical classification. (C) Number of variants found in the more frequently altered genes and their clinical classification.
Summary of the type and classification of germ line variants. (A) Absolute frequency of all detected GVs based on their clinical classification. (B) Absolute number of GVs classified by type of variant and colored per clinical classification. (C) Number of variants found in the more frequently altered genes and their clinical classification.
P/LP GVs
| Patient ID . | Paired material . | Gene name (RefSeq) . | HGVS.c . | HGVS.p . | dbSNP . | Germ line VAF . | Tumoral VAF . | ClinVar . | CADD . | MutationTaster . | GnomAD WG AF POPMAX . | ACMG-AMP criteria . | Clinical variant classification . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AQ5388 | Saliva | DDX41 (NM_016222.4) | c.804delG | p.Glu268fs | rs780979459 | 0.53 | 0.45 | NA | NA | NA | PVS1 + PM2 | LP | |
| AQ5389 | PRBM | DDX41 (NM_016222.4) | c.1015C>T | p.Arg339Cys | rs759862062 | 0.56 | 0.49 | NA | 32 | Disease causing (1) | NA | PM1 + PM5 + PP2 + PP3 | LP |
| AQ5357 | BMSC | ATM (NM_000051.4) | c.1110C>G | p.Tyr370∗ | rs376170600 | 0.52 | 0.51 | P | 25 | Disease causing automatic (1) | NA | PVS1 + PM2 + PP5 | P |
| AQ5355 | BMSC | ATM (NM_000051.4) | c.2672C>G | p.Ser891∗ | rs876660780 | 0.58 | 0.5 | P | 36 | Disease causing automatic (1) | NA | PVS1 + PM2 + PP5 | P |
| AQ5358 | BMSC | ATM (NM_000051.4) | c.4148C>A | p.Ser1383∗ | rs141087784 | 0.53 | 0.5 | P/LP | 37 | Disease causing automatic (1) | NA | PVS1 + PM2 + PP5 | P |
| AQ5329 | BMSC | CHEK2 (NM_007194.4) | c.593-1G>T | rs786203229 | 0.45 | 0.48 | P/LP | 34 | Disease causing (1) | NA | PVS1 + PP5 + PM2 | P | |
| AQ5380 | BMSC | FANCM (NM_020937.4) | c.5791C>T | p.Arg1931∗ | rs144567652 | 0.47 | 0.54 | C | 42 | Disease causing automatic (1) | 0.0007422 | PVS1 + PM2 | LP |
| AQ5336 | PRBM | FANCA (NM_000135.4) | c.2529C>G | p.Tyr843∗ | rs1247378731 | 0.49 | 0.45 | P | 33 | Disease causing automatic (1) | NA | PVS1 + PM2 + PP5 | P |
| AQ5325 | BMSC | SBDS (NM_016038.4) | c.258+2T>C | rs113993993 | 0.57 | 0.54 | P/LP | 33 | Disease causing automatic (1) | 0.003675 | PVS1 + PM2 + PP5 + PS3 | P | |
| AQ5361 | BMSC | DNAJC21 (NM_001012339.3) | c.544C>T | p.Arg182∗ | rs771063992 | 0.53 | 0.55 | P | 37 | Disease causing automatic (1) | 0.00001171 | PVS1 + PM2 + PP5 | P |
| AQ5347 | BMSC | CSF3R (NM_000760.4) | c.296_299delTCTC | p.Leu99fs | NA | 0.63 | 0.49 | NA | NA | NA | PVS1 + PM2 | LP |
| Patient ID . | Paired material . | Gene name (RefSeq) . | HGVS.c . | HGVS.p . | dbSNP . | Germ line VAF . | Tumoral VAF . | ClinVar . | CADD . | MutationTaster . | GnomAD WG AF POPMAX . | ACMG-AMP criteria . | Clinical variant classification . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AQ5388 | Saliva | DDX41 (NM_016222.4) | c.804delG | p.Glu268fs | rs780979459 | 0.53 | 0.45 | NA | NA | NA | PVS1 + PM2 | LP | |
| AQ5389 | PRBM | DDX41 (NM_016222.4) | c.1015C>T | p.Arg339Cys | rs759862062 | 0.56 | 0.49 | NA | 32 | Disease causing (1) | NA | PM1 + PM5 + PP2 + PP3 | LP |
| AQ5357 | BMSC | ATM (NM_000051.4) | c.1110C>G | p.Tyr370∗ | rs376170600 | 0.52 | 0.51 | P | 25 | Disease causing automatic (1) | NA | PVS1 + PM2 + PP5 | P |
| AQ5355 | BMSC | ATM (NM_000051.4) | c.2672C>G | p.Ser891∗ | rs876660780 | 0.58 | 0.5 | P | 36 | Disease causing automatic (1) | NA | PVS1 + PM2 + PP5 | P |
| AQ5358 | BMSC | ATM (NM_000051.4) | c.4148C>A | p.Ser1383∗ | rs141087784 | 0.53 | 0.5 | P/LP | 37 | Disease causing automatic (1) | NA | PVS1 + PM2 + PP5 | P |
| AQ5329 | BMSC | CHEK2 (NM_007194.4) | c.593-1G>T | rs786203229 | 0.45 | 0.48 | P/LP | 34 | Disease causing (1) | NA | PVS1 + PP5 + PM2 | P | |
| AQ5380 | BMSC | FANCM (NM_020937.4) | c.5791C>T | p.Arg1931∗ | rs144567652 | 0.47 | 0.54 | C | 42 | Disease causing automatic (1) | 0.0007422 | PVS1 + PM2 | LP |
| AQ5336 | PRBM | FANCA (NM_000135.4) | c.2529C>G | p.Tyr843∗ | rs1247378731 | 0.49 | 0.45 | P | 33 | Disease causing automatic (1) | NA | PVS1 + PM2 + PP5 | P |
| AQ5325 | BMSC | SBDS (NM_016038.4) | c.258+2T>C | rs113993993 | 0.57 | 0.54 | P/LP | 33 | Disease causing automatic (1) | 0.003675 | PVS1 + PM2 + PP5 + PS3 | P | |
| AQ5361 | BMSC | DNAJC21 (NM_001012339.3) | c.544C>T | p.Arg182∗ | rs771063992 | 0.53 | 0.55 | P | 37 | Disease causing automatic (1) | 0.00001171 | PVS1 + PM2 + PP5 | P |
| AQ5347 | BMSC | CSF3R (NM_000760.4) | c.296_299delTCTC | p.Leu99fs | NA | 0.63 | 0.49 | NA | NA | NA | PVS1 + PM2 | LP |
CADD, combined annotation dependent depletion; HGVS, Human Genome Variation Society; ID, identifier; PM, pathogenicity moderate; PP, pathogenicity supporting; PVS, pathogenicity very strong.
Detailed characteristics of carriers of P/LP GVs
| GV . | Patient ID . | Age, y . | Diagnosis . | Cytogenetics . | Somatic mutations . | Outcome . | Survival . |
|---|---|---|---|---|---|---|---|
| DDX41, c.804delG | AQ5388 | 72 | AML with maturation | 46,XY | DDX41 | Primary refractory AML, to 3 low-intensity regimes (DEC + sabatolimab, VEN + azacytidine, DHODi) | 44 mo (DOD) |
| DDX41, c.1015C>T | AQ5389 | 61 | AML, myelodysplasia related | 46,XY | DNMT3Ax2, STAG2, PTPN11, DDX41, ETV6, MLL-PTD, FLT3-TKD | CR1 after induction therapy (I + C + MIDO), with early relapse (<6 mo). CR2 after salvage therapy, followed by alloHCT, with subsequent second refractory relapse | 28 mo (DOD) |
| ATM, c.1110C>G | AQ5357 | 57 | Myeloid sarcoma | 46,XX | STAG2x2, TET2, ASXL1, EZH2, ATM | CR after induction therapy (I+C), followed by alloHCT (CR1). Extensive cGvHD | 64 mo (CR+) |
| ATM, c.2672C>G | AQ5355 | 59 | AML, myelodysplasia related | 46,XY,del(7)(?)[19]/46,XY[1] | RUNX1x2, ASXL1, EZH2, KIT, NF1 | CR after induction therapy (I + C), followed by alloHCT (CR1). Subsequent relapse (+13 mo) | 26 mo (DOD) |
| ATM, c.4148C>A | AQ5358 | 47 | Acute monocytic leukemia | 46,XX | IDH1, SRSF2 | Primary refractory AML to 4 therapy lines (I + C, HiDAC + idasanutlin, MIDAM-GO, and olutasidenib). CR obtained after alloHCT, performed in refractory status | 34 mo (CR+) |
| CHEK2, c.593-1G>T | AQ5329 | 45 | AML with minimal differentiation | 46,XX | SF3B1 | CR1 after induction therapy (I + C), followed by alloHCT (CR1) | 117 mo (CR+) |
| FANCM, c.5791C>T | AQ5380 | 56 | AML with minimal differentiation | 46,XY | RUNX1x2, FLT3-ITD, SRSF2, SETBP1 | CR1 after induction therapy (IDICE), with subsequent relapse, refractory to salvage therapy | 10 mo (DOD) |
| FANCA, c.2529C>G | AQ5336 | 58 | AML, myelodysplasia related | 46,XY | RUNX1, BCOR | CR1 after induction therapy (I + C), followed by alloHCT (CR1). Dead because of bilateral pneumonia | 9 mo (NRM) |
| SBDS, c.258+2T>C | AQ5325 | 45 | AML without maturation | 46,XX | None | CR1 after induction therapy (IDICE), followed by alloHCT | 134 mo (CR+) |
| DNAJC21, c.544C>T | AQ5361 | 58 | AML with minimal differentiation | - | RUNX1x2, NRASx2, KRAS | CR1 after induction therapy (I + C), followed by alloHCT (CR1) | 40 mo (CR+) |
| CSF3R, c.296_299delTCTC | AQ5347 | 60 | AML, myelodysplasia related | 79-90,XXX,-X,-3,-5,-5,-8,-10,-10,-13,-14,-15,+16,i(17)(q10),-17,-18,der(19),der(19),-21,-21,-22,+mar1,+mar2[cp18]/46,XX[2] | DNMT3A, IDH1, TP53 | Primary refractory AML to 2 lines of therapy (ICOG-07, FLAG-IDA). CR after alloHCT but early relapse after alloHCT (+3 mo) | 9 mo (DOD) |
| GV . | Patient ID . | Age, y . | Diagnosis . | Cytogenetics . | Somatic mutations . | Outcome . | Survival . |
|---|---|---|---|---|---|---|---|
| DDX41, c.804delG | AQ5388 | 72 | AML with maturation | 46,XY | DDX41 | Primary refractory AML, to 3 low-intensity regimes (DEC + sabatolimab, VEN + azacytidine, DHODi) | 44 mo (DOD) |
| DDX41, c.1015C>T | AQ5389 | 61 | AML, myelodysplasia related | 46,XY | DNMT3Ax2, STAG2, PTPN11, DDX41, ETV6, MLL-PTD, FLT3-TKD | CR1 after induction therapy (I + C + MIDO), with early relapse (<6 mo). CR2 after salvage therapy, followed by alloHCT, with subsequent second refractory relapse | 28 mo (DOD) |
| ATM, c.1110C>G | AQ5357 | 57 | Myeloid sarcoma | 46,XX | STAG2x2, TET2, ASXL1, EZH2, ATM | CR after induction therapy (I+C), followed by alloHCT (CR1). Extensive cGvHD | 64 mo (CR+) |
| ATM, c.2672C>G | AQ5355 | 59 | AML, myelodysplasia related | 46,XY,del(7)(?)[19]/46,XY[1] | RUNX1x2, ASXL1, EZH2, KIT, NF1 | CR after induction therapy (I + C), followed by alloHCT (CR1). Subsequent relapse (+13 mo) | 26 mo (DOD) |
| ATM, c.4148C>A | AQ5358 | 47 | Acute monocytic leukemia | 46,XX | IDH1, SRSF2 | Primary refractory AML to 4 therapy lines (I + C, HiDAC + idasanutlin, MIDAM-GO, and olutasidenib). CR obtained after alloHCT, performed in refractory status | 34 mo (CR+) |
| CHEK2, c.593-1G>T | AQ5329 | 45 | AML with minimal differentiation | 46,XX | SF3B1 | CR1 after induction therapy (I + C), followed by alloHCT (CR1) | 117 mo (CR+) |
| FANCM, c.5791C>T | AQ5380 | 56 | AML with minimal differentiation | 46,XY | RUNX1x2, FLT3-ITD, SRSF2, SETBP1 | CR1 after induction therapy (IDICE), with subsequent relapse, refractory to salvage therapy | 10 mo (DOD) |
| FANCA, c.2529C>G | AQ5336 | 58 | AML, myelodysplasia related | 46,XY | RUNX1, BCOR | CR1 after induction therapy (I + C), followed by alloHCT (CR1). Dead because of bilateral pneumonia | 9 mo (NRM) |
| SBDS, c.258+2T>C | AQ5325 | 45 | AML without maturation | 46,XX | None | CR1 after induction therapy (IDICE), followed by alloHCT | 134 mo (CR+) |
| DNAJC21, c.544C>T | AQ5361 | 58 | AML with minimal differentiation | - | RUNX1x2, NRASx2, KRAS | CR1 after induction therapy (I + C), followed by alloHCT (CR1) | 40 mo (CR+) |
| CSF3R, c.296_299delTCTC | AQ5347 | 60 | AML, myelodysplasia related | 79-90,XXX,-X,-3,-5,-5,-8,-10,-10,-13,-14,-15,+16,i(17)(q10),-17,-18,der(19),der(19),-21,-21,-22,+mar1,+mar2[cp18]/46,XX[2] | DNMT3A, IDH1, TP53 | Primary refractory AML to 2 lines of therapy (ICOG-07, FLAG-IDA). CR after alloHCT but early relapse after alloHCT (+3 mo) | 9 mo (DOD) |
cGvHD, chronic graft-versus-leukemia disease; CR1, first complete response; CR2, second complete response; CR+, sustained complete response; DEC, decitabine; DHODi, dihydroorotate dehydrogenase inhibitor; DOD, dead of progression; FLAG-IDA, fludarabine + high-dose cytarabine + G-CSF–idarubicin; HiDAC, high-dose cytarabine; I + C, idarubicin + cytarabine; ICOG-07, idarubicin,cytarabine, gemtuzumab ozogamicin; IDICE, idarubicin, intermediate-dose cytarabine, etoposide; MIDAM-GO, mitoxantrone, intermediate-dose cytarabine, gemtuzumab ozogamicin; MIDO, midostaurin; NRM, nonrelapse mortality; VEN, venetoclax.
GV associated to autosomal dominant cancer predisposition syndromes
ATM variants
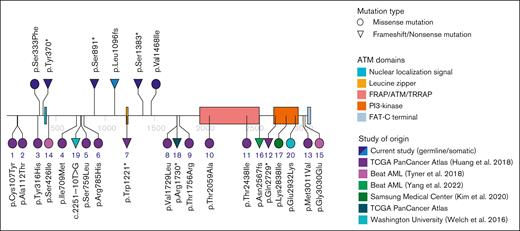
In 3 different patients we found nonsense variants in the ATM gene (Figure 4), all of which were validated by conventional Sanger sequencing (supplemental Figure 3). They are predicted to induce a loss of ATM function and are classified as pathogenic following the recently published ACMG-AMP criteria adapted for ATM gene.19 None of these 3 patients had a strong cancer family history. Nonetheless, patient AQ5357 was diagnosed with a myeloid sarcoma concurrent with a cervical cancer in a cone biopsy. This patient had, besides germ line ATM c.1110C>G; p.Tyr370∗, a somatic frameshift deletion in the same gene (ATM c.3288delG, p.Leu1096fs), together with comutations in STAG2 (double hit), ASXL1, EZH2, and TET2.
ATM variants found in patients with AML in different studies. All ATM mutations identified in our cohort are displayed in the upper part of the plot (dark blue for GVs, light blue for somatic variant). Variants in the lower part of the plot are all GVs reported in the literature. The number under each node relates to Table 4, which contains more information about each mutation.
ATM variants found in patients with AML in different studies. All ATM mutations identified in our cohort are displayed in the upper part of the plot (dark blue for GVs, light blue for somatic variant). Variants in the lower part of the plot are all GVs reported in the literature. The number under each node relates to Table 4, which contains more information about each mutation.
A second ATM variant, c.2672C>G, p.Ser891∗, was found in a 60-year-old man (AQ5355) who was diagnosed with AML-MR based on the presence of a 7q deletion and mutations in RUNX1 (double hit), ASXL1, EZH2, KIT, and NF1. The disease responded well to intensive chemotherapy, allowing the patient to proceed to an alloHCT. One year later, the patient relapsed and, while he was receiving azacytidine and venetoclax as salvage therapy, he was diagnosed with a locally advanced prostate cancer.
The third patient (AQ5358) was a 49-year-old woman with personal history of rheumatoid arthritis treated with methotrexate. She presented with a monocytic AML with germ line ATM c.1418C>A; p.Ser1383∗ and somatic mutations in IDH1 and SRSF2. She was primarily refractory to 3 lines of treatment, achieving a partial remission with isocitrate dehydrogenase 1 inhibitor, olutasidenib. In this situation she proceeded to a sequential alloHCT (fludarabine + high-dose cytarabine + granulocyte colony-stimulating factor [G-CSF]/high-dose melphalan) and remained in remission until last follow-up.
We also identified 2 missense variants that were considered VUS by ACMG-AMP criteria. Figure 4 shows all variants detected in our study together with other ATM variants reported in the literature (also refer to Table 4).
ATM variants (RefSeq NM_000051.3) reported in AML in different studies
| Variant ID . | Study of origin (Reference) . | Participants . | Source . | c.HGVS . | p.HGVS . | VAF . | Clinical interpretation . | AML subtype . |
|---|---|---|---|---|---|---|---|---|
| 1 | Beat AML (14) | 391 | Germ line | 11:g.108100039G>A | p.Cys107Tyr | >0.4 | VUS | NA |
| 2 | 11:g.108106399G>A | p.Ala112Thr | >0.4 | VUS | NA | |||
| 3 | 11:g.108117735T>C | p.Tyr316His | >0.4 | VUS | NA | |||
| 4 | 11:g.108126944T>G | p.Ile709Met | >0.4 | VUS | NA | |||
| 5 | 11:g.108128233G>A | p.Ser759Leu | >0.4 | VUS | NA | |||
| 6 | 11:g.108128311G>A | p.Arg785His | >0.4 | VUS | NA | |||
| 7 | 11:g.108153522G>A | p.Trp1221∗ | >0.4 | P | AML-DD | |||
| 8 | 11:g.108172382G>C | p.Val1729Leu | >0.4 | VUS | NA | |||
| 9 | 11:g.108172464C>G | p.Thr1756Arg | >0.4 | VUS | NA | |||
| 10 | 11:g.108186817A>G | p.Thr2059Ala | >0.4 | VUS | NA | |||
| 11 | 11:g.108200946C>T | p.Thr2438Ile | >0.4 | VUS | NA | |||
| 12 | 11:g.108206605C>T | p.Gln2729∗ | >0.4 | P | AML-MR | |||
| 13 | 11:g.108236095A>G | p.Met3011Val | >0.4 | VUS | NA | |||
| 14 | Beat AML20 | 531 | Somatic | 11:g.108121469G>T | p.Ser426Ile | 0.08 | VUS | NA |
| 15 | 11:g.108236153G>A | p.Gly3030Glu | 0.25 | VUS | NA | |||
| 16 | TCGA PanCancer Atlas (22) | 142 | Germ line | 11:g.108202673_108202676delCAAA | p.Asn2567fs | 0.42 | LP | NA |
| 17 | TCGA PanCancer Atlas | 200 | Somatic | p.Lys2838Ile | 0.07 | VUS | NA | |
| 18 | Washington University21 | 60 | Somatic | 11:g.108172385C>T | p.Arg1730∗ | 0.08 | LP/P | AML-DD |
| 19 | Samsung Medical Center (23) | 180 | NA | c.2251-10T>G | c.2251-10T>G | 0.52 | P | NA |
| 20 | c.8794G>A | p.Glu2932Lys | 0.4 | VUS | NA | |||
| Total | 971 |
| Variant ID . | Study of origin (Reference) . | Participants . | Source . | c.HGVS . | p.HGVS . | VAF . | Clinical interpretation . | AML subtype . |
|---|---|---|---|---|---|---|---|---|
| 1 | Beat AML (14) | 391 | Germ line | 11:g.108100039G>A | p.Cys107Tyr | >0.4 | VUS | NA |
| 2 | 11:g.108106399G>A | p.Ala112Thr | >0.4 | VUS | NA | |||
| 3 | 11:g.108117735T>C | p.Tyr316His | >0.4 | VUS | NA | |||
| 4 | 11:g.108126944T>G | p.Ile709Met | >0.4 | VUS | NA | |||
| 5 | 11:g.108128233G>A | p.Ser759Leu | >0.4 | VUS | NA | |||
| 6 | 11:g.108128311G>A | p.Arg785His | >0.4 | VUS | NA | |||
| 7 | 11:g.108153522G>A | p.Trp1221∗ | >0.4 | P | AML-DD | |||
| 8 | 11:g.108172382G>C | p.Val1729Leu | >0.4 | VUS | NA | |||
| 9 | 11:g.108172464C>G | p.Thr1756Arg | >0.4 | VUS | NA | |||
| 10 | 11:g.108186817A>G | p.Thr2059Ala | >0.4 | VUS | NA | |||
| 11 | 11:g.108200946C>T | p.Thr2438Ile | >0.4 | VUS | NA | |||
| 12 | 11:g.108206605C>T | p.Gln2729∗ | >0.4 | P | AML-MR | |||
| 13 | 11:g.108236095A>G | p.Met3011Val | >0.4 | VUS | NA | |||
| 14 | Beat AML20 | 531 | Somatic | 11:g.108121469G>T | p.Ser426Ile | 0.08 | VUS | NA |
| 15 | 11:g.108236153G>A | p.Gly3030Glu | 0.25 | VUS | NA | |||
| 16 | TCGA PanCancer Atlas (22) | 142 | Germ line | 11:g.108202673_108202676delCAAA | p.Asn2567fs | 0.42 | LP | NA |
| 17 | TCGA PanCancer Atlas | 200 | Somatic | p.Lys2838Ile | 0.07 | VUS | NA | |
| 18 | Washington University21 | 60 | Somatic | 11:g.108172385C>T | p.Arg1730∗ | 0.08 | LP/P | AML-DD |
| 19 | Samsung Medical Center (23) | 180 | NA | c.2251-10T>G | c.2251-10T>G | 0.52 | P | NA |
| 20 | c.8794G>A | p.Glu2932Lys | 0.4 | VUS | NA | |||
| Total | 971 |
Only bold numbers of participants have been summed for the total number of patients of study to avoid duplicated registries.
HGVS, Human Genome Variation Society.
DDX41 variants
We identified 2 P/LP variants in DDX41 in 2 male patients aged 61 and 72 years, respectively, without neither hematologic malignancy family history nor any clinical suspicion of hereditary syndrome.
The first variant, DDX41 c.804delG p.Glu268fs, has not been reported before but is predicted to induce a loss of function of the protein. The same patient (AQ5388) had a somatic DDX41 variant (c.1196G>T, p.Gly399Val, VAF 9.8%), without identification of any other mutated driver gene. Because the patient was not considered eligible for intensive chemotherapy, he was treated with 3 consecutive low-intensity lines of treatment (decitabine combined with sabatolimab, enrolled in a clinical trial; and azacytidine combined with venetoclax and a dihydroorotate dehydrogenase inhibitor in a clinical trial) achieving a relatively long survival (44 months from diagnosis).
The second variant detected, DDX41 c.1015C>T p.Arg339Cys, is a missense mutation already described in at least 2 other patients with AML.22 This case (AQ5389) had also the canonical somatic mutation of DDX41 (c.1574G>A p.Arg525His, VAF of 28%) and additional mutations in DNMT3A (double hit), STAG2, PTPN11, ETV6, FLT3-TKD, and a partial tandem duplication of KMT2A. He was treated with intensive chemotherapy, presenting an early relapse after first consolidation course, which could be rescued with fludarabine + high-dose cytarabine + G-CSF–idarubicin. In second complete remission (CR) the patient underwent an alloHCT, but he unfortunately relapsed 16 months later and died of progression.
A third variant was found in DDX41 (c.845G>A, p.Arg282His) with conflicting classification criteria. This patient was female and did not show any somatic mutation in DDX41, supporting the lack of evidence of causality.
CHEK2 variant
A CHEK2 c.593-1G>T variant was found in a 45-year-old woman diagnosed with AML-MR (AQ5329). This variant involves a canonical splice site and has previously been reported in a patient with hereditary cancer syndrome.23 She was diagnosed with AML after pancytopenia detection in a routine blood test before a surgical procedure. She presented with a borderline blast count of 20%, trilineage dysplasia, a normal karyotype, and the canonical K700E SF3B1 mutation. The presence of ring sideroblasts was not assessable because of empty iron deposits.
We also identified 2 recently reported23 missense variants of CHEK2 (c.1427C>T; p.Thr476Met and c.434G>A; p.Arg145Gln) but they do not achieve enough evidence of causality and have been classified as VUS.
Heterozygous variants in genes related to BM-failure syndromes
We also identified 5 heterozygous carriers of variants in genes associated to autosomal recessive hereditary BM syndromes, including Fanconi anemia (FANCA and FANCM), Shwachman-Diamond syndrome (SBDS and DNAJC21), and severe congenital neutropenia (SCN) (CSF3R).
FANCA c.2529C>G, p.Tyr843∗ was detected in a 58-year-old patient (AQ5336) with AML-MR (comutations in RUNX1 and BCOR) and development of an irreversible organizing pneumonia with a fatal outcome 5 months after undergoing alloHCT. The variant FANCM c.5791C>T, p.R1931∗ was detected in a 56-year-old man (AQ5380) diagnosed with AML-MR with double mutation of RUNX1, internal tandem duplication of FLT3, and mutations in SRSF2 and SETBP1. He died 10 months after diagnosis because of an early refractory relapse. DNAJC21 c.544C>T, p.Arg182∗ was found in a 59-year old woman (AQ5361) with concurrent mutations in NRAS and KRAS with history of localized intraductal breast carcinoma. A recurrent mutation in a Shwachman-Diamond syndrome–associated gene (SDBS c.258+2T>C) was found in a 45-year-old woman (AQ5361) with normal karyotype and absence of somatically mutated driver genes. Finally, we identified a frameshift variant (c.296_299delTCTC, p.Leu99fs) in immunoglobulin-like C2-type CSF3R extracellular domain in a 60-year-old patient with primary refractory AML harboring somatic mutations in DNMT3A, IDH1, and TP53 and complex karyotype. This case never presented neutropenia, neither at AML diagnosis.
Discussion
In this study we have interrogated the presence of GVs in a heterogenous cohort of patients with AML without suspicion of HHMS. Considering the genes related to myeloid neoplasms with germ line predisposition detailed in current classifications, we could only identify 2 variants in DDX41. This is consistent with the characteristics of our patients, with none of them presenting with suggestive phenotypic traits, previous cytopenia, or a strong family or personal cancer history. Of note, despite the high frequency of RUNX1 variants in this group, all of them have been confirmed to be somatic, even when found at VAFs of >40%. This supports the idea that long-standing thrombocytopenia or myeloid malignancy familial aggregation are important factors to suspect a germ line origin of mutations in this gene.
Nonetheless, the frequency of other GVs affecting genes recently proposed to be related to AML predisposition is remarkable. In the last years, there is growing evidence of the contribution of genes related to cancer predisposition in general that could also play a role in the development of AML. Myeloid predisposition GVs are estimated to be present in 5% to 14% of patients with AML14,24,25 and in 11% of patients with MDS.26 In our series, considering only 6 LP/P variants with a reported damaging effect in heterozygous state, this would represent a prevalence of 13%. This incidence could be even higher, because new AML-predisposing genes are expected to be recognized in the future, making broad-sequencing techniques preferable over targeted NGS panels. Of note, we did not find any difference in clinical outcome between carriers of GVs and noncarriers, although larger studies are needed to validate this observation.
We have also explored the usefulness of alternative germ line sources in AML. BMSC cultures are suitable irrespective of the tumor burden at sample collection and even in the post-alloHCT setting but take ∼3 weeks to grow, similar to skin fibroblast cultures.27 However, BMSC cultures do not need trained personnel performing a skin biopsy and can be retrospectively obtained from cryopreserved BM. PRBM requires disease full response, but even in this situation interpretation of variants is handicapped by measurable residual disease and chemotherapy-resistant CH. Evaluation of disease response with NGS targeted panels has unraveled how CH can persist or even emerge after therapy.28,29 Being aware of this, we have not considered GVs affecting DAT genes, but to what extent this should caution against the use of PRBM as surrogate of germ line source has not fully been elucidated. In a recent work, 40% of patients with AML treated with chemotherapy retained some of the mutations identified at diagnosis after CR.30 Median VAFs of post-CR CH was 14% and SRSF2 and IDH2 were included as the most frequently mutated genes along with the age-related, CH–associated DAT genes. We only detected 9 variants present at diagnosis in 5 PRBM samples (29%), probably because of lower sensitivity of WES. In our experience, 7 of these 9 variants showed a clear drop in VAF, suggesting a somatic origin. However, 2 variants affecting DNMT3A and TET2 were present in both diagnostic and PRBM with VAFs of >40%, precluding its assignation as GV or post-CR CH. Considering all this, we encourage to only use PRBM when proper germ line sources are not available and to confirm the results in other family members. Finally, saliva contains significant blood contamination, which hampers its use after alloHCT and in a setting of active leukemia.
In this work, we found a striking high incidence of nonsense ATM mutations, opposed to that reported in public repositories. Biallelic mutations of ATM gene are responsible for ataxia-telangiectasia syndrome, a condition associated to a high risk for development of lymphoid malignancies and carcinoma,31 although rare cases of AML have also been reported.32-36 In heterozygous state, many ATM germ line mutations have been associated with an increased risk for breast, prostate, and pancreatic cancer and melanoma.37-40 For this reason, ATM should be routinely tested in hereditary cancer NGS studies, although it was recently found that 25% of HHMS-targeted sequencing panels do not include this gene.41 Furthermore, ATM somatic mutations and deletions are recurrently found in B-cell neoplasia, although they are not considered a frequent event in myeloid malignancies, preventing its inclusion in myeloid diagnostic targeted sequencing panels.37-40,42 Heterozygote carriers of mutant ATM account for ∼1% of the population,43 whereas in our cohort they accounted for 6.4% (3 of 47) of patients. The analysis of GVs in 33 types of cancer had already noted ATM as a susceptibility gene for AML,24 although other large series have not reported many ATM variants. From a total of 971 reported subjects of study, we could only find 20 variants, 15 of which correspond to VUS (1.54%) and 5 to LP/P (0.51%). From 18 variants with confirmed somatic or germ line origin, 14 correspond to germ line origin (78%). Based on our results, we suggest the inclusion of this gene in myeloid diagnostic panels to facilitate the conduction of broader epidemiological studies and to clarify the role of ATM mutations as risk-alleles for AML.
We have also report 2 novel variants in DDX41, 1 germ line (c.804delG, p.Glu268fs) and 1 somatic (c.1196G>T, p.Gly399Val). These variants affected an individual without other driver genetic events that fulfills the characteristics attributed to this category,44 among others, a low blast count at diagnosis (27%) and long survival despite low-intensity treatments. In contrast, a second patient with mutated DDX41 and many somatic concurrent mutations presented a more aggressive disease. The factors modulating the acquisition of other genetic alterations and, thus, a more aggressive phenotype, must still be elucidated.
Finally, we detected a canonical splice site variant in CHEK2, coding for a disrupted phosphorylation substrate of ATM that has also been associated with a higher risk of breast and prostate cancer. Loss-of-function mutations in CHEK2 are being increasingly recognized as a risk factor for CH,45 myeloproliferative disorders, and MDS.46 Despite 1 study that only found an increased risk for MDS and not for de novo AML,47 other reports have described CHEK2 mutations in newly diagnosed AML.47 Moreover, an important proportion of patients presenting with de novo AML harbor myelodysplasia-related mutations,48 emphasizing the relevance of genomic background over clinical presentation to elucidate the origin of AML. In our study, the patient with a germ line CHEK2 mutation presented with trilineage dysplasia and a somatic mutation in SF3B1, a gene implicated in CH and secondary AML, which would support the predisposing effect of CHEK2 mutation for myelodysplasia–related gene mutations.
Regarding the second group of variants, individuals affected by hereditary BM-failure syndromes have a higher risk of developing hematologic malignancies, ranging from mild to moderate depending on the specific underlying variant and condition.49 It is a matter of discussion whether the heterozygous carriers of the LP/P variants that we have identified present cancer predisposition.50,51 An exception could be FANCM c.5791C>T, p.R1931∗, previously associated to an increased risk for developing breast cancer.52 Indeed, this variant has already been found in 3 other males with AML,14 that, together with our case, represent a significative cluster of affected patients, and may therefore be a risk allele for AML. Three of these patients were middle aged (44-58 years), whereas another developed AML at 75 years. All 4 patients had myelodysplasia–related features without a known family history of hematologic malignancies.
There is also controversy related to CSF3R. Homozygous or compound heterozygous germ line mutations in the extracellular domain of CSF3R are responsible for rare cases of SCN,53-55 which are characterized by a lack of response to G-CSF therapy. Moreover, acquisition of truncating or missense activating mutations affecting a critical region in the intracellular domain of CSF3R is a common phenomenon in patients with SCN, constituting a preleukemic stage that increases the risk of developing AML in these patients.56 These somatic mutations greatly cooperate with RUNX1 mutations in leukemogenesis. Similarly, somatic mutations clustering around the transmembrane region and intracellular domain of CSF3R have been described in the novo AML, being more frequent in AML with t(8;21)(q22;q22.1) and in AML with biallelic mutation of CEBPA.57-59 The variant we found in CSF3R (c.296_299delTCTC, p.Leu99fs) causes a truncating protein at residue 103, located at the beginning of the extracellular domain. To date, it has not been demonstrated that heterozygote carriers of mutations affecting the extracellular domain of CSF3R, as could be family members of SCN patients, have an increased risk for developing AML, although the number of such patients is very low. Besides, germ line p.Trp547∗ and p.Ala119Thr variants, both affecting the extracellular domain of the protein, have recently been proposed as risk alleles for hematologic malignancies.60 Functional studies would be necessary to evaluate the effect of this protein change in JAK/STAT5 pathway substrates and its possible relationship with leukemia predisposition.
In this study we have analyzed GVs of a heterogeneous group of patients with AML. Our results confirm the rationale behind a comprehensive screening of GVs in AML-MR and AML-DD, even when a clinical suspicion of HHMS is lacking or the disease presented late in life. Currently, specific recommendations for management and follow-up of individuals with particular GVs are limited. Moreover, it has to be noted that ACMG-AMP criteria were designed for variant interpretation in mendelian inheritance rare diseases. Refined criteria have been proposed for low-penetrant phenotypes61 or myeloid predisposition syndromes62 but, in many cases, lack of evidence hampers confident assignation of rules. All these findings make GVs recognition essential to improving our variant classification ability and to compiling evidence for a better care of patients and their relatives.
In conclusion, BMSCs and PRBM are alternative sources for GV identification that are suitable in most cases, although awareness of genes related to CH is necessary to correctly interpret results when PRBM is used. Germ line mutations in genes related to cancer predisposition are present in patients with AML-MR and AML-DD at a frequency consistent with other reports, even in absence of HHMS suspicion. Finally, >6% of our cohort presented ATM nonsense variants, appealing for further studies to explain its role in this disease.
Acknowledgments
The authors are grateful to Jose Antonio Guijarro, Alexandre Fortuny, and Ferran Nadeu for their help with data management and preparation of the oncoprint representation.
This study has been funded by Instituto de Salud Carlos III (ISCIII) through the projects “FIS PI16/01027” and “FIS PI19/01476” and cofunded by the European Union. It was also supported by grants from Fundació La Marató TV3 sobre Càncer 201 201930-31 and the award “Premi Fi de Residència” 2019 (Societat Catalana d’Hematologia i Hemoteràpia).
Authorship
Contribution: J.M., R.T., and I.G. performed the variant calling analysis; F.G., M.L.-G., A.B., S.P., D.C., and J.E. manually curated germ line variants; J.M.C.-M., A.B.-M., L.C.-C., J.M.C., S.C.-D., C.J.-V., A.C.-B., A.T., A.M.-R., and D.E. collected the clinical data; F.G., A.B., S.C.-D., M.D.-B., and J.E. analyzed the clinical data and wrote the manuscript; M.G.-H., J.R.A.M., I.L.-O., and M.G. interpreted results and provided feedback; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: A.M.-R. serves as a consultant or in an advisory role, for Bristol Myers Squibb (BMS), AbbVie, and Kite Gilead; received travel grants from Kite Gilead, Roche, Takeda, Janssen, and AbbVie; and serves as a speaker for AbbVie and Gilead. C.J.-V. received travel grants from AbbVie and Pfizer. M.D.-B. serves as a consultant for, in an advisory role for, received travel grants from, or served as speaker for BMS, AbbVie, Astellas, JazzPharma, Takeda, and Novartis. J.E. declares consultancy honoraria from AbbVie, Novartis, Astellas, Jazz Pharmaceuticals, BMS-Celgene, Pfizer, and Daichii-Sankyo, and received research grants from Novartis, Jazz Pharmaceuticals, and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Francesca Guijarro, Pathology Department, Hematopathology Section, Hospital Clinic Barcelona, Villarroel 170, Barcelona 08036, Spain; e-mail: fguijarro@clinic.cat.
References
Author notes
Sequencing data are available from the National Center for Biotechnology Information Sequence Read Archive under accession number PRJNA994311.
Data are available on request from the corresponding author, Francesca Guijarro (fguijarro@clinic.cat).
The full-text version of this article contains a data supplement.