Key Points
A UM171 CB transplant leads to lower nonrelapse mortality compared with a standard CB and MUD transplant.
A UM171 cord transplant has a higher progression-free and GVHD-free relapse-free survival compared with a MUD transplant.
Abstract
Cord blood (CB) transplantation is hampered by low cell dose and high nonrelapse mortality (NRM). A phase 1-2 trial of UM171-expanded CB transplants demonstrated safety and favorable preliminary efficacy. The aim of the current analysis was to retrospectively compare results of the phase 1-2 trial with those after unmanipulated CB and matched-unrelated donor (MUD) transplants. Data from recipients of CB and MUD transplants were obtained from the Center for International Blood and Marrow Transplant Research (CIBMTR) database. Patients were directly matched for the number of previous allogeneic hematopoietic stem cell transplants (alloHCT), disease and refined Disease Risk Index. Patients were further matched by propensity score for age, comorbidity index, and performance status. Primary end points included NRM, progression-free survival (PFS), overall survival (OS), and graft-versus-host disease (GVHD)-free relapse-free survival (GRFS) at 1 and 2 years after alloHCT. Overall, 137 patients from CIBMTR (67 CB, 70 MUD) and 22 with UM171-expanded CB were included. NRM at 1 and 2 years was lower, PFS and GRFS at 2 years and OS at 1 year were improved for UM171-expanded CBs compared with CB controls. Compared with MUD controls, UM171 recipients had lower 1- and 2-year NRM, higher 2-year PFS, and higher 1- and 2-year GRFS. Furthermore, UM171-expanded CB recipients experienced less grades 3-4 acute GVHD and chronic GVHD compared with MUD graft recipients. Compared with real-world evidence with CB and MUD alloHCT, this study suggests that UM171-expanded CB recipients may benefit from lower NRM and higher GRFS. This trial was registered at www.clinicaltrials.gov as #NCT02668315.
Introduction
Despite many improvements, allogeneic hematopoietic stem cell transplant (alloHCT) remains hampered by nonrelapse mortality (NRM), relapse of underlying hematologic malignancy and impairment of quality of life by chronic graft-versus-host disease (GVHD).1 Peripheral blood stem cells (PBSCs) from HLA-matched related and unrelated donors have become the most commonly used stem cell source for adults who undergo transplantation for hematologic malignancies, but the associated risk of chronic GVHD frequently impedes return to normal life.2 In addition, one-third of patients will not have an HLA-matched donor.3 In the 1990s, cord blood (CB) emerged as an alternative source of stem cells for such patients.4 CB presents several advantages, such as the rapid availability of a graft that is already cryopreserved (particularly useful during the COVID-19 pandemic), permissive HLA mismatches improving access for racial minorities, a low risk of chronic GVHD, and a low risk of relapse in high-risk diseases.3,5-7 Unfortunately, CB-use is deterred by its low cell dose with consequent prolonged cytopenias, increased risk of infections, longer hospitalization, and ultimately higher NRM than other transplants.3 Moreover, the small size of most CBs in the banks forces physicians to select the few larger (∼5% of stored units),8 and thus poorly HLA-matched CBs to circumvent prolonged cytopenias. However, this practice is counterproductive as each HLA mismatch increases NRM.9 Data from the Center for International Blood and Marrow Transplant Research (CIBMTR), the European Group for Blood and Marrow Transplantation, and Eurocord have shown that any allele mismatch at HLA A, B, C, or DRB1 (8 loci) contributes to a higher NRM.9 However, many transplant centers are still matching at low resolution (antigen matching) for HLAs A and B and at high resolution for DRB1 (allele matching), thus matching at only 6 loci.10 Furthermore, double CB transplants became the norm for adults as it permitted selection of smaller CBs; however, neutrophil engraftment was still delayed and NRM remained high.11,12 Thus, in the past decade, transplant physicians’ interest in CB has declined significantly, especially with the advent of haploidentical transplants, which have a low risk of NRM with the in vivo depletion of T cells by posttransplant cyclophosphamide.3 However, if CB’s NRM issue could be solved, CB’s advantages could make it a very desirable stem cell source because of its low risk of relapse and chronic GVHD.
UM171 is a small molecule that enhances hematopoietic stem cell renewal13 by degrading histone deacetylase 1/2 and lysine-specific demethylase 1 to prevent differentiation in culture.14 From 2016 to 2018, patients with high-risk hematologic malignancies lacking an HLA-identical donor were enrolled on a phase 1-2 clinical trial at 2 hospitals in Canada.15 Once engraftment by the UM171 CB was documented in a double CB setting, part 2 of the trial enrolled 22 patients who received a single UM171-expanded CB unit with a dose de-escalation design to determine the minimal CB unit cell dose that could achieve prompt engraftment. Two myeloablative (as defined by CIBMTR, https://www.manula.com/manuals/cibmtr/fim/1/en/topic/q155-315-pre-hct-preparative-regimen-conditioning?q=regimen) conditioning regimens were permitted: (i) cyclophosphamide 120 mg/kg, fludarabine 75 mg/m2, 12 Gy total body irradiation and (ii) cyclophosphamide 50 mg/kg, thiotepa 10 mg/kg, fludarabine 150 mg/m2, 4 Gy total body irradiation). Patients received CD34+ selected cells placed into culture for 7 days with UM171 and growth factors, as well as the CD34- cells, which were cryopreserved and infused into the patient on the day of transplant, followed by daily granulocyte colony-stimulating factor starting 2 days after transplant until engraftment. GVHD prophylaxis consisted of a calcineurin inhibitor (CNI) with mycophenolate mofetil (MMF). Antithymocyte globulin (ATG) and alemtuzumab were not permitted. Results of this phase 1-2 trial demonstrated that a CB as small as 0.52 × 105/kg could achieve prompt engraftment; 50% of the patients received a more closely HLA-matched CB because of expansion, and 82% of patients received a ≥ 6/8 HLA-matched CB (allele matching at A, B, C, DRB1), which is better than the most often used 5/8 CB in the conventional setting. The median time to reach 100 and 500 neutrophils per μL was 9.5 days and 18 days, respectively. All patients rapidly achieved 100% donor chimerism in all cell lineages. NRM was remarkably low at 4.5% translating into impressive overall survival (OS) and progression-free survival (PFS) at 1 year of 86% (95% confidence interval [CI], 69-97) and 73% (95% CI, 53-89), respectively, especially considering the underlying high-risk diseases. Indeed, 23% of patients on this trial had experienced disease relapse/progression after a prior alloHCT, and 36% had high or very high Disease Risk Index (DRI)16 malignancies. For example, OS at 1 year is expected to be <50% and <40% for high and very high DRI hematologic malignancies, respectively.16
Based on these promising results, we felt it was important to compare these results to conventional CB transplantation but also to the most commonly used graft source, PBSC obtained from a matched-unrelated donor (MUD).17 We therefore, performed an independent matched–control analysis in collaboration with the CIBMTR to compare outcomes of our 22 single UM171 CB transplants to 2 contemporary control cohorts, identified from the CIBMTR Research database, of conventional single/double ≥ 4/6 HLA-matched CB and 8/8-MUD PBSC transplants.
Methods
Study design
This observational, retrospectively matched cohort study examined the long-term outcomes of alloHCT with either CB or PBSCs in adults for hematologic malignancies. Data linkage was performed to identify clinical trial participants in the CIBMTR registry for data reconciliation purposes. Linkage was performed by CIBMTR with National Marrow Donor Program/Be The Match Institutional Review Board approval. Variables used for linkage included transplant center name, disease, date of HCT, and patient age and sex. Of the 22 trial participants, 7 had not been reported by the transplant center to the CIBMTR registry (for a period of several months there was a lack of qualified personnel at one of the transplant centers, and thus transplant data were not communicated to the CIBMTR during this time); for these patients, data from the clinical trial was used. The CIBMTR statistical team identified matched participants from their database to the 22 UM171 treatment participants using a blend of direct matching on a small number of variables influential in determining outcomes combined with other variables matched using the propensity score method.
Setting
The UM171 cohort underwent transplant between 2016 to 2018. CIBMTR controls included patients at centers in the Unites States and Canada who received an alloHCT during 2014 to 2019, with the additional years of HCT being needed to identify adequate number of controls. A minimum follow-up of 2 years after transplant for all patients was required.
Inclusion/exclusion criteria for controls
Patients were eligible if they had a diagnosis of acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myeloid leukemia, non-Hodgkin lymphoma, or Hodgkin lymphoma (HL), were between the ages of 18 and 65 years, had a Karnofsky performance status score ≥70, and received myeloablative conditioning as defined by the CIBMTR. For the CB control group, single and double CB transplants were permitted, the CB had to be ≥ 4/6 HLA-matched (low resolution A, B, and high resolution DRB1), and GVHD prophylaxis needed to consist of a CNI with MMF. PBSC recipients had to have an 8/8 HLA MUD, and GVHD prophylaxis had to entail a CNI with methotrexate or MMF and could also include ATG or alemtuzumab. Exclusion criteria were CB recipients who received ATG or alemtuzumab, patients who did not give consent for research, CB recipients who underwent transplant in the 2 Canadian centers that enrolled patients on the UM171 CB protocol, unknown refined DRI for patients undergoing a first alloHCT, acute promyelocytic leukemia, AML with unknown European Leukemia Net (ELN) risk,18 and patients undergoing a second alloHCT in first complete remission.
Matching
The CIBMTR identified matched participants using direct matching for number of alloHCTs (1 or ≥ 2) and underlying malignancy (AML, ALL, CML, non-HL, HL). Refined DRI was used for patients receiving first alloHCT, and disease status defined as intermediate or advanced for those receiving second alloHCT. CIBMTR modified ELN risk criteria, based on data availability in the registry, were used for patients with AML (supplemental Table 1). Variables used for propensity score matching were patient age (continuous), hematopoietic cell transplantation comorbidity index (0, 1-2, 3-5), and Karnofsky performance status (<90, 90-100). A logistic regression model was used to model the propensity score. For each UM171 patient, the CIBMTR attempted to identify 4 CB and 4 PBSC controls with the smallest difference in the propensity score. When matching was not possible for all criteria, the number of alloHCT, type of malignancy, and refined DRI or disease status were prioritized over ELN disease risk and propensity score to identify at least 1 matched control.
End points
The primary end points were NRM, PFS, OS, and GVHD-free relapse-free survival (GRFS) at 1 and 2 years after HCT. Grades 3-4 acute GVHD, chronic GVHD requiring systemic immunosuppression, relapse/progression, or death were considered events for GRFS.19 Secondary end points included neutrophil engraftment (first of 3 consecutive dates with an absolute neutrophil count of ≥500/mm3) and platelet recovery (first of 3 consecutive dates with platelet count of ≥20 × 109/L, unsupported by platelet transfusions for 7 consecutive days) at 30, 42, and 100 days; primary graft failure (failure to achieve neutrophil engraftment by day 42); acute GVHD 2-4 and 3-4 at 100 days, 1 and 2 years; relapse/progression of underlying disease and chronic GVHD at 1 and 2 years after HCT. Acute GVHD was defined using established criteria,20 and chronic GVHD was graded according to National Institutes of Health consensus criteria.21
Statistical analysis
GRFS, OS, and PFS were estimated by the Kaplan-Meier method. All other outcomes were calculated using cumulative incidence estimates. All probability estimates have corresponding 95% CIs. Competing risk for NRM was relapse and NRM was a competing risk for relapse. Competing risk of GVHD was death without GVHD. Marginal Cox regression models were used for analyzing NRM and GRFS to accommodate matched pairs. Log-rank test was used to compare Kaplan-Meier curves and Gray test for cumulative incidence curves.
Results
Overall, 137 CIBMTR control patients from 60 centers and 22 patients in the UM171-expanded cord clinical trial were included.
UM171 CBs compared with conventional CBs
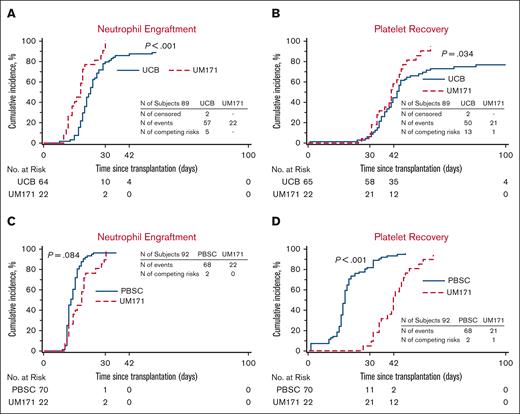
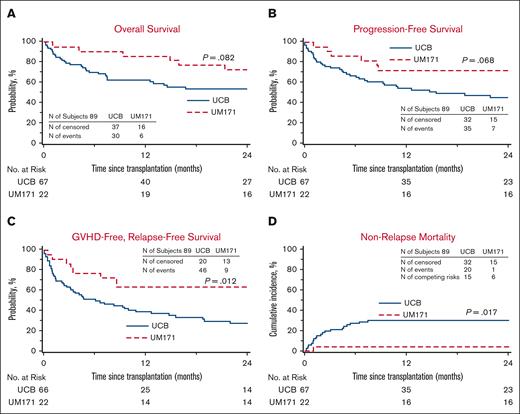
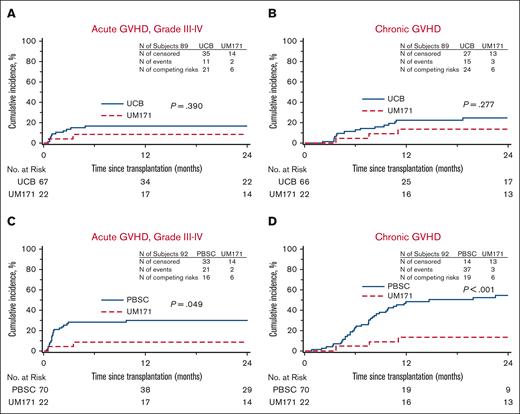
In this cohort, 67 controls were identified out of a possible 88 (1 case had 3 controls, 1 case had 2 controls, and 6 cases had 1 control each). Patient characteristics are listed in Table 1 and no significant difference was noted between the cohorts. In univariate analysis (Table 2), the patients receiving UM171 CBs had less 1- and 2-year NRM, improved 2-year PFS, improved 1-year OS, and improved 1- and 2-year GRFS compared with controls. The median time to neutrophil engraftment was shorter in the cases with similar time to platelet recovery. Furthermore, more cases achieved neutrophil engraftment at all time points and platelet recovery at 100 days (100% vs 89% for neutrophils, P < .01; and 96% vs 77% for platelets, P = .03) compared with controls. There was no case of graft failure in the UM171 cohort as opposed to 5.1% (95% CI, 0-10.7) in the control group (P = .28). The UM171 CB cohort was 87% less likely to experience NRM and 63% less likely to experience grade 3-4 acute GVHD, chronic GVHD requiring immunosuppression, relapse, or death (Table 3). Cumulative incidences of neutrophil and platelet engraftment were superior for the UM171 group (Figure 1). There was a trend for improved OS and PFS by Kaplan-Meier estimates, but this did not reach statistical significance (Figure 2). The main cause of death in both cohorts was primary disease. Moreover, the log-rank test demonstrated greater GRFS (P = .012; at 2 years: 64% vs 28%; Figure 2) and the Gray test showed a lower cumulative incidence of NRM (P = .017; at 2 years: 5% vs 30%; Figure 2) both in favor of the UM171 cohort. Cumulative incidences of relapse (supplemental Figure 1) and chronic GVHD (Figure 3) were very similar in both cohorts.
Characteristics of the UM171, CB, and MUD PBSC controls
| Characteristic . | UM171 CB . | CB controls . | MUD PBSC controls . | ||
|---|---|---|---|---|---|
| . | N (%) . | P UM171 CB vs CB controls . | N (%) . | P UM171 CB vs PBSC controls . | |
| Number of patients | 22 | 67 | 70 | ||
| Patient age, median (range), y | 45 (19-63) | 45 (19-63) | .68∗ | 39 (18-63) | .38∗ |
| First alloHCT | .30† | .35† | |||
| No | 5 (23) | 9 (13) | 10 (14) | ||
| Yes | 17 (77) | 58 (87) | 60 (86) | ||
| Disease | .81† | .89† | |||
| AML | 8 (36) | 30 (45) | 31 (44) | ||
| ALL | 8 (36) | 23 (34) | 23 (33) | ||
| CML | 1 (5) | 4 (6) | 4 (6) | ||
| NHL/HL | 5 (23) | 10 (15) | 12 (17) | ||
| Refined Disease Risk Index | .31† | .76† | |||
| Low | 4 (18) | 4 (6) | 8 (11) | ||
| Intermediate | 8 (36) | 35 (52) | 32 (46) | ||
| High | 4 (18) | 16 (24) | 16 (23) | ||
| Very high | 1 (5) | 3 (4) | 4 (6) | ||
| N/A, second alloHCT | 5 (23) | 9 (13) | 10 (14) | ||
| Disease status for second alloHCT (AML/ALL) | .43† | .59† | |||
| Intermediate | 3 (14) | 7 (10) | 7 (10) | ||
| Advanced | 2 (9) | 2 (3) | 3 (4) | ||
| N/A, first alloHCT | 17 (77) | 58 (87) | 30 (86) | ||
| Modified ELN for AML | .77† | .84† | |||
| Favorable | 0 (0) | 1 (1) | 1 (1) | ||
| Intermediate | 6 (27) | 18 (27) | 20 (29) | ||
| Adverse | 2 (9) | 11 (16) | 10 (14) | ||
| N/A | 14 (64) | 37 (55) | 39 (56) | ||
| HCT-CI | .89† | .79† | |||
| 0 | 8 (36) | 23 (34) | 21 (30) | ||
| 1-2 | 6 (27) | 22 (33) | 18 (26) | ||
| ≥ 3 | 8 (36) | 22 (33) | 31 (44) | ||
| KPS | .42† | .87† | |||
| < 90 | 13 (59) | 33 (49) | 40 (57) | ||
| 90-100 | 9 (41) | 34 (51) | 30 (43) | ||
| Characteristic . | UM171 CB . | CB controls . | MUD PBSC controls . | ||
|---|---|---|---|---|---|
| . | N (%) . | P UM171 CB vs CB controls . | N (%) . | P UM171 CB vs PBSC controls . | |
| Number of patients | 22 | 67 | 70 | ||
| Patient age, median (range), y | 45 (19-63) | 45 (19-63) | .68∗ | 39 (18-63) | .38∗ |
| First alloHCT | .30† | .35† | |||
| No | 5 (23) | 9 (13) | 10 (14) | ||
| Yes | 17 (77) | 58 (87) | 60 (86) | ||
| Disease | .81† | .89† | |||
| AML | 8 (36) | 30 (45) | 31 (44) | ||
| ALL | 8 (36) | 23 (34) | 23 (33) | ||
| CML | 1 (5) | 4 (6) | 4 (6) | ||
| NHL/HL | 5 (23) | 10 (15) | 12 (17) | ||
| Refined Disease Risk Index | .31† | .76† | |||
| Low | 4 (18) | 4 (6) | 8 (11) | ||
| Intermediate | 8 (36) | 35 (52) | 32 (46) | ||
| High | 4 (18) | 16 (24) | 16 (23) | ||
| Very high | 1 (5) | 3 (4) | 4 (6) | ||
| N/A, second alloHCT | 5 (23) | 9 (13) | 10 (14) | ||
| Disease status for second alloHCT (AML/ALL) | .43† | .59† | |||
| Intermediate | 3 (14) | 7 (10) | 7 (10) | ||
| Advanced | 2 (9) | 2 (3) | 3 (4) | ||
| N/A, first alloHCT | 17 (77) | 58 (87) | 30 (86) | ||
| Modified ELN for AML | .77† | .84† | |||
| Favorable | 0 (0) | 1 (1) | 1 (1) | ||
| Intermediate | 6 (27) | 18 (27) | 20 (29) | ||
| Adverse | 2 (9) | 11 (16) | 10 (14) | ||
| N/A | 14 (64) | 37 (55) | 39 (56) | ||
| HCT-CI | .89† | .79† | |||
| 0 | 8 (36) | 23 (34) | 21 (30) | ||
| 1-2 | 6 (27) | 22 (33) | 18 (26) | ||
| ≥ 3 | 8 (36) | 22 (33) | 31 (44) | ||
| KPS | .42† | .87† | |||
| < 90 | 13 (59) | 33 (49) | 40 (57) | ||
| 90-100 | 9 (41) | 34 (51) | 30 (43) | ||
HCT-CI, hematopoietic cell transplantation comorbidity index; KPS, Karnofsky Performance Status; N/A, not applicable; N, number.
Kruskal-Wallis test hypothesis testing.
Pearson chi-square test hypothesis testing.
Univariate analysis of UM171–expanded CB cohort compared with CB and MUD PBSC controls
| Outcomes . | UM171 CB (N = 22) . | CB controls (N = 67) . | P UM171 CB vs CB controls . | MUD PBSC controls (N = 70) . | P UM171 CB vs PBSC controls . | |||
|---|---|---|---|---|---|---|---|---|
| N . | Prob % (95% CI) . | N . | Prob % (95% CI) . | . | N . | Prob % (95% CI) . | . | |
| Nonrelapse mortality | 22 | 67 | 69 | |||||
| 1-y | 17 | 4.5 (0.0-17.3) | 36 | 30.0 (19.6-41.6) | <.01 | 40 | 17.5 (9.4-27.4) | .05 |
| 2-y | 17 | 4.5 (0.0-17.3) | 24 | 30.0 (19.6-41.6) | <.01 | 25 | 19.4 (10.8-29.9) | .03 |
| PFS | 22 | 67 | 69 | |||||
| 1-y | 16 | 72.7 (52.7-88.8) | 35 | 54.7 (42.7-66.5) | .11 | 39 | 57.8 (45.9-69.1) | .18 |
| 2-y | 16 | 72.7 (52.7-88.8) | 23 | 45.6 (33.5-58.0) | .02 | 24 | 45.4 (33.3-57.7) | .02 |
| OS | 22 | 67 | 70 | |||||
| 1-y | 19 | 86.4 (69.3-97.2) | 40 | 62.4 (50.5-73.6) | .01 | 47 | 69.4 (58.0-79.7) | .06 |
| 2-y | 16 | 72.7 (52.7-88.8) | 27 | 53.6 (41.3-65.7) | .09 | 33 | 59.2 (47.1-70.8) | .23 |
| GVHD-free, relapse-free survival | 22 | 66 | 69 | |||||
| 1-y | 14 | 63.6 (42.9-82) | 25 | 39.3 (27.9-51.3) | .04 | 16 | 24.0 (14.7-34.8) | <.01 |
| 2-y | 14 | 63.6 (42.9-82) | 14 | 27.9 (17.3-39.9) | <.01 | 7 | 16.0 (7.9-26.3) | <.01 |
| Relapse | 22 | 67 | 69 | |||||
| 1-y | 17 | 22.7 (7.8-42.7) | 36 | 15.2 (7.6-25.0) | .46 | 40 | 24.7 (15.2-35.7) | .85 |
| 2-y | 17 | 22.7 (7.8-42.7) | 24 | 24.3 (14.3-36.0) | .88 | 25 | 35.2 (23.9-47.4) | .26 |
| Acute GVHD 2-4 | 22 | 67 | 70 | |||||
| 100-d | 9 | 59.1 (37.5-79) | 24 | 45.1(33.2-57.2) | .26 | 34 | 50 (38.3-61.7) | .47 |
| 1-y | 6 | 63.6 (42-82.7) | 18 | 48.2 (36.2-60.3) | .21 | 25 | 51.6 (39.8-63.3) | .33 |
| 2-y | 4 | 63.6 (42-82.7) | 11 | 48.2 (36.2-60.3) | .21 | 20 | 51.6 (39.8-63.3) | .33 |
| Acute GVHD 3-4 | 22 | 67 | 70 | |||||
| 100 d | 21 | 4.5 (0.0-17.3) | 43 | 15.1 (7.5-24.8) | .10 | 49 | 28.6 (18.6-39.7) | <.01 |
| 1-y | 18 | 9.1 (0.8-24.7) | 35 | 16.7 (8.7-26.2) | .33 | 39 | 30.1 (19.9-41.4) | .01 |
| 2-y | 15 | 9.1 (0.8-24.7) | 23 | 16.7 (8.7-26.2) | .33 | 30 | 30.1 (19.9-41.4) | .01 |
| Chronic GVHD | 22 | 66 | 70 | |||||
| 1-y | 17 | 13.6 (2.6-31.2) | 26 | 22.6 (13.0-33.9) | .33 | 20 | 49 (37-61.1) | <.01 |
| 2-y | 14 | 13.6 (2.6-31.2) | 18 | 24.8 (14.6-36.6) | .24 | 10 | 55.2 (42.6-67.5) | <.01 |
| Neutrophil engraftment | 22 | 64 | 70 | |||||
| Median d (range) | 22 | 18 (10-30) | 57 | 21 (8-53) | <.01 | 68 | 13 (9-24) | <.01 |
| 30-d | 2 | 100 (31-100) | 10 | 79.7 (68.8-88.7) | <.01 | 2 | 97.1 (90.9-99.9) | .22 |
| 42-d | 2 | 100 (31-100) | 4 | 87.5 (77.9-94.6) | <.01 | 1 | 97.1 (90.9-99.9) | .22 |
| 100-d | 2 | 100 (31-100) | 1 | 89.1 (79.8-95.7) | <.01 | 1 | 97.1 (90.9-99.9) | .22 |
| Platelet recovery | 22 | 65 | 70 | |||||
| Median d (range) | 21 | 42 (27-62) | 50 | 42 (1-180) | .84 | 68 | 18 (1-48) | <.01 |
| 30-d | 22 | 4.5 (0-17.3) | 58 | 6.2 (1.6-13.3) | .77 | 12 | 82.9 (73-90.8) | <.01 |
| 42-d | 12 | 54.5 (33.2-75) | 35 | 40 (28.4-52.2) | .25 | 3 | 94.3 (87.1-98.7) | <.01 |
| 100-d | 1 | 95.5 (75.5-100) | 5 | 76.9 (65.3-86.7) | .03 | 1 | 97.1 (90.2-100) | .81 |
| Graft failure | ||||||||
| 42-d | 22 | 0 | 59 | 5.1 (0-10.7) | .28 | 68 | 0 | NE |
| Outcomes . | UM171 CB (N = 22) . | CB controls (N = 67) . | P UM171 CB vs CB controls . | MUD PBSC controls (N = 70) . | P UM171 CB vs PBSC controls . | |||
|---|---|---|---|---|---|---|---|---|
| N . | Prob % (95% CI) . | N . | Prob % (95% CI) . | . | N . | Prob % (95% CI) . | . | |
| Nonrelapse mortality | 22 | 67 | 69 | |||||
| 1-y | 17 | 4.5 (0.0-17.3) | 36 | 30.0 (19.6-41.6) | <.01 | 40 | 17.5 (9.4-27.4) | .05 |
| 2-y | 17 | 4.5 (0.0-17.3) | 24 | 30.0 (19.6-41.6) | <.01 | 25 | 19.4 (10.8-29.9) | .03 |
| PFS | 22 | 67 | 69 | |||||
| 1-y | 16 | 72.7 (52.7-88.8) | 35 | 54.7 (42.7-66.5) | .11 | 39 | 57.8 (45.9-69.1) | .18 |
| 2-y | 16 | 72.7 (52.7-88.8) | 23 | 45.6 (33.5-58.0) | .02 | 24 | 45.4 (33.3-57.7) | .02 |
| OS | 22 | 67 | 70 | |||||
| 1-y | 19 | 86.4 (69.3-97.2) | 40 | 62.4 (50.5-73.6) | .01 | 47 | 69.4 (58.0-79.7) | .06 |
| 2-y | 16 | 72.7 (52.7-88.8) | 27 | 53.6 (41.3-65.7) | .09 | 33 | 59.2 (47.1-70.8) | .23 |
| GVHD-free, relapse-free survival | 22 | 66 | 69 | |||||
| 1-y | 14 | 63.6 (42.9-82) | 25 | 39.3 (27.9-51.3) | .04 | 16 | 24.0 (14.7-34.8) | <.01 |
| 2-y | 14 | 63.6 (42.9-82) | 14 | 27.9 (17.3-39.9) | <.01 | 7 | 16.0 (7.9-26.3) | <.01 |
| Relapse | 22 | 67 | 69 | |||||
| 1-y | 17 | 22.7 (7.8-42.7) | 36 | 15.2 (7.6-25.0) | .46 | 40 | 24.7 (15.2-35.7) | .85 |
| 2-y | 17 | 22.7 (7.8-42.7) | 24 | 24.3 (14.3-36.0) | .88 | 25 | 35.2 (23.9-47.4) | .26 |
| Acute GVHD 2-4 | 22 | 67 | 70 | |||||
| 100-d | 9 | 59.1 (37.5-79) | 24 | 45.1(33.2-57.2) | .26 | 34 | 50 (38.3-61.7) | .47 |
| 1-y | 6 | 63.6 (42-82.7) | 18 | 48.2 (36.2-60.3) | .21 | 25 | 51.6 (39.8-63.3) | .33 |
| 2-y | 4 | 63.6 (42-82.7) | 11 | 48.2 (36.2-60.3) | .21 | 20 | 51.6 (39.8-63.3) | .33 |
| Acute GVHD 3-4 | 22 | 67 | 70 | |||||
| 100 d | 21 | 4.5 (0.0-17.3) | 43 | 15.1 (7.5-24.8) | .10 | 49 | 28.6 (18.6-39.7) | <.01 |
| 1-y | 18 | 9.1 (0.8-24.7) | 35 | 16.7 (8.7-26.2) | .33 | 39 | 30.1 (19.9-41.4) | .01 |
| 2-y | 15 | 9.1 (0.8-24.7) | 23 | 16.7 (8.7-26.2) | .33 | 30 | 30.1 (19.9-41.4) | .01 |
| Chronic GVHD | 22 | 66 | 70 | |||||
| 1-y | 17 | 13.6 (2.6-31.2) | 26 | 22.6 (13.0-33.9) | .33 | 20 | 49 (37-61.1) | <.01 |
| 2-y | 14 | 13.6 (2.6-31.2) | 18 | 24.8 (14.6-36.6) | .24 | 10 | 55.2 (42.6-67.5) | <.01 |
| Neutrophil engraftment | 22 | 64 | 70 | |||||
| Median d (range) | 22 | 18 (10-30) | 57 | 21 (8-53) | <.01 | 68 | 13 (9-24) | <.01 |
| 30-d | 2 | 100 (31-100) | 10 | 79.7 (68.8-88.7) | <.01 | 2 | 97.1 (90.9-99.9) | .22 |
| 42-d | 2 | 100 (31-100) | 4 | 87.5 (77.9-94.6) | <.01 | 1 | 97.1 (90.9-99.9) | .22 |
| 100-d | 2 | 100 (31-100) | 1 | 89.1 (79.8-95.7) | <.01 | 1 | 97.1 (90.9-99.9) | .22 |
| Platelet recovery | 22 | 65 | 70 | |||||
| Median d (range) | 21 | 42 (27-62) | 50 | 42 (1-180) | .84 | 68 | 18 (1-48) | <.01 |
| 30-d | 22 | 4.5 (0-17.3) | 58 | 6.2 (1.6-13.3) | .77 | 12 | 82.9 (73-90.8) | <.01 |
| 42-d | 12 | 54.5 (33.2-75) | 35 | 40 (28.4-52.2) | .25 | 3 | 94.3 (87.1-98.7) | <.01 |
| 100-d | 1 | 95.5 (75.5-100) | 5 | 76.9 (65.3-86.7) | .03 | 1 | 97.1 (90.2-100) | .81 |
| Graft failure | ||||||||
| 42-d | 22 | 0 | 59 | 5.1 (0-10.7) | .28 | 68 | 0 | NE |
N, number; Prob, probability.
Marginal cox regression analysis for UM171 CB vs CB and MUD PBSC controls
| Outcomes UM171 CB vs CB controls . | HR (95% CI) . | P value . |
|---|---|---|
| Nonrelapse mortality | 0.13 (0.02-0.89) | .04 |
| GVHD-free, relapse-free survival | 0.37 (0.17-0.84) | .02 |
| Outcomes UM171 CB vs CB controls . | HR (95% CI) . | P value . |
|---|---|---|
| Nonrelapse mortality | 0.13 (0.02-0.89) | .04 |
| GVHD-free, relapse-free survival | 0.37 (0.17-0.84) | .02 |
| Outcomes UM171 CB vs MUD PBSC controls . | HR (95% CI) . | P value . |
|---|---|---|
| Nonrelapse mortality | 0.20 (0.04-1.10) | .06 |
| GVHD-free, relapse-free survival | 0.27 (0.12-0.60) | <.01 |
| Outcomes UM171 CB vs MUD PBSC controls . | HR (95% CI) . | P value . |
|---|---|---|
| Nonrelapse mortality | 0.20 (0.04-1.10) | .06 |
| GVHD-free, relapse-free survival | 0.27 (0.12-0.60) | <.01 |
Engraftment. (A-B) Cumulative incidences of neutrophil (A) and platelet (B) engraftment of the UM171 CB cohort (UM171) and the CIBMTR CB controls (UCB). Neutrophil and platelet engraftment are improved in the UM171 CB cohort by Gray test. (C-D) Cumulative incidences of neutrophil (A) and platelet (B) engraftment of the UM171 CB cohort and the CIBMTR MUD PBSC controls (PBSC). Platelet engraftment is delayed for UM171 CB compared with MUD PBSC by Gray test.
Engraftment. (A-B) Cumulative incidences of neutrophil (A) and platelet (B) engraftment of the UM171 CB cohort (UM171) and the CIBMTR CB controls (UCB). Neutrophil and platelet engraftment are improved in the UM171 CB cohort by Gray test. (C-D) Cumulative incidences of neutrophil (A) and platelet (B) engraftment of the UM171 CB cohort and the CIBMTR MUD PBSC controls (PBSC). Platelet engraftment is delayed for UM171 CB compared with MUD PBSC by Gray test.
Outcomes of UM171 CB Cohort and CIBMTR CB Controls. (A-B) Kaplan-Meier estimates of OS (A) and progression-free survival (B). (C) GRFS by Kaplan-Meier estimate of the UM171 CB cohort (UM171) appears superior to that of the CIBMTR CB controls (UCB) when compared with log-rank test. (D) Cumulative incidence of NRM of the UM171 CB cohort is lower than that of the CIBMTR CB controls when compared by Gray test.
Outcomes of UM171 CB Cohort and CIBMTR CB Controls. (A-B) Kaplan-Meier estimates of OS (A) and progression-free survival (B). (C) GRFS by Kaplan-Meier estimate of the UM171 CB cohort (UM171) appears superior to that of the CIBMTR CB controls (UCB) when compared with log-rank test. (D) Cumulative incidence of NRM of the UM171 CB cohort is lower than that of the CIBMTR CB controls when compared by Gray test.
GVHD. (A-B) Cumulative incidences of grade 3-4 acute GVHD (A) and chronic GVHD (B) of the UM171 CB cohort (UM171) and the CIBMTR CB controls (UCB). (C-D) Cumulative incidences of grade 3-4 acute GVHD (C) and chronic GVHD (D) of the UM171 CB cohort appear lower than those reported for the CIBMTR MUD PBSC controls (PBSC) by Gray test.
GVHD. (A-B) Cumulative incidences of grade 3-4 acute GVHD (A) and chronic GVHD (B) of the UM171 CB cohort (UM171) and the CIBMTR CB controls (UCB). (C-D) Cumulative incidences of grade 3-4 acute GVHD (C) and chronic GVHD (D) of the UM171 CB cohort appear lower than those reported for the CIBMTR MUD PBSC controls (PBSC) by Gray test.
UM171 CBs compared with HLA MUD PBSCs
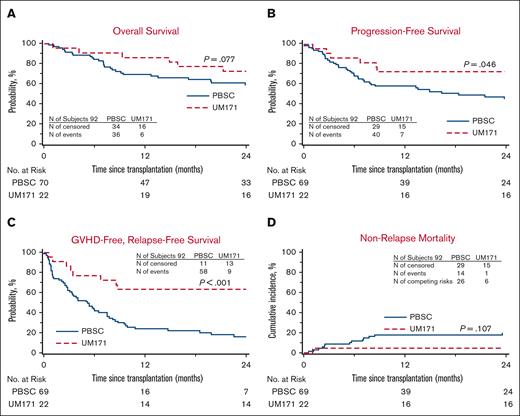
In this cohort, 70 controls were identified out of a possible 88 (1 case had 3 controls, 1 case had 2 controls, and 5 cases had 1 control each). Patient characteristics are listed in Table 1 and no significant difference was noted between the 2 groups. In univariate analysis (Table 2), the UM171 group had less 1- and 2-year NRM, improved 2-year PFS, and better 1- and 2-year GRFS compared with the controls. The UM171-expanded CB cases also experienced less grade 3-4 acute GVHD and chronic GVHD at both time points. Median times to neutrophil engraftment and platelet recovery were longer in the UM171-expanded CB cases. Furthermore, the UM171-expanded CB cases were less likely than the PBSC controls to achieve platelet recovery by 30 and 42 days, but by day 100, the probability of platelet engraftment was equivalent in both groups. Graft failure did not occur in either group. The hazard ratio (HR) for NRM was not significantly different; however, the UM171 CB cohort was 73% less likely to experience grade 3-4 acute GVHD, chronic GVHD requiring immunosuppression, relapse, or death compared to the MUD PBSC group (Table 3). Cumulative incidences of neutrophil engraftment were similar in both groups, but the cumulative incidence of platelet recovery was superior for the MUD PBSC cohort (Figure 1). The Kaplan-Meier estimate for PFS was statistically better for the UM171 cohort (P = .046; at 2 years: 73% vs 45%; Figure 4); however, this translated only into a trend for better OS (P = .077; at 2 years 73% vs 59%; Figure 4). PFS improvement was likely due to the additional effect of the small, nonstatistically significant benefit of relapse (supplemental Figure 1) and NRM favoring the UM171 group (Figure 4). The primary cause of death in both groups was the underlying malignancy. Furthermore, the log-rank test was significantly in favor of the UM171 CB group for GRFS (at 2 years: 64% vs 16%; P < .001; Figure 4), accounted for by the difference in chronic GVHD (Figure 3), acute grade 3-4 GVHD (Figure 3), and PFS (Figure 4).
Outcomes of UM171 CB Cohort and CIBMTR MUD PBSC controls. (A) Kaplan-Meier estimates of OS in the UM171 CB cohort (UM171) and the CIBMTR MUD PBSC controls (PBSC) compared by log-rank test. (B) PFS appears superior by Kaplan-Meier estimate for the UM171 CB cohort compared with that of the CIBMTR MUD PBSC controls by log-rank test. (C) GRFS by Kaplan-Meier estimate appears superior for the UM171 CB cohort compared with that of the CIBMTR MUD PBSC controls by log-rank test. (D) Cumulative incidence of NRM of the UM171 CB and MUD PBSC cohorts.
Outcomes of UM171 CB Cohort and CIBMTR MUD PBSC controls. (A) Kaplan-Meier estimates of OS in the UM171 CB cohort (UM171) and the CIBMTR MUD PBSC controls (PBSC) compared by log-rank test. (B) PFS appears superior by Kaplan-Meier estimate for the UM171 CB cohort compared with that of the CIBMTR MUD PBSC controls by log-rank test. (C) GRFS by Kaplan-Meier estimate appears superior for the UM171 CB cohort compared with that of the CIBMTR MUD PBSC controls by log-rank test. (D) Cumulative incidence of NRM of the UM171 CB and MUD PBSC cohorts.
Discussion
A matched-paired analysis examining long-term outcomes of alloHCT with a UM171 CB compared to a conventional CB and to MUD PBSCs in adults with hematologic malignancies revealed that UM171 CB transplants achieved more rapid neutrophil engraftment, less NRM, and improved GRFS compared with contemporary conventional CB transplants. Importantly, CBs’ advantages appeared preserved in the UM171 cohort, with similar rates of chronic GVHD and relapse when compared with conventional CB. When compared with MUD PBSC transplants, the UM171 CB cohort had lower NRM, chronic GVHD, grade 3-4 acute GVHD, and improved PFS and GRFS. Engraftment was slower with UM171 CBs compared with PBSCs, but this did not appear to affect NRM.
The phase 1-2 trial from which the data for UM171 expanded CB transplants was taken was designed to test feasibility, safety, and preliminary efficacy with the main goal of decreasing NRM to allow the advantages of CB, including the low risk of chronic GVHD and relapse, to predominate making CB once again a desirable graft source. We planned to achieve a lower NRM by selecting smaller, better HLA-matched CBs and by accelerating engraftment. The trial appeared to be a success, with a very low risk of NRM at 4.5%. The conventional CB transplants used for comparison represent contemporary CB transplants, which should reflect the current improved recommendations for CB selection criteria10 and supportive care.22,23 Despite this, NRM was lower in the patients receiving UM171 CB, and a significant proportion of these patients received CBs that would be considered too small for clinical use, thus further validating the benefit of the UM171 technology. Additional reasons why outcome may be improved with a UM171-manipulated CB is the associated rapid T-cell reconstitution, greater T-cell diversity,24 and lower risk of severe infections after transplant24 compared with a conventional CB graft.
There were several limitations to this study. The sample size was small for the UM171 cohort. Four controls were unable to be identified for each case, requiring prioritization of matching criteria. This inability to identify the initially planned number of controls stems at least in part from the very high-risk diseases in the UM171 group who would be expected to have a poor outcome (eg, 1 patient had refractory relapsed ALL after a failed initial alloHCT). Data reconciliation was impossible to perform for all UM171 cases, as 7 cases were not reported to the CIBMTR. In addition, most UM171 recipients were treated in a single center and followed prospectively as opposed to the retrospective data collection for the control patients who underwent transplant in many different centers, possibly introducing a bias.
Various techniques to augment the number of stem and progenitor cells have been reported. Omidubicel (previously known as NiCord) was recently approved by the US Food and Drug Administration and is the expanded CB product with the most experience. It was studied in an initial phase 1-2 study, demonstrating very rapid neutrophil engraftment at 11.5 days,25 earlier than a UM171 CB. However, when the omidubicel group was compared with a similar CIBMTR cohort of conventional CB transplants, NRM appeared lower, but a higher risk of relapse in the omidubicel group translated into a similar long-term outcome. These results were confirmed in a randomized prospective trial of omidubicel vs standard CB transplant, affirming very rapid neutrophil engraftment.26 With 62 patients in the omidubicel arm, the difference in NRM was not statistically different but showed a trend favoring omidubicel (11% vs 24%). Relapse risk, OS, disease-free survival, and GRFS were similar in both groups. Interestingly, 74% of patients in the omidubicel arm and 73% in the control arm received a 4/6 HLA-matched CB, thus no obvious improvement in HLA match is apparent. In the phase 1-2 UM171 CB trial, more than 80% of patients received a ≥ 6/8 HLA-matched CB, which is better than the most used 5/8 matched CBs with our conventional CB transplants (local data). Furthermore, many centers are still selecting CB based on low-resolution typing for A and B without taking into consideration HLA C. In contrast, we matched at high resolution for A, B, C, and DRB1, which could explain why we observed a statistically significant improvement in NRM with only 22 patients. The initial set price for omidubicel is $338 000 and the sum is yet unknown for a UM171-expanded graft. The cost will obviously need to be taken into consideration when selecting a stem cell source.
In conclusion, our results suggest that UM171-expanded CB recipients have improved select clinical outcomes compared with conventional CB and MUD PBSC recipients. Importantly, we were able to confirm that the major limitation of CB, high NRM, is improved with UM171 expansion without compromising the low risk of relapse and chronic GVHD of CB transplants. Despite these promising results, the small number of patients in the UM171 cohort precludes any firm conclusions, and a phase 3 randomized trial will be required to confirm these results.
Acknowledgments
The authors thank the Center for International Blood and Marrow Transplant Research (CIBMTR) member centers in the United States and throughout the world for reporting patients to the CIBMTR (refer to https://www.cibmtr.org/About/WhoWeAre/Centers/Pages/index.aspx) and contributing to this study. The initial phase 1-2 study of UM171 expanded cord blood (CB) transplant, which is the basis for this matched-paired analysis, was funded by the Stem Cell Network (FY17/CT1), the Canadian Institutes of Health Research (3264569) and the Canadian Cancer Society Research Institute (703199). ExCellThera contributed the funding for this matched-paired analysis with the CIBMTR. The authors acknowledge Catherine Paquin, research nurse, for all her work pertaining to this study and the members of the Center of Excellence for Cell Therapy at Maisonneuve-Rosemont Hospital for manufacturing of the UM171 expanded CB grafts. They thank Hema-Quebec for their contribution to CB selection. Finally, they recognize the support received by all the members of the Hematology-Oncology service at Maisonneuve Rosemont Hospital, Montreal, Canada during the UM171 clinical trials.
Authorship
Contribution: S.C. was responsible for study design, data collection, data analysis, and interpretation, and she wrote the first draft as well as the final version of the manuscript; N.B. and I.A. contributed to data collection and review of the raw data; L. Burns and M.-J.Z., and X.T. contributed to study design, data collection and assembly, and data analysis and interpretation; and all the authors contributed to the review of the final data and the writing and review of the manuscript.
Conflict-of-interest disclosures: S.C. has received consulting fees from ExCellThera. S.C., J.R., S.L., and G.S. are eligible to receive royalties if UM171 is commercialized. G.S. is a major stockholder and chief executive/scientific officer of ExCellThera. S.C. and J.R. have received funding for clinical trials from ExCellThera. ExCellThera holds the license for UM171. L.J.B., M.-J.Z., and X.T. have collaborated on research with Gamida Cell, Inc. L. Burns has also collaborated on research with Astellas, bluebird bio, Gamida Cell, Kyowa Kirin, Mesoblast, Sanofi, and Vertex. The remaining authors declare no competing financial interests.
Correspondence: Sandra Cohen, Maisonneuve Rosemont Hospital, 5415 boul. de l’Assomption, Montréal, QC, H1T 2M4, Canada; e-mail: sandra.cohen@umontreal.ca; and Guy Sauvageau, Maisonneuve Rosemont Hospital, 5415 boul. de l’Assomption, Montréal, QC, H1T 2M4, Canada; e-mail: guy.sauvageau@umontreal.ca.
References
Author notes
CIBMTR supports accessibility of research in accord with the National Institutes of Health Data Sharing Policy and the National Cancer Institute Cancer Moonshot Public Access and Data Sharing Policy. CIBMTR only releases deidentified data sets that comply with all relevant global regulations regarding privacy and confidentiality.
Data are available upon request from the investigators. The initial phase 1-2 trial was published in Lancet Hematology (doi: https://doi.org/10.1016/S2352-3026(19)30202-9). The data published in that study were used for the matched-paired analysis in this study.
The online version of this article contains a data supplement.