Abstract
Immunogenicity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination is diminished in hematopoietic stem cell transplant (HSCT) recipients. To summarize current evidence and identify risk factors for attenuated responses, 5 electronic databases were searched since database inceptions through 12 January 2023 for studies reporting humoral and/or cellular immunogenicity of SARS-CoV-2 vaccination in the HSCT population. Using descriptive statistics and random-effects models, extracted numbers of responders and pooled odds ratios (pORs) with 95% confidence intervals (CIs) for risk factors of negative immune responses were analyzed (PROSPERO: CRD42021277109). From 61 studies with 5906 HSCT recipients, after 1, 2, and 3 doses of messenger RNA (mRNA) SARS-CoV-2 vaccines, the mean antispike antibody seropositivity rates (95% CI) were 38% (19-62), 81% (77-84), and 80% (75-84); neutralizing antibody seropositivity rates were 52% (40-64), 71% (54-83), and 78% (61-89); and cellular immune response rates were 52% (39-64), 66% (51-79), and 72% (52-86). After 2 vaccine doses, risk factors (pOR; 95% CI) associated with antispike seronegativity were male recipients (0.63; 0.49-0.83), recent rituximab exposure (0.09; 0.03-0.21), haploidentical allografts (0.46; 0.22-0.95), <24 months from HSCT (0.25; 0.07-0.89), lymphopenia (0.18; 0.13-0.24), hypogammaglobulinemia (0.23; 0.10-0.55), concomitant chemotherapy (0.48; 0.29-0.78) and immunosuppression (0.18; 0.13-0.25). Complete remission of underlying hematologic malignancy (2.55; 1.05-6.17) and myeloablative conditioning (1.72; 1.30-2.28) compared with reduced-intensity conditioning were associated with antispike seropositivity. Ongoing immunosuppression (0.31; 0.10-0.99) was associated with poor cellular immunogenicity. In conclusion, attenuated humoral and cellular immune responses to mRNA SARS-CoV-2 vaccination are associated with several risk factors among HSCT recipients. Optimizing individualized vaccination and developing alternative COVID-19 prevention strategies are warranted.
Introduction
Before the era of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination, coronavirus disease 2019 (COVID-19) could lead to high mortality from 17% to 21% in autologous or allogeneic hematopoietic stem cell transplant (HSCT) recipients because of impaired humoral and cellular immunity.1 After the rapid development of SARS-CoV-2 vaccination platforms, the efficacy and safety of 3 messenger RNA (mRNA) vaccine doses have been proven in the general population,2,3 but HSCT recipients were excluded from most vaccine trials. Moreover, studies of solid organ transplant recipients and patients with multiple myeloma revealed low immunogenicity of the mRNA SARS-CoV-2 vaccination, with seroconversion rates ranging from 49% to 69% and from 56% to 94%, respectively.4,5
The emergence of the SARS-CoV-2 Omicron variant of concern (VOC) reduces the efficacy of first-generation vaccine platforms.6 Neutralizing monoclonal antibodies (mAbs) as preexposure prophylaxis have similarly shown reduced efficacy over time, and as of January 2023, their emergency use authorization has been revoked.7 Although long-term immune responses after vaccination require further exploration,8 booster SARS-CoV-2 vaccine doses have been recommended, with additional doses for patients with immunocompromising conditions.9 However, immune responses can be diminished despite repeated vaccinations in patients with hematologic malignancies undergoing HSCT.10 In this updated systematic review and meta-analysis, we summarize current evidence on humoral immunogenicity, determined by antispike Abs and neutralizing Abs (nAbs), and cellular immunogenicity of first-generation SARS-CoV-2 vaccines in HSCT recipients, as well as risk factors associated with poor immune responses.
Methods
Data sources and searches
Two authors (T.M. and K.M.) independently performed a systematic search in MEDLINE, Embase, ISI Web of Science, Cochrane Library, and ClinicalTrials.gov databases from their inceptions to 12 January 2023. Search terms included “SARS-CoV-2,” “COVID-19,” “vaccine,” “BNT162b2,” “mRNA-1273,” “Ad26.COV2.S,” “AZD1222,” “hematopoietic stem cell transplant,” and “hematologic malignancy”; full strings of which are available in the supplement (supplemental Methods). After combining the search results, the duplicates were excluded. This study was conducted without language limitation according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and the International Prospective Register of Systematic Reviews (PROSPERO) registration (CRD42021277109).11
Study selection
All retrievable studies were independently reviewed by the 2 authors (T.M. and K.M.). Clinical and observational studies reporting humoral and/or cellular immunogenicity of SARS-CoV-2 vaccines in HSCT recipients were eligible for review. Preprints, case reports, editorials, reviews, conference abstracts, and studies with <10 participants were excluded. For studies with overlapping populations, the study with the larger population or with more recent data was included. Studies limited to HSCT recipients with prior COVID-19 were excluded. Conflicts were resolved via mutual consensus among reviewers.
Data extraction and quality assessment
The primary outcome was a humoral immune response, including antispike Abs and nAbs, after SARS-CoV-2 vaccination. The secondary outcomes were a functional cellular immune response and risk factors associated with poor humoral and cellular immunogenicity of vaccination. A priori, potential risk factors were divided into 3 groups: (1) host characteristics, including age >60 years, sex, underlying hematologic diseases (myeloid malignancy, lymphoid malignancy, acute leukemia, and nonmalignancy), rituximab exposure within 6 to 12 months, and remission status of underlying diseases; (2) HSCT characteristics, including matched sibling donors, matched unrelated donors, haploidentical donors, myeloablative conditioning (MAC) vs reduced-intensity conditioning (RIC) regimens, and timing from HSCT to vaccination; and (3) post-HSCT characteristics, including any graft-versus-host disease (GVHD), acute GVHD, chronic GVHD, concurrent chemotherapy for relapsed disease, ongoing immunosuppressive therapy, lymphopenia (absolute lymphocyte count <1.0 × 103 cells per μL), and hypogammaglobulinemia (immunoglobulin G level <6 g/L).
Positive responses were determined based on the definitions and the test cutoffs used in each study. Antispike seropositivity rates based on commercial or in-house immunoassays quantitatively measuring Abs, including immunoglobulin G, against SARS-CoV-2 spike protein; nAb seropositivity rates based on surrogate or cell-based viral neutralization assays; and cellular immune response rates based on immunoassays or flow cytometry detecting interferon gamma production from peripheral blood mononuclear cells after stimulation with SARS-CoV-2 spike protein were reviewed (supplemental Tables 10-13). The numbers of responders, total participants, and odds ratios (ORs) with 95% confidence intervals (CIs) for potential risk factors were extracted. If ORs were not available, crude numbers were extracted for OR calculation. Google Translate was used during the screening processes for non-English publications. Study quality was evaluated using the Newcastle-Ottawa scale (NOS) (supplemental Table 1).12
Data synthesis and analysis
The random-effects meta-analysis using generalized linear mixed models to estimate the pooled immune response rates with 95% CIs was performed using R version 4.2 (R Foundation, Vienna, Austria).13 The pooled ORs (pORs; 95% CIs) and the pooled mean differences (standard errors) for potential risk factors were generated using the DerSimonian and Laird random-effects models.14 Sensitivity analyses were performed by removing low-quality studies based on the NOS. Publication bias was evaluated using funnel plot inspection and Egger regression in models with ≥10 studies.15 Concerned estimates (Egger regression P < .1) were adjusted using the Duval and Tweedie trim-and-fill method.16 The I2 value (0%-100%), based on the I2 statistics, indicated a low (I2 < 25%), moderate (I2 = 25%-60%), or substantial heterogeneity (I2 > 60%) in each model.14 Levels of evidence for the pORs were assessed based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE).17
Results
Study and patient characteristics
The initial search generated 6144 results, 4866 of which were unique. Of these, 4689 were excluded by screening titles and abstracts. Of the 177 English full-text articles assessed for eligibility, 116 articles were excluded because they were review articles, preprints, or duplicate cohorts; included <10 participants; included participants with prior COVID-19; or had no outcomes of interest. A total of 61 studies18-78 were included in the systematic review, of which 52 studies were included in the meta-analysis, representing 5906 HSCT recipients (4647 allogeneic, 945 autologous, and 314 unclassified-type HSCT recipients; Figure 1; supplemental Tables 2-4). Eleven studies reported vaccine responses after mRNA (BNT162b2, Pfizer-BioNTech; mRNA-1273, Moderna) and/or adenoviral vector (AZD1222, AstraZeneca; Ad26.COV2.S, Janssen) vaccines,33,34,42,45,50,58,61,64,65,70,73 whereas 50 studies provided data on immune responses after mRNA vaccines only.18-32,35-41,43,44,46-49,51-57,59,60,62,63,66-69,71,72,74-78 Among studies with mRNA vaccines only, 23 studies provided data on the risk factors associated with diminished immunogenicity20,21,24,26,27,29,30,37,38,40,46,48,30,52,54,55,57,67,71,72,74-76,78 (supplemental Tables 14 and 15).
PRISMA study selection flowchart. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
PRISMA study selection flowchart. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Humoral immunogenicity
mRNA vaccines
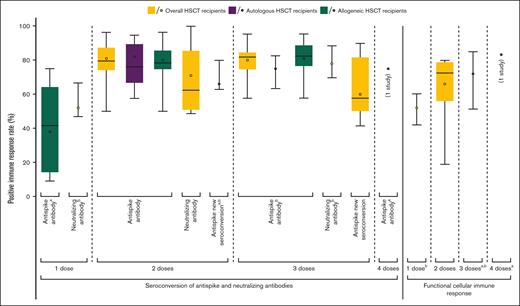
After 1 dose of BNT162b2 or mRNA-1273, the mean antispike seropositivity rate was 38% (95% CI, 19-62; 5 studies)34,47,48,63,68 (Figure 2; supplemental Table 10; supplemental Figure 1A). None of the included studies reported antispike seropositivity after 1 dose of the mRNA vaccine in autologous HSCT recipients. The mean nAb seropositivity rate was 52% (95% CI, 40-64; 2 studies)38,68 (Figure 2; supplemental Table 11; supplemental Figure 1B). Only 1 study reported a nAb seropositivity rate of 64% in autologous HSCT recipients,38 whereas the mean nAb seropositivity rate in allogeneic HSCT recipients was 49% (95% CI, 36-62; 2 studies).38,68 The mean time to Ab testing was 29.4 (95% CI, 22.2-36.5) days after vaccination.
Humoral and cellular immune responses to SARS-CoV-2 vaccination among HSCT recipients. Dark horizontal lines indicate medians; colored dots, weighted means; boxes, interquartile ranges (IQRs); whiskers, ranges. Only data in the allogeneic HSCT group are available in some models (indicated by a). Because of <5 included studies, some box plots cannot be graphed (indicated by b).
Humoral and cellular immune responses to SARS-CoV-2 vaccination among HSCT recipients. Dark horizontal lines indicate medians; colored dots, weighted means; boxes, interquartile ranges (IQRs); whiskers, ranges. Only data in the allogeneic HSCT group are available in some models (indicated by a). Because of <5 included studies, some box plots cannot be graphed (indicated by b).
After 2 doses of mRNA vaccines, the mean antispike and nAb seropositivity rates were 81% (95% CI, 77-84; 39 studies)20-22,24-29,32,35,37,39,40,43,46-48,51-60,62,63,66,67,69,71,72,74-76,78 and 71% (95% CI, 54-83; 6 studies),19,28,32,38,44,51 respectively (Figure 2; supplemental Tables 10 and 11; supplemental Figure 1C-D). HSCT recipients without antispike seropositivity after a first dose of mRNA vaccine achieved seroconversion after a second dose in the mean of 66% (95% CI, 53-76; 2 studies)48,63 (supplemental Table 12; supplemental Figure 1E). Regardless of prior serostatus, the mean antispike seropositivity rate after 2 doses of mRNA vaccines among autologous HSCT recipients was 82% (95% CI, 72-89; 11 studies),20-22,26,29,40,55,57,59,69,75 whereas the mean antispike seropositivity rate in allogeneic HSCT recipients was 80% (95% CI, 76-83; 31 studies).20,24-29,32,37,40,43,46-48,52,54-60,63,66,67,71,72,74-76,78 Only 1 study reported a nAb seropositivity rate of 100% after 2 doses of mRNA vaccines in autologous HSCT recipients,38 whereas the mean nAb seropositivity rate in allogeneic HSCT recipients was 69% (95% CI, 54-80; 5 studies).19,28,32,38,44 The mean time to Ab testing was 30.1 days (95% CI, 26.9-33.2) after the second dose. Seropositivity rates after 2 doses of mRNA vaccines as measured by various antispike and nAb laboratory testing methods are summarized in supplemental Tables 7 and 8.
After 3 doses of mRNA vaccines, the mean antispike and nAb seropositivity rates were 80% (95% CI, 75-84; 10 studies)18,23,31,36,41,47,49,51,52,77 and 78% (95% CI, 61-89; 2 studies),19,51 respectively (Figure 2; supplemental Tables 10 and 11; supplemental Figure 1F,H). HSCT recipients without antispike seropositivity after 2 doses of mRNA vaccines achieved seroconversion after a third dose in the mean of 60% (95% CI, 47-71; 6 studies)18,23,36,49,52,77 (supplemental Table 12; supplemental Figure 1I). Regardless of prior serostatus, the mean antispike seropositivity rate after 3 doses of mRNA vaccines among autologous HSCT recipients was 75% (95% CI, 60-86; 2 studies),18,41 whereas the mean antispike seropositivity rate in allogeneic HSCT recipients was 81% (95% CI, 76-86; 9 studies).18,23,31,36,41,47,49,52,77 When excluding 1 study that exclusively enrolled HSCT recipients who did not achieve seroconversion after 2 doses of mRNA vaccines,18 the antispike seropositivity rate after 3 doses of mRNA vaccines in autologous HSCT recipients was 83% based on the other 1 study (supplemental Figure 1J).41 Nevertheless, none of the included studies specifically reported nAb seropositivity after 3 doses of mRNA vaccines in the autologous HSCT group. The mean time to Ab testing was 49.2 days (95% CI, 17.3-81.0) after the third dose. Considering the shorter times to Ab testing after the first and second doses, the mean antispike seropositivity rate at ∼4 weeks after the third dose was 82% (95% CI, 77-86; 4 studies)36,41,47,51 (supplemental Figure 1G). One study reported an antispike seropositivity rate after 4 doses of BNT162b2 in 12 HSCT recipients: 9 (75%) were seropositive, and 3 (25%) were seronegative at the median of 60.0 days (interquartile range, 16.0-117.0) after the fourth dose.31
Other vaccine platforms
At the time of this search, there were no studies reporting humoral immune responses from other vaccine platforms alone. Nine studies reported antispike seropositivity rates after mixed series using mRNA and adenoviral vector (AZD1222, AstraZeneca; Ad26.COV2.S, Janssen) vaccines (supplemental Table 10). In these studies, the mean antispike seropositivity rate was 77% (95% CI, 69-83).33,42,45,50,58,64,65,70,73
Risk factors for attenuated antispike humoral immune response after 2 doses of SARS-CoV-2 vaccines
Host characteristics
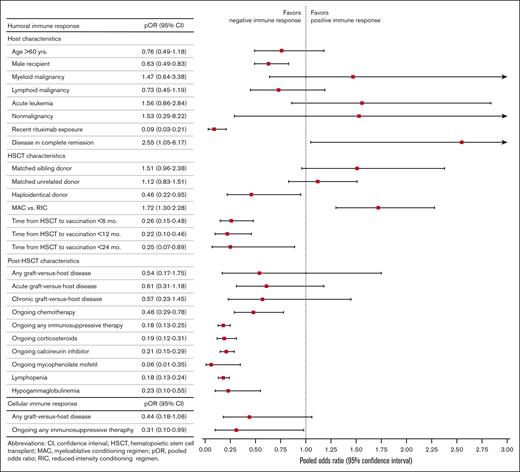
Male sex (pOR, 0.63; 95% CI, 0.49-0.83; P < .01; I2 = 7%; 11 cohorts from 10 studies)21,24,37,48,52,57,67,71,72,78 and rituximab exposure within 6 to 12 months (pOR, 0.09; 95% CI, 0.03-0.21; P < .01; I2 = 0%; 4 studies)21,26,52,74 were associated with seronegativity. Complete remission of the patient’s underlying hematologic malignancy was associated with seropositivity (pOR, 2.55; 95% CI, 1.05-6.17; P = .04; I2 = 0%; 3 cohorts from 2 studies).71,75 Age >60 years and underlying diseases were not significantly associated with humoral immune responses (Table 1; Figure 3; supplemental Table 14; supplemental Figure 2A).
Summary of risk factors associated with attenuated humoral and cellular immunogenicity after 2 doses of SARS-CoV-2 vaccination in HSCT recipients
| Risk factors . | pORs for positive immune responses (95% CI) . | No. of cohorts∗ . | Levels of evidence (GRADE) . |
|---|---|---|---|
| Humoral immune response | |||
| Host characteristics | |||
| Age >60 y | 0.76 (0.49-1.18) | 621,48,54,57,78 (1 study with 2 cohorts)57 | Moderate |
| Male recipient | 0.63 (0.49-0.83) | 1121,24,37,48,52,57,67,71,72,78 (1 study with 2 cohorts)57 | Moderate |
| Myeloid malignancy | 1.47 (0.64-3.38) | 824,26,29,48,52,71,72,78 | Moderate |
| Lymphoid malignancy | 0.73 (0.45-1.19) | 1121,26,29,48,52,57,71,72,78 (2 studies with 2 cohorts)26,57 | Moderate |
| Acute leukemia | 1.56 (0.86-2.84) | 426,71,72,78 | Moderate |
| Nonmalignancy | 1.53 (0.29-8.22) | 352,71,72 | Moderate |
| Rituximab exposure within 6-12 mo | 0.09 (0.03-0.21) | 421,26,52,74 | Moderate |
| Complete remission status of underlying malignancy/indication for HSCT | 2.55 (1.05-6.17) | 371,75 (1 study with 2 cohorts)75 | Moderate |
| HSCT characteristics | |||
| Donor | |||
| Matched sibling donor | 1.51 (0.96-2.38) | 724,26,48,52,71,72,78 | Moderate |
| Matched unrelated donor | 1.12 (0.83-1.51) | 724,26,48,52,71,72,78 | Moderate |
| Haploidentical donor | 0.46 (0.22-0.95) | 524,26,48,52,71 | Moderate |
| Conditioning regimen | |||
| MAC vs RIC | 1.72 (1.30-2.28) | 726,48,52,57,71,72,78 | Moderate |
| Time from transplant to vaccination | |||
| <6 mo | 0.26 (0.15-0.48) | 421,27,40,54 | Moderate |
| <12 mo | 0.22 (0.10-0.46) | 1420,21,24,27,40,48,52,55,57,71,74,76 (2 studies with 2 cohorts)55,57 | Moderate |
| <24 mo | 0.25 (0.07-0.89) | 437,48,71,76 | Moderate |
| Post-HSCT characteristics | |||
| Any GVHD | 0.54 (0.17-1.75) | 448,52,71,75 | Moderate |
| Acute GVHD | 0.61 (0.31-1.18) | 357,72,78 | Moderate |
| Chronic GVHD | 0.57 (0.23-1.45) | 737,40,57,71,72,74,78 | Moderate |
| Ongoing chemotherapy | 0.48 (0.29-0.78) | 426,46,52 (1 study with 2 cohorts)26 | Moderate |
| Ongoing any IST | 0.18 (0.13-0.25) | 1026,37,46,48,52,71,72,75,78 (1 study with 2 cohorts)26 | Moderate |
| Ongoing corticosteroids | 0.19 (0.12-0.31) | 626,57,71,72,74,78 | Moderate |
| Ongoing calcineurin inhibitor | 0.21 (0.15-0.29) | 626,57,71,72,74,78 | Moderate |
| Ongoing mycophenolate mofetil | 0.06 (0.01-0.35) | 226,71 | Moderate |
| Lymphopenia (<1000 cells per μL) | 0.18 (0.13-0.24) | 548,52,54,57 (1 study with 2 cohorts)57 | Moderate |
| Hypogammaglobulinemia (IgG <6 g/L) | 0.23 (0.10-0.55) | 352,57 (1 study with 2 cohorts)57 | Moderate |
| Cellular immune response | |||
| Post-HSCT characteristics | |||
| Any GVHD | 0.44 (0.18-1.06) | 237,46 | Moderate |
| Ongoing any IST | 0.31 (0.10-0.99) | 430,37,38,46 | Low |
| Risk factors . | pORs for positive immune responses (95% CI) . | No. of cohorts∗ . | Levels of evidence (GRADE) . |
|---|---|---|---|
| Humoral immune response | |||
| Host characteristics | |||
| Age >60 y | 0.76 (0.49-1.18) | 621,48,54,57,78 (1 study with 2 cohorts)57 | Moderate |
| Male recipient | 0.63 (0.49-0.83) | 1121,24,37,48,52,57,67,71,72,78 (1 study with 2 cohorts)57 | Moderate |
| Myeloid malignancy | 1.47 (0.64-3.38) | 824,26,29,48,52,71,72,78 | Moderate |
| Lymphoid malignancy | 0.73 (0.45-1.19) | 1121,26,29,48,52,57,71,72,78 (2 studies with 2 cohorts)26,57 | Moderate |
| Acute leukemia | 1.56 (0.86-2.84) | 426,71,72,78 | Moderate |
| Nonmalignancy | 1.53 (0.29-8.22) | 352,71,72 | Moderate |
| Rituximab exposure within 6-12 mo | 0.09 (0.03-0.21) | 421,26,52,74 | Moderate |
| Complete remission status of underlying malignancy/indication for HSCT | 2.55 (1.05-6.17) | 371,75 (1 study with 2 cohorts)75 | Moderate |
| HSCT characteristics | |||
| Donor | |||
| Matched sibling donor | 1.51 (0.96-2.38) | 724,26,48,52,71,72,78 | Moderate |
| Matched unrelated donor | 1.12 (0.83-1.51) | 724,26,48,52,71,72,78 | Moderate |
| Haploidentical donor | 0.46 (0.22-0.95) | 524,26,48,52,71 | Moderate |
| Conditioning regimen | |||
| MAC vs RIC | 1.72 (1.30-2.28) | 726,48,52,57,71,72,78 | Moderate |
| Time from transplant to vaccination | |||
| <6 mo | 0.26 (0.15-0.48) | 421,27,40,54 | Moderate |
| <12 mo | 0.22 (0.10-0.46) | 1420,21,24,27,40,48,52,55,57,71,74,76 (2 studies with 2 cohorts)55,57 | Moderate |
| <24 mo | 0.25 (0.07-0.89) | 437,48,71,76 | Moderate |
| Post-HSCT characteristics | |||
| Any GVHD | 0.54 (0.17-1.75) | 448,52,71,75 | Moderate |
| Acute GVHD | 0.61 (0.31-1.18) | 357,72,78 | Moderate |
| Chronic GVHD | 0.57 (0.23-1.45) | 737,40,57,71,72,74,78 | Moderate |
| Ongoing chemotherapy | 0.48 (0.29-0.78) | 426,46,52 (1 study with 2 cohorts)26 | Moderate |
| Ongoing any IST | 0.18 (0.13-0.25) | 1026,37,46,48,52,71,72,75,78 (1 study with 2 cohorts)26 | Moderate |
| Ongoing corticosteroids | 0.19 (0.12-0.31) | 626,57,71,72,74,78 | Moderate |
| Ongoing calcineurin inhibitor | 0.21 (0.15-0.29) | 626,57,71,72,74,78 | Moderate |
| Ongoing mycophenolate mofetil | 0.06 (0.01-0.35) | 226,71 | Moderate |
| Lymphopenia (<1000 cells per μL) | 0.18 (0.13-0.24) | 548,52,54,57 (1 study with 2 cohorts)57 | Moderate |
| Hypogammaglobulinemia (IgG <6 g/L) | 0.23 (0.10-0.55) | 352,57 (1 study with 2 cohorts)57 | Moderate |
| Cellular immune response | |||
| Post-HSCT characteristics | |||
| Any GVHD | 0.44 (0.18-1.06) | 237,46 | Moderate |
| Ongoing any IST | 0.31 (0.10-0.99) | 430,37,38,46 | Low |
IgG, immunoglobulin G; IST, immunosuppressive therapy.
Both autologous HSCT and allogeneic HSCT cohorts from the same studies, for which data were accounted for 2 cohorts, had been included in some models.
pORs of potential risk factors associated with attenuated humoral and cellular immunogenicity after 2 doses of SARS-CoV-2 vaccination in HSCT recipients.
pORs of potential risk factors associated with attenuated humoral and cellular immunogenicity after 2 doses of SARS-CoV-2 vaccination in HSCT recipients.
HSCT characteristics
Haploidentical HSCT was associated with seronegativity (pOR, 0.46; 95% CI, 0.22-0.95; P = .04; I2 = 38%; 5 studies).24,26,48,52,71 Intervals of <2 years from HSCT to vaccination were associated with seronegativity with pORs for post-HSCT durations <6, <12, and <24 months of 0.26 (95% CI, 0.15-0.48; P < .01; I2 = 0%; 4 studies),21,27,40,54 0.22 (95% CI, 0.10-0.46; P < .01; I2 = 80%; 14 cohorts from 12 studies),20,21,24,27,40,48,52,55,57,71,74,76 and 0.25 (95% CI, 0.07-0.89; P = .03; I2 = 68%; 4 studies),37,48,71,76 respectively. Compared with RIC, MAC regimens were associated with a better humoral immune response to vaccination (pOR, 1.72; 95% CI, 1.30-2.28; P < .01; I2 = 0%; 7 studies)26,48,52,57,71,72,78 (Table 1; Figure 3; supplemental Table 14; supplemental Figure 2B).
Post-HSCT characteristics
Lymphopenia and hypogammaglobulinemia were associated with seronegativity, with pORs of 0.18 (95% CI, 0.13-0.24; P < .01; I2 = 14%; 5 cohorts from 4 studies)48,52,54,57 and 0.23 (95% CI, 0.10-0.55; P < .01; I2 = 69%; 3 cohorts from 2 studies),52,57 respectively. After HSCT, ongoing chemotherapy for relapsed disease (pOR, 0.48; 95% CI, 0.29-0.78; P < .01; I2 = 0%; 4 cohorts from 3 studies)26,46,52 and any immunosuppression (pOR, 0.18; 95% CI, 0.13-0.25; P < .01; I2 = 20%; 10 cohorts from 9 studies),26,37,46,48,52,71,72,75,78 including corticosteroids (pOR, 0.19; 95% CI, 0.12-0.31; P < .01; I2 = 15%; 6 studies),26,57,71,72,74,78 calcineurin inhibitors (pOR, 0.21; 95% CI, 0.15-0.29; P < .01; I2 = 0%; 6 studies),26,57,71,72,74,78 and mycophenolate mofetil (pOR, 0.06; 95% CI, 0.01-0.35; P < .01; I2 = 0%; 2 studies),26,71 were associated with seronegativity (Table 1; Figure 3; supplemental Table 14; supplemental Figure 2C). HSCT recipients who were seropositive after 2 doses of mRNA vaccines had significantly higher absolute lymphocyte counts than those who were seronegative (pooled mean difference, 0.88 × 103 cells per μL; standard error, 0.36 × 103 cells per μL; P = .02; I2 = 90%; 3 cohorts from 2 studies)71,75 (supplemental Tables 16 and 17; supplemental Figure 5). GRADE for potential risk factors associated with poor humoral immune response to vaccination is available in supplemental Table 5.
Cellular immunogenicity
After 1 dose of BNT162b2 or mRNA-1273, the pooled mean of cellular immune response rates was 52% (95% CI, 39-64; 2 studies).37,38 After 2 doses of mRNA vaccines, the pooled mean of cellular immune response rates was 66% (95% CI, 51-79; 8 studies)30,32,37,38,46,47,58,67 (Figure 2; supplemental Table 13; supplemental Figure 3A-B). Five studies used interferon gamma enzyme-linked immunosorbent spot assays.30,32,38,58,67 Cellular immune response rates after 2 doses of mRNA vaccines, as measured using various immunological methods, are summarized in supplemental Table 9.
After 3 doses of mRNA vaccines, the pooled mean of cellular immune response rates was higher at 72% (95% CI, 52-86; 3 studies)31,36,47 (Figure 2; supplemental Table 13; supplemental Figure 3C). After 4 doses of BNT162b2, 1 study reported a cellular immune response rate of 83%.31 None of the included studies specifically reported cellular immune response in the autologous HSCT group.
Risk factors for attenuated cellular immune response after 2 doses of SARS-CoV-2 vaccines
Four studies provided data related to risk factors associated with poor cellular immunogenicity after 2 doses of mRNA vaccines.30,37,38,46 Ongoing immunosuppression was associated with poor cellular immune response (pOR, 0.31; 95% CI, 0.10-0.99; P = .05; I2 = 55%; 4 studies)30,37,38,46 (Table 1; Figure 3; supplemental Table 15; supplemental Figure 4). GRADE for potential risk factors associated with poor cellular immune response to vaccination is available in supplemental Table 6.
Sensitivity analysis and publication bias
The quality assessment based on the NOS did not identify any studies as poor quality. Hence, sensitivity analysis by removing studies with poor quality was not applicable. Egger test and inspection of funnel plots indicated potential publication bias with regard to the antispike seropositivity rate after 2 doses (P = .04) but not for 3 doses of mRNA vaccines or for male sex, time from HSCT to vaccination <12 months, or ongoing immunosuppressive therapy (supplemental Figure 6). We were unable to assess publication bias for other factors because of the insufficient number of studies. Adjusted by the trim-and-fill method,16 the antispike seropositivity rate after 2 doses of mRNA vaccines was 76% (95% CI, 72-80) (supplemental Figure 7).
Discussion
This systematic review and meta-analysis recapitulates the cumulative evidence regarding immunological responses to SARS-CoV-2 vaccines and risk factors associated with attenuated responses among HSCT recipients. From studies of 1 to 3 doses of mRNA vaccines, the antispike seropositivity rate increased from 38% after 1 dose to 81% and 80% after 2 and 3 doses, respectively. Although we found no obvious difference in overall seropositivity rates after second and third doses of mRNA vaccines, in studies of patients who were previously seronegative, the new seroconversion rate after a third dose was 60%, which might be expected to achieve a seropositivity rate of 91% overall. The nAb seropositivity rate increased from 52% after 1 dose to 71% and 78% after 2 and 3 doses, respectively. In studies of cellular immunogenicity, the positive functional cellular immune response rate increased from 52% after 1 dose to 66%, 72%, and 83% after 2, 3, and 4 doses, respectively. These findings can be compared with data from the general population, in whom the antispike seropositivity, nAb seropositivity, and positive functional cellular immune responses are seen in 99% to 100%, 97% to 99%, and 72% to 91% of individuals after 2 vaccine doses, and in 94% to 98%, 90% to 96%, and 89% to 100% of individuals after 3 vaccine doses, respectively.3,79,80
Importantly, the role of subsequent vaccine doses is not only to increase the proportion of recipients with protective humoral and cellular immunity relative to previous doses but also to maintain protective immunity against waning of Ab or cellular immune responses between doses.81 Our study is not able to quantify this benefit of repeated vaccination, which would be measured by tracking patients who stopped vaccination after 1 or 2 doses and comparing them over time with patients who continued to receive additional doses.
Several factors have been identified to be associated with an attenuated humoral immune response after 2 doses of mRNA vaccines: male sex; recent rituximab exposure; haploidentical HSCT; lymphopenia; hypogammaglobulinemia; vaccination timed within 2 years post-HSCT; or concomitant chemotherapy or immunosuppression, such as corticosteroids, calcineurin inhibitors, and mycophenolate mofetil. Ongoing immunosuppression is also associated with an attenuated cellular immune response to vaccination.
Until achieving post-HSCT adaptive immune reconstitution (IR), which usually requires 6 to 24 months or longer, HSCT recipients are at high risk of infections and respond to vaccination poorly.82-84 Repeated childhood and adult vaccination series are indispensable for augmented seroprotection,85-88 because IR is more consolidated over time.89 From our meta-analysis, 2 to 3 doses of SARS-CoV-2 vaccines given after HSCT could lead to seroconversion in ∼80% of HSCT recipients, compared with 38% to 52% after 1 dose. However, those vaccinated <24 months after HSCT responded poorly to SARS-CoV-2 vaccination, possibly because restoration of the B-cell compartment remained incomplete.82,83
Markers of B-cell deficiency/dysfunction (lymphopenia, hypogammaglobulinemia), HSCT factors associated with delayed B-cell IR (rituximab, haploidentical allografts),83,90-92 and post-HSCT factors that may synergistically suppress B-cell function (ongoing chemotherapy, corticosteroids, and corticosteroid-sparing agents)90 are all associated with seronegativity after 2 vaccine doses, highlighting the crucial importance of B-cell IR for establishing protection from vaccination after HSCT. Efforts to better forecast, promote, or assess B-cell IR could help with individualizing the timing of post-HSCT vaccines.
T-cell IR can also take years after HSCT.83 We have shown that ongoing use of immunosuppression for GVHD prophylaxis, with T-cell inhibitory effects,83,93 is associated with an attenuated cellular immune response to SARS-CoV-2 vaccines.
Interestingly, we found a superior humoral immune response to vaccination among HSCT recipients who had received more intensive MAC compared with those who received RIC. Although RIC might shorten the time to IR after HSCT, vaccine response in RIC recipients may be confounded by more fragile conditions at baseline, the need for T-cell lymphodepletion with antithymocyte globulin or alemtuzumab, and a higher GVHD risk.94
Male HSCT recipients, compared with female recipients, demonstrate weaker post-HSCT immunity, shorter survival, and an increased risk of GVHD, regardless of donor sex.95,96 Our finding that male sex is associated with an attenuated humoral immune response to SARS-CoV-2 vaccination aligns with this constellation of sex-specific risks.97
mRNA SARS-CoV-2 vaccines are useful for establishing humoral and cellular immune protection against SARS-CoV-2 after HSCT, but this protection may be incomplete. Despite the anticipated reduced immunogenicity of mRNA vaccines in HSCT recipients, it is crucial not to delay SARS-CoV-2 vaccination using the best available mRNA vaccines. Recent evidence during the Omicron era supported the administration of the full 3-dose primary series, with or without an additional booster, as it could prevent COVID-19 for up to 6 months after vaccination in 91.2% and 78.4% of allogeneic HSCT recipients, respectively. Among those infected, the reported mortality rate was 2.7%.98 However, concerning impaired immunization in HSCT recipients within the first 2 years after HSCT or with risk factors, the optimized SARS-CoV-2 vaccination schedule is yet to be determined.
Subgroups identified as more vulnerable to poor immune responses to vaccination would also benefit from additional preventive strategies. Neutralizing mAb preexposure prophylaxis was previously safe and effective in this group.99,100 Unfortunately, emergency use authorizations for neutralizing mAbs for preexposure prophylaxis, postexposure prophylaxis, and treatment have all been halted by the US Food and Drug Administration after these products lost efficacy against the Omicron VOC, especially more immune-evasive subvariants.7,9 Preclinical studies suggested a new therapeutic strategy to enhance neutralizing activity against the Omicron VOC using bivalent or bispecific neutralizing mAbs by simultaneously engaging the SARS-CoV-2 spike protein through both antigen-binding fragments.101,102 Nevertheless, accelerated clinical studies of novel effective neutralizing mAbs are urgently needed, and we would support broad authorization of any new neutralizing mAb product with a favorable safety profile for use as preexposure prophylaxis, postexposure prophylaxis, and/or treatment in especially vulnerable HSCT recipients. Authorization for multiple indications should be accompanied by plans to further study the effectiveness of each indication after the product is distributed.
Our aggregate data provide updated and more robust information relative to a previous meta-analysis.103 Most studies reported immunogenicity after 2 doses of vaccination, limiting our ability to identify risk factors associated with attenuated immunity after the full 3-dose primary series recommended for patients who are immunocompromised, although we presume that similar factors associated with IR would also affect the response to additional doses. Because all the included studies used first-generation, monovalent SARS-CoV-2 vaccines, our results are helpful for many countries in which bivalent booster vaccines are not available. The immunogenicity of bivalent boosters must be further studied for patients who underwent HSCT, and equity in global vaccine distribution for individuals with and without these risk factors remains paramount for protecting the most vulnerable around the world.104 Additional limitations of our analysis are the small proportion of autologous HSCT recipients in the included studies, limited data on donor immunity that may benefit immunization in allogeneic HSCT recipients,105 focus on mRNA vaccines that are not available in all parts of the world, and variation in laboratory techniques and evaluative criteria for immunogenicity. Gold-standard laboratory methods have not been defined. Finally, although failure to detect a positive result of measured humoral or cellular immunity may suggest a lack of immune response, the clinical significance of test positivity in terms of infection, disease severity, and mortality in the HSCT population remains uncertain. Applying the tests outside the research context should be interpreted with caution.
Humoral and cellular immunogenicity of SARS-CoV-2 vaccination are attenuated in HSCT recipients, with factors associated with incomplete or delayed post-HSCT IR portending a worse immune response. Recognizing these factors presents an opportunity to individualize vaccination and other preventive strategies. Additional studies, including those with bivalent boosters in HSCT recipients, are needed to define the ideal vaccination schedule. Furthermore, we anticipate that the recommended vaccination schedule may change with the emergence of new variants and the development of new variant-specific booster vaccines. As we continue to grapple with the evolving challenges of COVID-19 for patients who are immunocompromised, we call for continued efforts to develop and distribute effective neutralizing mAbs for preexposure prophylaxis and other indications.
Acknowledgments
The authors extend their gratitude to librarians at Faculty of Medicine, Chiang Mai University, Faculty of Medicine, Chulalongkorn University, and Johns Hopkins University School of Medicine for their assistance retrieving full manuscripts that were not available on the databases.
No funding is to be disclosed for the study.
Authorship
Contribution: T.M. and K.S. designed the study, performed literature search, extracted data, performed quality assessment of the studies, analyzed the data, and wrote and critically reviewed the manuscript; K.M. designed the study, performed literature search, extracted data, and wrote and critically reviewed the manuscript; A.T. and N.M. extracted data, performed quality assessment of the studies, and wrote and critically reviewed the manuscript; N.C. and S.N. performed literature search, and wrote and critically reviewed the manuscript; A.S. analyzed data, and wrote and critically reviewed the manuscript; K.P., S.L., J.T., B.L., N.L., P.T., N.W., R.P., and N.H. wrote and critically reviewed the manuscript; N.P. and O.S.K. designed the study, analyzed the data, and wrote and critically reviewed the manuscript; and C.M. designed the study, performed literature search, extracted and analyzed the data, and wrote and critically reviewed the manuscript.
Conflict-of-interest disclosure: S.N. received a grant from the Fisher Center Discovery Program, Johns Hopkins University. N.W. and R.P. received grants from the Health Systems Research Institute (Thailand) and Rachadapiseksompotch Fund, Chulalongkorn University outside the submitted work. N.P. received grants and salary support from the Health Systems Research Institute (Thailand), the Fisher Center Discovery Program, Johns Hopkins University, the Cystic Fibrosis Foundation, and Cidara outside the submitted work, and served on the advisory board for Shionogi Inc and data review committee for Pulmocide Ltd. The remaining authors declare no competing financial interests.
Correspondence: Chatphatai Moonla, Department of Medicine, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, 1873 Rama IV Rd, Pathumwan, Bangkok 10330, Thailand; e-mail: chatphatai.moonla@gmail.com.
References
Author notes
∗T.M. and K.S. contributed equally to this study.
T.M., K.S., and C.M. have full access to all the data and take responsibility for the integrity and accuracy of data analysis. The data for this systematic review and meta-analysis are publicly available in the supplement online content based on the study design.
Study protocol and data set are available on request from the corresponding author, Chatphatai Moonla (chatphatai.moonla@gmail.com).
The full-text version of this article contains a data supplement.