Key Points
A pediatric-inspired regimen for ALL based on regionally available drugs is feasible in Central America.
The modified CALGB10403 is associated with a 2-year OS of 72.1%.
Abstract
Mexico and Central America have a high incidence of acute lymphoblastic leukemia (ALL) in adolescents and young adults. Historically, this patient group has been treated using adult-based regimens, which entails a high rate of treatment-related mortality and a poor overall survival (OS). The use of the CALGB 10403, a pediatric-inspired regimen, has been proven effective in this patient subgroup. Nonetheless, low- and middle-income countries (LMICs) may present limited access to standard care treatments implemented elsewhere, warranting the need for further research to improve outcomes among vulnerable populations. In this study, we present the outcomes in terms of safety and effectiveness of using a modified CALGB 10403 regimen to reflect drug and resource availability in LMICs. Modifications included the use of Escherichia coli asparaginase,6-mercaptopurine instead of thioguanine and the use of rituximab among patients with CD20+. A total of 95 patients with a median age of 23 (range, 14-49) years treated with this modified scheme were prospectively assessed at 5 centers in Mexico and 1 in Guatemala. Among these, 87.8% achieved a complete response after induction. During follow-up, 28.3% of patients relapsed. Two-year OS rate was 72.1%. Factors associated with worse OS included hyperleukocytosis (hazard ratio [HR], 4.28; 95% confidence interval [CI], 1.81-10.10) and postinduction minimal residual disease (HR, 4.67; 95% CI, 1.75-12.44). Most patients presented hepatotoxicity (51.6% and 53.7% during induction and consolidation, respectively), and the treatment-related mortality was 9.5%. Overall, results highlight that implementing a modified CALGB 10403 regimen in Central America is feasible, and it is associated with improvements in clinical outcomes and a manageable safety profile.
Introduction
High incidence rates for acute lymphoblastic leukemia (ALL) have been reported in Central America. Though the exact incidence in the region is unknown, it has been estimated to reach 5 per 100 000 person years in Mexico alone. This contrasts considerably against data from the United States, where the incidence rate for this malignancy is considerably lower (1.8 per 100 000 person years).1,2
Epidemiological data show that ALL represents 51% of all acute leukemias in adults in Mexico.3 A previous multicenter registry gathered data from 559 adults diagnosed with ALL across 5 reference centers in Mexico City and identified adolescents and young adults (AYAs) as the subgroup with the highest incidence, comprising 67.3% of all diagnosed cases.4 Data retrieved from this registry showcased that most patients were undergoing treatment with traditional adult regimens; the most frequently used was HyperCVAD. In terms of outcomes, the study identified a high treatment-related mortality (17.3%) and poor overall survival (OS) in the general study population (3-year survival rate of 22%) as well as in the AYA subgroup (3-year survival rate of 25.7%).4
Among patients living in the United States, ALL is more frequent among Hispanics, with higher incidence rates in this ethnic subgroup than among non-Hispanic patients (2.2-2.9 vs 1.3-1.7 per 100 000 person years, respectively). Furthermore, although data in this regard have been inconsistent, some reports underscore a worse prognosis for Hispanics with ALL, particularly for patients with Mexican and Central American backgrounds.5 The reason behind these observations has not been fully elucidated, though some reports have identified that the Hispanic/Latino ethnicity is associated with high-risk molecular and genetic subgroups, including variants in GATA3 and Philadelphia-like (Ph-like) ALL.6,7 Other studies have also showcased a higher risk for chemotherapy-related toxicity among Hispanics, particularly when undergoing treatment with methotrexate and L-asparaginase.8-10
Several study groups have identified a considerable benefit from treating AYAs diagnosed with ALL using pediatric schemes, which are based on L-asparaginase and report lower myelotoxicity.11 The CALGB 10403 scheme represents an effort by 3 different cooperative groups in the United States to adapt the Children's Oncology Group therapeutic scheme for treating AYAs. Results from using CALGB 10403 have been promising thus far, achieving 3-year survival rates of 73% in this challenging patient subgroup.12
This study sought to assess the safety and effectiveness of a modified CALGB 10403 regimen in patients diagnosed with ALL in Central America. We hypothesized that a modified version of CALGB, adapted to local resources, would reduce treatment-related mortality and improve long-term clinical outcomes among patients diagnosed with ALL in Central America.
Methods
Study design and participants
This was a prospective, multicentric observational study conducted at 5 centers in Mexico and 1 in Guatemala. Eligible patients were aged from 14 to 49 years, had been diagnosed with Ph– B- or T-cell ALL from January 2017 to December 2020. The decision to extend the cutoff age from 39 years, used in the CALGB 101409, to 49 years was based on the information from other pediatric protocols that have proved effective in selected populations aged ≤55 years (reference). The study was approved by the institutional ethics and research committees, and the participants gave a written informed consent in accordance with local practice.
Treatment
Patients included in this study received a modified CALGB 10403 regimen, which included the following adaptations to reflect regional availability and access to therapeutic agents: pegaspargase was replaced with Escherichia coli L-asparaginase; thioguanine was replaced with 6-mercaptopurine, and rituximab was incorporated in patients who had CD20+ (defined as CD20 expression ≥20% via flow cytometry). Patients were given the option of purchasing pegaspargase by their own means, if financially feasible. All patients received intrathecal chemotherapy during the intensive and maintenance stages of treatment, either with triple therapy or methotrexate, in accordance with local practices. The initial scheme included 12 lumbar punctions with intrathecal chemotherapy. Minimal residual disease (MRD) was assessed locally via flow cytometry at the end of the induction stage, after the first consolidation, and before initiating the maintenance stage. Some of the participating institutions routinely measure CRLF2 expression via flow cytometry using the mean fluorescence intensity of fresh bone marrow samples; even though the assessment was not performed in all patients, it was reported with the results, given the relevance of this finding.
In October 2019, an interim analysis showed that of patients who relapsed, 50% presented with central nervous system (CNS) involvement. Therefore, several modifications were incorporated; these included eliminating extended induction, changing prednisone for dexamethasone during induction, and including 22 lumbar punctions instead of 12. A detailed account of the modified therapeutic scheme is presented in Table 1. The protocol suggests the use of antimicrobial prophylaxis in all of the patients during induction and consolidation, as follows: acyclovir 400 mg every 12 hours, trimethoprim-sulphamethoxazole from 160 to 800 mg 3 times per week, and fluconazole 400 mg once per day that should be held during the 48 hours around the vincristine dose. Of note, during episodes of severe neutropenia or hospital admission, the antifungal prophylaxis could be switched to voriconazole or caspofungin based on the drug availability. The use of ciprofloxacin 500 mg 2 times per day or levofloxacin 500 mg per day was recommended only during the episodes of severe neutropenia. All of the patients with febrile neutropenia received treatment based on international guidelines for febrile neutropenia. Concerning allogeneic hematopoietic stem cell transplant (allo-HCT), all patients with t(4:11) should undergo allo-HCT in the first complete remission (CR1). Other high-risk features like MRD persistence are at the discretion of the treating physician/patient. All patients in the second remission (CR2) should be considered for an allotransplant.
Modifed CALGB 10403 regimen
| Induction |
| Vincristine: 1.5mg/m2 (max, 2 mg) on days 1, 8, 15, and 22 |
| Daunorubicin: 25 mg/m2 on days 1, 8, 15, and 22 |
| Dexamethasone: 10 mg/m2 from days 1 to 7 and from days 15 to 21 |
| E coli asparaginase: 6000 UI/m2 on days 3, 5, 7, 9, 11, and 13 or pegaspargase: 2500 UI/m2 on day 4 (max, 3750 UI) |
| Rituximab: 375 mg/m2 on day 8 |
| Consolidation 1 |
| Rituximab: 375 mg/m2 on days 1 and 29 |
| Cyclophosphamide: 1000 mg/m2 on days 1 and 29 |
| Cytarabine: 75 mg/m2 on days 1-4, 8-11, 29-32, and 36-39 |
| 6-Mercaptopurine: 60 mg/m2 on days 1-14 and 29-42 |
| Vincristine: 1.5 mg/m2 (max, 2mg) on days 8, 15, 43, and 50 |
| E coli asparaginase: 6000 UI/m2 on days 15, 17, 19, 21, 23, 25, 43, 45, 47, 49, 51 and 53 or pegaspargase: 2500 UI/m2 on days 15 and 43 (max, 3750 UI) |
| Consolidation 2 |
| Rituximab: 375 mg/m2 on days 1 and 21 |
| Methotrexate: 100 mg/m2 on day 1, 150 mg/m2 on day 11, 200 mg/m2 on day 21, 250 mg/m2 on day 31, and 300 mg/m2 on day 41 |
| E coli asparaginase: 6000 UI/m2 on days 2, 4, 6, 8, 10, 12, 22, 24, 26, 28, 30 and 32 or pegaspargase: 2500 UI/m2 on days 2 and 22 (max, 3750 UI) |
| Delayed intensification |
| Rituximab: 375 mg/m2 on days 1, 29, and 50 |
| Vincristine: 1.5 mg/m2 (max, 2 mg) on days 1, 8, 15, 43, and 50 |
| Doxorubicin: 25 mg/m2 on days 1, 8, and 15 |
| Dexamethasone: 10 mg/m2 from days 1 to 7 and from days 15 to 21 |
| E coli asparaginase: 6000 UI/m2 on days 3, 5, 7, 9, 11, 13, 43, 45, 47, 49, 51 and 53 or pegaspargase: 2500 UI/m2 on days 4 and 43 (max, 3750 UI) |
| Cyclophosphamide: 1000 mg/m2 on day 29 |
| Cytarabine: 75 mg/m2 from days 29 to 32 and from days 36 to 39 |
| 6-mercaptopurine: 60 mg/m2 from days 29 to 42 |
| Maintenance (2 y) |
| Vincristine: 1.5 mg/m2 (max, 2 mg) on day 1, monthly |
| Dexamethasone: 6 mg/m2 from days 1 to 5, monthly |
| 6-mercaptopurine: 75 mg/m2 daily |
| Methotrexate: 20 mg/m2 weekly |
| Induction |
| Vincristine: 1.5mg/m2 (max, 2 mg) on days 1, 8, 15, and 22 |
| Daunorubicin: 25 mg/m2 on days 1, 8, 15, and 22 |
| Dexamethasone: 10 mg/m2 from days 1 to 7 and from days 15 to 21 |
| E coli asparaginase: 6000 UI/m2 on days 3, 5, 7, 9, 11, and 13 or pegaspargase: 2500 UI/m2 on day 4 (max, 3750 UI) |
| Rituximab: 375 mg/m2 on day 8 |
| Consolidation 1 |
| Rituximab: 375 mg/m2 on days 1 and 29 |
| Cyclophosphamide: 1000 mg/m2 on days 1 and 29 |
| Cytarabine: 75 mg/m2 on days 1-4, 8-11, 29-32, and 36-39 |
| 6-Mercaptopurine: 60 mg/m2 on days 1-14 and 29-42 |
| Vincristine: 1.5 mg/m2 (max, 2mg) on days 8, 15, 43, and 50 |
| E coli asparaginase: 6000 UI/m2 on days 15, 17, 19, 21, 23, 25, 43, 45, 47, 49, 51 and 53 or pegaspargase: 2500 UI/m2 on days 15 and 43 (max, 3750 UI) |
| Consolidation 2 |
| Rituximab: 375 mg/m2 on days 1 and 21 |
| Methotrexate: 100 mg/m2 on day 1, 150 mg/m2 on day 11, 200 mg/m2 on day 21, 250 mg/m2 on day 31, and 300 mg/m2 on day 41 |
| E coli asparaginase: 6000 UI/m2 on days 2, 4, 6, 8, 10, 12, 22, 24, 26, 28, 30 and 32 or pegaspargase: 2500 UI/m2 on days 2 and 22 (max, 3750 UI) |
| Delayed intensification |
| Rituximab: 375 mg/m2 on days 1, 29, and 50 |
| Vincristine: 1.5 mg/m2 (max, 2 mg) on days 1, 8, 15, 43, and 50 |
| Doxorubicin: 25 mg/m2 on days 1, 8, and 15 |
| Dexamethasone: 10 mg/m2 from days 1 to 7 and from days 15 to 21 |
| E coli asparaginase: 6000 UI/m2 on days 3, 5, 7, 9, 11, 13, 43, 45, 47, 49, 51 and 53 or pegaspargase: 2500 UI/m2 on days 4 and 43 (max, 3750 UI) |
| Cyclophosphamide: 1000 mg/m2 on day 29 |
| Cytarabine: 75 mg/m2 from days 29 to 32 and from days 36 to 39 |
| 6-mercaptopurine: 60 mg/m2 from days 29 to 42 |
| Maintenance (2 y) |
| Vincristine: 1.5 mg/m2 (max, 2 mg) on day 1, monthly |
| Dexamethasone: 6 mg/m2 from days 1 to 5, monthly |
| 6-mercaptopurine: 75 mg/m2 daily |
| Methotrexate: 20 mg/m2 weekly |
Max, maximum.
Definitions
Patients at high risk were defined as those who presented with hyperleukocytosis (white blood cell count > 30 × 109/μL for B-ALL; or > 100 × 109/μL for T-ALL) and had a high-risk cytogenetic profile, which was defined as presence of 11q23/KMT2A rearrangements, complex karyotype (≥5 alterations), or hypodiploidy (<44 chromosomes).
Hepatic function tests were considered abnormal when bilirubin levels were 1.5 times higher than the upper normal limit or when transaminase levels were 2.0 times higher than the upper normal limit. All events related to toxicity were recorded and graded according to the Common Terminology Criteria for Adverse Events version 5.0.13
End points
CR was defined as <5% blasts in a bone marrow smear and no evidence of extramedullary disease with hematological recovery, defined as >1000 neutrophils × 109/L and >100 platelets × 109/L. Induction-related mortality was defined as any death occurring after day 1 of induction therapy and before the next cycle. We defined refractoriness when CR was not achieved after 2 cycles of induction chemotherapy. Relapse was defined as the presence of >5% blasts in the bone marrow or extramedullary disease at any point, after achieving CR. OS refers to the time, in months, from diagnosis until patient death or last follow-up. Relapse-free survival (RFS) refers to the time, in months, from CR to relapse.
Statistical analysis
Baseline characteristics were summarized as medians, with interquartile ranges and percentages for descriptive purposes. Differences between categorical variables were assessed using the χ2 test or Fisher exact test. Differences between medians were analyzed using the Mann-Whitney U test. Risk factors were assessed by calculating the odds ratio (OR) along with 95% confidence intervals (CIs). Time-to-event variables were estimated using the Kaplan-Meier method, and comparisons among the prognostic subgroups were analyzed using the log-rank test. Statistical significance was determined as P < .05. All analyses were performed using the SPSS software version 22 (SPSS Inc, Chicago, IL).
Results
Study population
From January 2017 to December 2020, a total of 95 patients were included in the study, with 73 from Mexico and 22 from Guatemala. The median age for the entire study population was 23 (range, 14-49) years. Baseline characteristics for patients included are summarized in Table 2. Among the included patients, 45.3% were considered to be at high risk because of hyperleukocytosis or high-risk karyotype (10.5% of patients had a nonevaluable karyotype). Among patients with karyotype alterations, the most frequent included were hypoploidy (7.4%), followed by KMT2A rearrangements (4.2%). Baseline hepatic alterations were present in 31.6% of patients.
Baseline characteristics of the study population
| Characteristic . | Median (range) or N (%) . |
|---|---|
| Age, y | 23 y (14-49) |
| >29 | 22 (23.2%) |
| 14-24 | 55 (57.8%) |
| 25-39 | 35 (36.8%) |
| >39 | 5 (5.2%) |
| Female/male | 39 (41.1%)/56 (58.9%) |
| Overweight/obesity∗ | 33 (34.7%)/11 (11.6%) |
| Leukemia phenotype | B cell: 88 (92.6%)/T cell: 7 (7.4%) |
| CD20+ | 36 of 68 (52%) |
| White blood cell count (× 109/μL) | 20.0 (0.29-427.7) |
| Hyperleukocytosis | 38 (40.9%) |
| CNS disease at diagnosis | 2 (2.1%) |
| Karyotype | |
| Normal | 81 (72.6%) |
| High risk | 14 (14.8%) |
| Characteristic . | Median (range) or N (%) . |
|---|---|
| Age, y | 23 y (14-49) |
| >29 | 22 (23.2%) |
| 14-24 | 55 (57.8%) |
| 25-39 | 35 (36.8%) |
| >39 | 5 (5.2%) |
| Female/male | 39 (41.1%)/56 (58.9%) |
| Overweight/obesity∗ | 33 (34.7%)/11 (11.6%) |
| Leukemia phenotype | B cell: 88 (92.6%)/T cell: 7 (7.4%) |
| CD20+ | 36 of 68 (52%) |
| White blood cell count (× 109/μL) | 20.0 (0.29-427.7) |
| Hyperleukocytosis | 38 (40.9%) |
| CNS disease at diagnosis | 2 (2.1%) |
| Karyotype | |
| Normal | 81 (72.6%) |
| High risk | 14 (14.8%) |
Overweight is a body mass index ≥25 kg/m2; obesity is a body mass index ≥30 kg/m2.
Regarding treatment, most patients received E coli asparaginase (95.8%), and only 4 received pegaspargase. In terms of induction steroid, 2 out of 3 patients included in the study received prednisone.
Safety
The most frequently observed toxicities during induction included hypofibrinogenemia, hepatotoxicity, and hyperglycemia. Table 3 summarizes the most relevant toxicities observed during induction and consolidation graded based on the severity. Most patients who presented hepatotoxicity had hypofibrinogenemia (81.6%), and this proportion was even higher if only patients with grade 3 or 4 hepatotoxicity (95.2%) were considered. Among patients with obesity, 63.6% had hepatotoxicity during induction, and this was grade 3 or 4 in 36.4%. Dose adjustments because of toxicity were required in 23.9% of patients during induction and in 36.8% during consolidation. The dose reductions were mainly because of hepatotoxicity and based on the adjustment of the recommended dosage, with toxicity for each drug. Fifty-nine patients (62%) completed the full protocol as planned. Meanwhile, 36 patients discontinued the protocol prematurely, with only 4 because of toxicity.
Most relevant adverse events during induction and consolidations
| Adverse event . | Induction . | Consolidations . | ||
|---|---|---|---|---|
| All grades, % . | Grade 3 or 4, % . | All grades, % . | Grade 3 or 4, % . | |
| Low fibrinogen | 61.1 | 44.2 | 43.9 | 15.8 |
| Liver toxicity (all) | 51.6 | 22.1 | 53.7 | 20.0 |
| Transaminase elevation | 37.8 | 14.7 | 20 | 4.2 |
| Bilirubin elevation | 36.8 | 21.1 | 44.2 | 18.9 |
| Hyperglycemia | 43.7 | 14.7 | 25 | 5.3 |
| Hypertrigliceridemia | 34.7 | 5.3 | 54.3 | 14.7 |
| Neuropathy | 13.7 | 6.3 | 24.4 | 1.1 |
| Thrombosis | 10.5 | 2.1 | 6.1 | 2.1 |
| Hypersensitivity | 2.1 | 1.1 | 3.7 | 3.7 |
| Pancreatitis | 2.1 | 2.1 | 2.4 | 1.1 |
| Adverse event . | Induction . | Consolidations . | ||
|---|---|---|---|---|
| All grades, % . | Grade 3 or 4, % . | All grades, % . | Grade 3 or 4, % . | |
| Low fibrinogen | 61.1 | 44.2 | 43.9 | 15.8 |
| Liver toxicity (all) | 51.6 | 22.1 | 53.7 | 20.0 |
| Transaminase elevation | 37.8 | 14.7 | 20 | 4.2 |
| Bilirubin elevation | 36.8 | 21.1 | 44.2 | 18.9 |
| Hyperglycemia | 43.7 | 14.7 | 25 | 5.3 |
| Hypertrigliceridemia | 34.7 | 5.3 | 54.3 | 14.7 |
| Neuropathy | 13.7 | 6.3 | 24.4 | 1.1 |
| Thrombosis | 10.5 | 2.1 | 6.1 | 2.1 |
| Hypersensitivity | 2.1 | 1.1 | 3.7 | 3.7 |
| Pancreatitis | 2.1 | 2.1 | 2.4 | 1.1 |
Only 38.1% of the patients who developed grade 3 or 4 hepatotoxicity during induction presented this adverse event during consolidation. However, 57.9% of patients who developed grade 3 or 4 hepatotoxicity during consolidation had not presented this adverse event during induction.
The rate of febrile neutropenia during induction was 55.8%, compared with 32.9% during consolidation. During induction, 31.5% of febrile neutropenia events had life-threatening consequences (grade 4 or 5), similar to consolidation, in which 31.2% of events were grade 4 or 5. Most patients who developed febrile neutropenia during consolidation had a single episode (63%), whereas 37% had 2 or 3 episodes.
Mortality during induction was 7.4%. Furthermore, 2 patients died during consolidation because of treatment-related complications. Overall, treatment-related mortality in this study was 9.5% in all cases associated with infections. Among the 9 patients who died because of treatment complications, unspecified sepsis was the leading cause of death; 1 died because of COVID-19 infection, and no fungal infections were identified. Two patients developed pancreatitis during the induction, and those patients underwent an intricate course with multiple infections and died because of complications. Of note, among the 9 patients, only 2 (22.2%) underwent treatment at the intensive care unit (ICU). In addition, during the consolidation, 2 patients died in the extrahospital setting in CR because of presumed infections.
When analyzing risk factors associated with death during induction, a significantly higher risk was identified for patients who presented hyperleukocytosis (13.5% vs 1.8%; OR, 10.50; 95% CI, 1.21-91.15; P = .015) and pancreatitis (100% vs 4.3%; P = .003).
Effectiveness
After induction, a CR was observed in 83.2% of study participants. A total of 72.6% of cases were assessed for MRD. After induction, 75.4% of patients had MRD < 0.1% and 36.2% had MRD < 0.01%. Receiving a complete induction without dose adjustments was significantly associated with achieving MRD < 0.1% (OR, 3.38; 95% CI, 1.08-10.56; P = .04). After consolidation, MRD was assessed in 45.2% of cases; of those, 37.2% had a negative MRD result.
The relapse rate during follow-up was 24.0%; sites of relapse included bone marrow (62.5%), CNS (25.0%), and both bone marrow and CNS (12.5%). Factors associated with CNS relapse included hyperleukocytosis (OR, 6.2; 95% CI, 1.21-31.76; P = .027) and development of hyperglycemia during induction (OR, 5.31; 95% CI, 1.03-27.24; P = .038). Only 8.4% of patients received consolidation with allo-HCT.
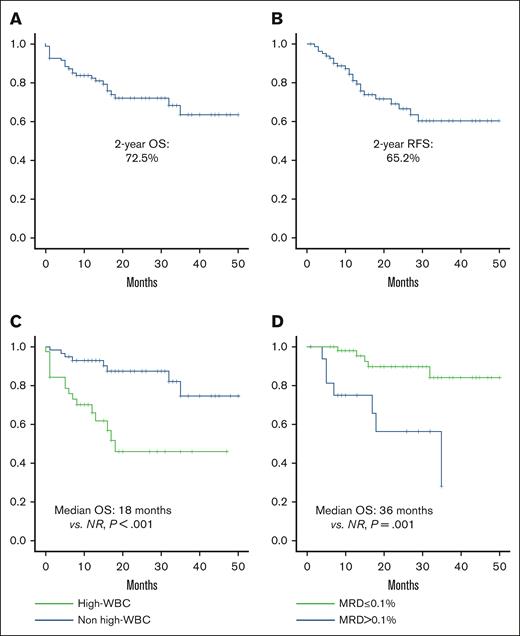
Median follow-up for the entire study population was 26.5 months (range, 11.7-59.2). Two-year survival rate was 72.1%, and 2 year-RFS was 68.4% (Figure 1A-B). Factors associated with poor OS included hyperleukocytosis (18 months vs nonreached [NR]; P = .001; hazard ratio [HR], 4.28; 95% CI, 1.81-10.10; P = .001) and postinduction MRD >0.1% (35 months vs NR [P = .001]; HR, 5.75; 95% CI, 1.82-18.18; P = .003; Figure 1C-D).
Overall and relapse-free survival. (A) OS; (B) RFS; (C) OS in patients with or without leukocytosis; (D) OS based on postinduction MRD.
Overall and relapse-free survival. (A) OS; (B) RFS; (C) OS in patients with or without leukocytosis; (D) OS based on postinduction MRD.
Factors associated with poor RFS included overweight/obesity (24 months vs NR [P = .044]; HR, 2.36; 95% CI, 0.99-5.59; P = .45), hyperleukocytosis (18 months vs NR [P < .001]; HR, 4.04; 95% CI, 1.73-9.44; P = .001), and postinduction MRD >0.1% (18 months vs NR [P = .001]; HR, 4.67; 95% CI, 1.75-12.44; P = .003). After relapse, the median OS was 8.35 months; no differences were observed in terms of OS according to the type of relapse.
The study population included 5 patients aged >39 years. Among these patients, all achieved a CR. MRD was assessed in 4 patients, of whom 1 had MRD < 0.01%, 2 had MRD from 0.01% to 0.1%, and 1 had MRD > 0.1%. Concerning the treatment-related toxicity, 2 patients presented hepatotoxicity during induction and another 1 during consolidation; the 3 patients underwent a dose adjustment. Neither patient had pancreatitis, thrombosis, or death due to complications. One patient was switched to a different protocol because of a persistent positive MRD result. The median follow-up for this patient subgroup was 16.4 months (range, 13.42-40.56). At the time of data cutoff, all 5 patients were alive and only 1 had a relapse.
Discussion
In this study, a pediatric-inspired regimen was adapted to reflect local drug access and availability in Central America, and results show that this modified regimen was effective for treatment of ALL in this population. Nonetheless, results are promising compared with those of a historical cohort of Mexican patients with ALL, which included 376 AYA patients, of whom 49% underwent HyperCVAD (Table 4); the use of the modified CALGB 10403 regimen in this study decreases treatment-related mortality (26% vs 9.5%) and relapse rate (61.7% vs 24%) and improves median OS (14.1 months vs NR). Previous data in this population show a 3-year survival rate of 25.7%, whereas data from our study show a 2-year survival rate of 72.1.4,12 Furthermore, the poor survival in the historical cohort contrasts with the international reports, which show a 5-year OS of 60% in the AYA population either with HyperCVAD or Augmented Berlin-Frankfurt-Munster regimen.14 In contrast, in the original report that presented the results of the CALGB 10403 regimen, only 15% of the study population were Hispanic (N = 45), and survival outcomes of this subgroup were similar to that of non-Hispanic patients.
Comparison of the current of the modified CALGB10403 with the original publication and a historical cohort of the Mexican AYA population
| . | Study publication . | CALGB 10403 original publication . | Historical cohort Mexican AYA population . |
|---|---|---|---|
| No. of patients | 95 | 295 | 376 |
| Age, median (range) | 23 y (14-49) | 24 y (17-39) | 22 y (14-39) |
| High risk, n (%) | High risk (45) Hyperleukocytosis (41) Unfavorable cytogenetics (15) Aberrant CRLF2 expression n = 14 (50) | Unfavorable cytogenetics 18 (7) Aberrant CRLF2 expression n = 131 (21) WBC < 30 × 109/L > 30 (26) | 182 (48) |
| Induction death rate (%) | 7 (7) | 9 (3) | 26 (7) |
| CR after induction (%) | 79 (83) | 263 (89) | 301 (80) |
| OS (events = deaths) | No events, 25 of 95 2 y OS, 72.1% (95% CI, 61.6-82.4) | No events, 105 of 295 3 y OS, 73% (95% CI, 68-78) | No events, 410 of 559 3 y OS, 26% Median OS, 14.1 mo (11.7-16.4) |
| Treatment-related deaths | No events, 9 of 95 (9) | No events, 8 of 295 (3) | No events, 98 of 376 (26) |
| Allo HSCT | 8 of 95 (8) in CR1 or CR2 | 20 of 263 (8) in CR1 | 25 of 376 (7) in CR1 or CR2 |
| . | Study publication . | CALGB 10403 original publication . | Historical cohort Mexican AYA population . |
|---|---|---|---|
| No. of patients | 95 | 295 | 376 |
| Age, median (range) | 23 y (14-49) | 24 y (17-39) | 22 y (14-39) |
| High risk, n (%) | High risk (45) Hyperleukocytosis (41) Unfavorable cytogenetics (15) Aberrant CRLF2 expression n = 14 (50) | Unfavorable cytogenetics 18 (7) Aberrant CRLF2 expression n = 131 (21) WBC < 30 × 109/L > 30 (26) | 182 (48) |
| Induction death rate (%) | 7 (7) | 9 (3) | 26 (7) |
| CR after induction (%) | 79 (83) | 263 (89) | 301 (80) |
| OS (events = deaths) | No events, 25 of 95 2 y OS, 72.1% (95% CI, 61.6-82.4) | No events, 105 of 295 3 y OS, 73% (95% CI, 68-78) | No events, 410 of 559 3 y OS, 26% Median OS, 14.1 mo (11.7-16.4) |
| Treatment-related deaths | No events, 9 of 95 (9) | No events, 8 of 295 (3) | No events, 98 of 376 (26) |
| Allo HSCT | 8 of 95 (8) in CR1 or CR2 | 20 of 263 (8) in CR1 | 25 of 376 (7) in CR1 or CR2 |
HSCT, hematopoietic stem cell transplant; WBC, white blood cell.
Although we included patients aged >49 years, the median age was similar to that in the original CALGB 10403 report (23 vs 24 years). Of note, high-risk features such as hyperleukocytosis (40.9% vs 25.7%) and unfavorable cytogenetics (14.8% vs 7%) were more frequent in our population. Even though patients in this study were not routinely assessed for a Ph-like genetic profile, in 14 patients whose CRLF2 expression was assessed via flow cytometry, 50% presented an aberrant expression. In the original CALGB 10403 study, 21.4% presented CRLF2 aberrant expression and had a considerable impact in terms of prognosis (HR, 2.65; 95% CI, 1.51-4.66). Data from MD Anderson CC have shown that among patients with Ph-like leukemias, 68% were Hispanic.6 Another report in Mexico showed that 41.3% of adults diagnosed with ALL have CRLF2 overexpression, via flow cytometry, and this was associated with worse outcomes.15 These results support the hypothesis that the population in Mexico and Central America has a more aggressive biology of the disease and could explain the increased risk of relapse in this population.
Regarding safety, the use of pediatric-inspired regimens has been associated with higher rates of hepatic and metabolic toxicity.14,16 Furthermore, it has been previously reported that Hispanic background (OR, 3.03) and obesity (OR, 2.87) are risk factors associated with asparaginase-related hepatotoxicity.10 Interestingly, although hepatotoxicity was a frequent adverse event in our study, we did not identify a higher rate than that in other similar studies. This observation could stem from the fact that obesity rate (body mass index > 30 kg/m2) was lower in our study population than that in the original CALGB 10403 study (11.6% vs 31.9%).12 Approximately, one-third of patients aged >15 years in Mexico have obesity, so the patients included in this study are not representative of the rate of obesity described in our population. It is likely that this reflects the decision by individual attending physicians to avoid treatment with L-asparaginase in patients with obesity. Interestingly, a report by the PETHEMA Group from the Spanish Society of Hematology identified a lower rate of hepatotoxicity when using native E coli asparaginase than that when using pegaspargase, while maintaining similar efficacy.17E. coli L-asparaginase is currently unavailable in several countries; nonetheless, it represents an affordable and accessible option for patients in low- and middle-income countries (LMICs). In contrast, the use of E coli asparaginase has been associated with development of hypersensitivity reactions in up to 60% of cases, compared with 24% when using pegaspargase.18 Data from our study show a low rate of hypersensitivity reactions (<10%), though it may be the result of underreporting for very mild or localized reactions. Furthermore, an important limitation stems from the fact that we did not screen for the development of antibodies that can lead to asparaginase silent inactivation, and this could potentially impact clinical outcomes.
Among patients included in this study, 14.7% presented grade 3 to 4 hypertriglyceridemia during consolidation therapy. Nonetheless, this could also be understated because many patients did not receive an active routine follow-up for triglyceride levels. Other studies have reported grade 3 or 4 hypertriglyceridemia rates ranging from 11% to 43.8%12,19; however, the clinical effect of asymptomatic hypertriglyceridemia is yet to be established.
Even though pediatric-inspired regimens have been associated with a lower rate of myelotoxicity, it is important to underscore that results from our study show a higher rate of treatment-related mortality than that reported in the CALGB 10403 study (9.5% vs 3%). Additionally, a significant difference in the rate of febrile neutropenia during induction was noticed (55.8% vs 22%-23.9%). Therefore, this situation could explain the increased risk of induction mortality (7.3% vs 3%) in our population. Contrastingly, induction-related death of <1% has been reported for HyperCVAD and ABFM in a report of a highly specialized referral center (Rytting). Among Latin American countries, the risk of death during induction therapy in patients with ALL can reach from 12% to 26% in LMICs. Infections are the main cause for morbidity and mortality in 87.4% of cases. This could be explained by more economically vulnerable populations and a lack of support treatment.20-22
Nevertheless, compared with the historical cohort that reported treatment-related mortality of 26%, the use of a pediatric-inspired regimen in this study resulted in a significant decrease in treatment-related mortality. Consequently, this treatment strategy will positively affect outcomes in our population. Of note, the improvement in this study compared with that in the historical cohort could also reflect the experience gathered over the years that has led us toward an improvement in care. Nonetheless, it is important to highlight that only 22.2% of patients who died from complications had access to an ICU, and 22.2% died in extrahospital settings. Therefore, supportive care remains an unmet need in the continuum of care of patients diagnosed with acute leukemias in LMICs.
The prognostic role of MRD assessment was reproduced in this study, although with several limitations. Assessment of MRD currently represents the most relevant prognostic factor for patients with ALL as well as a cornerstone tool for guiding therapy decision making, including transplants.23 One limitation regarding this data from our study stems from the fact that MRD was measured locally and without standardization in some of the included study sites. The second most important limitation is the lack of postconsolidation MRD determination in less than half of the included patients. Postinduction MRD assessments are relevant in terms of prognosis; however, postconsolidation MRD will guide important therapeutic decisions, including targeting therapy to eradicate MRD and/or provide consolidation with allo-HCT.24
The relapse rate in our study vs that in the original CALGB protocol was lower (24% vs 34.2%); however, the follow-up was shorter, and we reported the disease-free survival in 2 years instead of 3 years. Nonetheless, it has improved significantly compared with the historical cohort, in which the median disease-free survival was 16.9 months, and 62% of patients who participated had disease relapse. Given the high-risk features at diagnosis and the increased rate of positive MRD status compared with that in other populations, a longer follow-up is needed to confirm these findings. Although our data show a low transplant rate, this is similar to findings from the original CALGB 10403 study, in which only 20 patients among a sample of 318 received consolidation with allo-HCT during CR1. In this regard, a recent study demonstrated improved OS for patients treated with CALGB 10403 compared with those who received allo-HCT.25 Other study groups, including PETHEMA, have shown that even among patients who were at high risk with undetectable MRD, the use of chemotherapy improves survival compared with allo-HCT (5-year survival rate 72% vs 54%, respectively).26
Lastly, our future direction should be focused on improving supportive care, including ICU access, and identifying the patients with a positive MRD status with an increased risk of disease relapse. As such, the importance of standardizing and implementing routine MRD assessments at the ideal clinical time remains an unmet need in many regions. Contrastingly, the management of patients who persist with positive MRD status is a challenge in the region. Different strategies have been developed to reduce the relapse rate. Currently, blinatumomab is the most promising, given that it decreases the risk of relapse independently from the MRD status (reference). However, the cost limits the widespread use of the drug in LMICs. Therefore, implementing clinical trials based on the cost-effectiveness of bispecific antibiotics or academic cell therapy (chimeric antigen receptor-T) could be the future direction of this population.
In conclusion, a CALGB 10403 regimen modified to reflect local resources substantially improves OS compared with historic data among AYAs diagnosed with ALL in Mexico and Guatemala. Implementing the use of a pediatric-inspired regimen is feasible in LMICs, with a manageable safety profile and low treatment-related mortality.
Acknowledgment
The authors thank the Acute Leukemia Working Group (Grupo de Trabajo de Leucemias Agudas) from the Mexican Society of Hematology for the support for the publications.
Authorship
Contribution: J.R.-P., W.S., and R.D.-G. conceived and designed the study; J.R.-P. and R.D.-G. analyzed and interpreted the data and performed the statistical analysis; J.R.-P., Y.L.L.-T., V.I.U.-C., M.E.M.L.-P., K.A.E.-B., L.F.A., Á.C.-G., C.B.-D., S.I.I.-A., Y.N.-Y., J.M.S.-A., E.I.A., L.M.-G., and R.D.-G. provided the study material and/or patients; J.R.-P., V.I.U.-C., L.F.A., and W.S. drafted the manuscript; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: V.I.U.-C. received honoraria (speaker) from Amgen. Á.C.-G. received honoraria (speaker/advisory board) from Takeda, Vifor, Astellas, Amgen, and AbbVie. S.I.I.-A. received honoraria (speaker/advisory board) from Janssen, AbbVie, Sanofi, and Boehringer-Ingelheim. Y.N.-Y. received honoraria (speaker/advisory board) from Janssen and AbbVie. E.I.A. received honoraria (speaker/advisory board) from Asofarma. L.M.-G. received honoraria (speaker/advisory borad) from Astellas, Pfizer, Novartis, Aspen, AstraZeneca, Amgen, Sanofi, and Takeda. W.S. has consulted for Amgen, GlaxoSmithKline, Jazz, Kite, Newave, Pfizer, and Servier. R.D.-G. received honoraria (speaker/advisory board) from AbbVie, Amgen, Astellas, and Servier. The remaining authors declare no conflicts of interests.
Correspondence: Roberta Demichelis-Gómez, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Vasco de Quiroga 15, Belisario Domínguez, Tlalpan, 14080 Mexico City, CP, Mexico; e-mail: roberta.demichelisg@incmnsz.mx.
References
Author notes
Original data about the complete regimen or clinical data are available on request from the corresponding author, Roberta Demichelis-Gómez (roberta.demichelisg@incmnsz.mx). Individual participant data will not be shared.