Abstract
The overweight/obesity epidemic is a serious public health concern that affects >40% of adults globally and increases the risk of numerous chronic diseases, such as type 2 diabetes, heart disease, and various cancers. Multiple myeloma (MM) is a lymphohematopoietic cancer caused by the uncontrolled clonal expansion of plasma cells. Recent studies have shown that obesity is a risk factor not only for MM but also monoclonal gammopathy of undetermined significance (MGUS), a precursor disease state of MM. Furthermore, obesity may promote the transition from MGUS to MM. Thus, in this review, we summarize the epidemiological evidence regarding the role of obesity in MM and MGUS, discuss the biologic mechanisms that drive these disease processes, and detail the obesity-targeted pharmacologic and lifestyle interventions that may reduce the risk of progression from MGUS to MM.
Introduction
Multiple myeloma (MM) accounts for 2% of all cancer cases and 15% of all lymphohematopoietic cancers in the United States.1 It is characterized by elevated serum monoclonal protein and associated CRAB features (hypercalcemia, renal insufficiency, anemia, and bony lesions). Incidence increases with age, is highest in those aged >40 years, and appears to be higher in men and African Americans.1 Other risk factors postulated to be involved in the development of MM include exposure to benzene2 and pesticides,3 obesity,4-9 and genetic factors.10-12
In many cases, monoclonal gammopathy of undetermined significance (MGUS) precedes MM and, compared with MM, is characterized by a lower serum level of monoclonal protein (<3 g/dL), bone marrow (BM) clonal plasma cells <10%, lack of CRAB features, and absence of other B-cell proliferative disorders.13 MGUS occurs in 3% of people aged ≥50 years and in 5% of those aged ≥70 years.14 It is characterized by 2 major subtypes: (1) immunoglobulin M (IgM) MGUS, which arises from CD30+ lymphoplasmacytic cells (15%), and (2) non-IgM MGUS, which arises from mature plasma B cells (85%). Non-IgM MGUS poses a higher risk of transformation to MM,15 and the overall rate of progression from MGUS to MM is ∼1% per year, regardless of the duration of follow-up.16 The prevalence of MGUS is higher in men and African Americans, which may be partially attributable to the higher rates of MM observed in this population.17
Obesity is an evergrowing global problem that affects >40% of adults and significantly increases the risk of chronic diseases such as type 2 diabetes, heart disease, and cancer.4,18-22 Obesity has been increasingly found to be associated with the development of both MGUS and MM, and recent evidence suggests that it may further promote the transition from MGUS to MM.21,23,24 Consequently, the purpose of this review is threefold: (1) review the literature and describe the current evidence regarding the role of obesity in promoting the transition from MGUS to MM; (2) discuss the biologic mechanisms that may be implicated in driving this association; and (3) detail how this association could be leveraged clinically, with consideration of both pharmacologic and lifestyle interventions to reduce the risk of progression from MGUS to MM.
Epidemiologic studies
Obesity as a risk factor for the development of MGUS
Obesity is postulated to be a risk factor for the development of MGUS; however, data from observational studies have been conflicting. In vivo studies of diet-induced obesity have been associated with the development of MGUS in wild-type mice, with increases in insulin-like growth factor (IGF) level, which is implicated in both obesity and myeloma.25 In epidemiologic studies, the impact of obesity on the development of MGUS has been heterogeneous (summarized in Table 1), with some studies showing an increased risk of MGUS in obese patients and others showing no significance.
Summary of epidemiological studies regarding the association between MGUS and obesity
| Study and/or authors . | n . | Study design . | Results . |
|---|---|---|---|
| Southern Community Cohort Study20 | 60 patients with MGUS and 1936 controls | Case control study | Higher risk of MGUS in obese subjects (OR, 1.8; 95% CI, 1.03-3.14) |
| National Health and Nutritional Examination Survey26 | 365 patients with MGUS and 12 117 controls | Cohort study | MGUS prevalence appeared to increase with increasing BMI in both men and women but trend did not reach statistical significance |
| Age, Gene/Environment Susceptibility- Reykjavik (AGES)22 | 300 patients with MGUS and 5425 controls | Case control study | No association between MGUS and 11 different markers of obesity |
| Schimdt et al27 | 260 patients with MGUS and 4527 controls | Case control study | No association between MGUS and BMI |
| Boursi et al28 | 2363 patients with MGUS and 9193 controls | Case control study | Statistically significant association between obesity and MGUS (OR, 1.15; 95% CI, 1.02-1.29); however, this dissipated after adjustment (OR, 1.08; 95% CI, 0.96-1.22) |
| Study and/or authors . | n . | Study design . | Results . |
|---|---|---|---|
| Southern Community Cohort Study20 | 60 patients with MGUS and 1936 controls | Case control study | Higher risk of MGUS in obese subjects (OR, 1.8; 95% CI, 1.03-3.14) |
| National Health and Nutritional Examination Survey26 | 365 patients with MGUS and 12 117 controls | Cohort study | MGUS prevalence appeared to increase with increasing BMI in both men and women but trend did not reach statistical significance |
| Age, Gene/Environment Susceptibility- Reykjavik (AGES)22 | 300 patients with MGUS and 5425 controls | Case control study | No association between MGUS and 11 different markers of obesity |
| Schimdt et al27 | 260 patients with MGUS and 4527 controls | Case control study | No association between MGUS and BMI |
| Boursi et al28 | 2363 patients with MGUS and 9193 controls | Case control study | Statistically significant association between obesity and MGUS (OR, 1.15; 95% CI, 1.02-1.29); however, this dissipated after adjustment (OR, 1.08; 95% CI, 0.96-1.22) |
OR, odds ratio; CI, confidence interval.
A major drawback of these observational studies are confounding data, leading to ambiguous interpretations. Diet quality appears to be driven by socioeconomic status (SES), such that healthy foods, such as whole grains, lean meats, fish, and fresh vegetables and fruits, are more likely to be consumed by those with higher SESs.29 Furthermore, adiposity is closely linked to the consumption of energy-dense foods, such as refined grains and added fats, which may be associated with lower SESs.29-31 In some of these epidemiologic studies, body mass index (BMI) effects dissipated after adjusting for SES factors.27 It is possible that there are causal relationships among SES, diet quality, and adiposity that still need to be reappraised.
Obesity as a risk factor for the progression of MGUS
There has been increasing evidence that obesity is also associated with MM and may promote the transition from MGUS to MM.32 Results from epidemiological studies suggest that there likely is a positive association between various measurements of obesity and disease progression (summarized in Table 2).
Summary of epidemiological studies regarding the association between MM and obesity and between the progression from MGUS to MM and obesity
| Study and/or authors . | n . | Study design . | Results . |
|---|---|---|---|
| Larsson et al33 | 11 cohort studies with 13 120 patients with MM and 4 case-control studies with 1166 patients with MM and 8247 controls | Meta-analysis | Risk of MM was higher among those who were overweight (cohort studies: RR, 1.12; 95% CI, 1.07-1.18; case-control studies: OR, 1.43; 95% CI, 1.23-1.68) and obese (cohort studies: RR, 1.27; 95% CI, 1.15-1.41; case-control studies: OR, 1.82; 95% CI, 1.47-2.26) |
| Wallin et al34 | 15 cohort studies with 10 827 patients with MM and 6 566 684 controls | Meta-analysis | Risk of MM was elevated among those who were overweight (RR, 1.12; 95% CI, 1.07-1.18) and obese (RR, 1.21; 95% CI, 1.08-1.35) |
| Chang et al21 | 7878 patients with MGUS, with 329 subjects whose condition progressed to MM | Cohort study | Those who were overweight (HR, 1.55; 95% CI, 1.16-2.06) and obese (HR, 1.98; 95% CI, 1.47-2.68) exhibited a higher risk of progression, in a dose response manner, than those who were not, via multivariable analysis |
| Kleinstern et al35 | 594 patients with MGUS, with 57 subjects whose condition progressed to MM, AL amyloidosis, lymphoma, or Waldenstrom macroglobulinemia | Cohort study | BMI ≥25 was associated with an increased risk of disease progression (HR, 2.14; 95% CI, 1.05-4.36) |
| AEGES, Thordardottir et al22 | 300 patients with MGUS, with 29 subjects whose condition progressed to MM or other lymphoproliferative disorders | Cohort study | Obese subjects had a higher risk of progression to MM or other lymphoproliferative diseases (HR, 2.66, 95%; CI, 1.17-6.05) but not when progression to MM was evaluated alone, suggesting an underpowered analysis |
| Thompson et al36 | 200 patients with MGUS, with 100 subjects whose condition progressed to MM or related malignancies | Case control study | No impact of obesity on MGUS progression |
| Veld et al37 | 40 patients with MGUS and 32 patients with MM | Case control study | FDG/PET quantifying abdominal adiposity showed that patients with MM had higher total amounts of AT and higher metabolic activity within their AT compared to patients with MGUS (P < .02). Visceral adipose metabolic activity had a high sensitivity (90.6%) and specificity (92.5%) for progression |
| Study and/or authors . | n . | Study design . | Results . |
|---|---|---|---|
| Larsson et al33 | 11 cohort studies with 13 120 patients with MM and 4 case-control studies with 1166 patients with MM and 8247 controls | Meta-analysis | Risk of MM was higher among those who were overweight (cohort studies: RR, 1.12; 95% CI, 1.07-1.18; case-control studies: OR, 1.43; 95% CI, 1.23-1.68) and obese (cohort studies: RR, 1.27; 95% CI, 1.15-1.41; case-control studies: OR, 1.82; 95% CI, 1.47-2.26) |
| Wallin et al34 | 15 cohort studies with 10 827 patients with MM and 6 566 684 controls | Meta-analysis | Risk of MM was elevated among those who were overweight (RR, 1.12; 95% CI, 1.07-1.18) and obese (RR, 1.21; 95% CI, 1.08-1.35) |
| Chang et al21 | 7878 patients with MGUS, with 329 subjects whose condition progressed to MM | Cohort study | Those who were overweight (HR, 1.55; 95% CI, 1.16-2.06) and obese (HR, 1.98; 95% CI, 1.47-2.68) exhibited a higher risk of progression, in a dose response manner, than those who were not, via multivariable analysis |
| Kleinstern et al35 | 594 patients with MGUS, with 57 subjects whose condition progressed to MM, AL amyloidosis, lymphoma, or Waldenstrom macroglobulinemia | Cohort study | BMI ≥25 was associated with an increased risk of disease progression (HR, 2.14; 95% CI, 1.05-4.36) |
| AEGES, Thordardottir et al22 | 300 patients with MGUS, with 29 subjects whose condition progressed to MM or other lymphoproliferative disorders | Cohort study | Obese subjects had a higher risk of progression to MM or other lymphoproliferative diseases (HR, 2.66, 95%; CI, 1.17-6.05) but not when progression to MM was evaluated alone, suggesting an underpowered analysis |
| Thompson et al36 | 200 patients with MGUS, with 100 subjects whose condition progressed to MM or related malignancies | Case control study | No impact of obesity on MGUS progression |
| Veld et al37 | 40 patients with MGUS and 32 patients with MM | Case control study | FDG/PET quantifying abdominal adiposity showed that patients with MM had higher total amounts of AT and higher metabolic activity within their AT compared to patients with MGUS (P < .02). Visceral adipose metabolic activity had a high sensitivity (90.6%) and specificity (92.5%) for progression |
FDG/PET, fluorodeoxyglucose/positron emission tomography; RR, relative risk.
Because MM and MGUS are more likely to be implicated in older adults and prior literature has demonstrated BMI to be a suboptimal measure of adiposity in this group,38 a case can be made that adiposity, as measured based on the waist circumference and abdominal fat, may be a better marker for study than BMI alone.39 Furthermore, given that African Americans, who have a twofold higher risk of MGUS and MM, have higher muscle mass, resulting in higher BMIs, evaluating other measures of adiposity rather than BMI may be insightful. Besides how the exposure is measured, the results from these observational studies may be biased for other reasons. Firstly, the studies mentioned used both self-reported22,33,34 and collected BMIs,21,22,33-37 which may not be comparable, given the recall bias of the participants. Secondly, MGUS is usually asymptomatic and, thus, diagnosed incidentally. As such, it is difficult to pinpoint the exact time of disease origination, which can complicate studies evaluating the progression to MM.
Obesity-related biologic pathways that affect the MGUS to MM transition
Cytokines and adipokines
Adipose tissue (AT) is a dynamic endocrine organ composed of adipocytes that secrete inflammatory cytokines, such as interleukin-6 (IL-6), growth hormones, such as IGF-1 and 2, and adipokines such as adiponectin, leptin, resistin, and adipsin, which are all key modulators of homeostasis and vital functions.40
In the chronic inflammatory state of obesity, the AT produces many proinflammatory cytokines such as IL-6, IL-1β, and tumor necrosis factor α (TNF-α), which inhibit MM cell apoptosis and promote survival via the expression of proteins such as myeloid leukemia cell differentiation protein 1, B-cell lymphoma extralarge (BCL-xL), and c-Myc. IL-6 has been implicated in the carcinogenesis of various cancer types, and its production is increased in obesity, because ∼15% to 30% of the total IL-6 is produced by AT. IL-6 can also enhance DNA methyltransferase-1 activity, which leads to p53 deactivation in MM cells.41 Additionally, TNF-α provides positive feedback on IL-6 production by inducing the expression of adhesion molecules on MM cells that bind to BM stromal cells to promote IL-6 secretion, which further stimulates the growth of MM cells.42
Obesity and its associated metabolic diseases also lead to hyperinsulinemia and increased levels of IGF-1 and IGF-2. Signaling through the insulin growth factor receptor prevents apoptosis and promotes cell growth and survival.43,44 Increased fat mass also leads to higher levels of estrogen, which increases IGF-1 receptor expression and IGF-1 signaling, which in turn sensitizes cells to estrogen, resulting in a positive-feedback cycle.45
The adipokine adiponectin is thought to be a protective factor, given its anti-inflammatory and insulin-sensitizing properties and its inhibition of osteoclast differentiation and maturation via the mammalian target of rapamycin pathway.46 It is inversely associated with obesity and negatively correlated with visceral fat, BMI, waist circumference, and hip circumference.47 Studies have shown that adiponectin levels are lower in patients with MM and patients with MGUS that progresses to MM than in patients with MGUS that does not progress.48,49 Adiponectin-deficient mice also harbor a greater tumor burden and increased bone disease among mouse models of myeloma.48 It has been postulated that adiponectin can activate adenosine monophosphate–activated protein kinase (AMPK), thereby enhancing MM apoptosis.50 Adiponectin also inhibits MM cell proliferation through the signal transducer and activator of transcription 3, mitogen-activated protein kinase, β-catenin, and phosphatidylinositol 3-kinase (PI3K) pathways. Furthermore, decreased levels of adiponectin have been associated with higher levels of IL-6 and overproduction of TNF-α in the BM, both of which act as crucial proinflammatory cytokines in the development of myeloma.47 In contrast, higher levels of adiponectin may decrease TNF-α and IL-6 secretion via the inhibition of nuclear factor κB (NF-κB) and promote anti-inflammatory cytokine expression of IL-10 and IL-1RA.51
Patients with MM also have higher serum levels of the adipokine leptin, which is increased in obesity.52 Previous studies have demonstrated that the upregulation of leptin may lead to MM cell proliferation and reduced efficacy of chemotherapeutics.53 Coculture of myeloma cells and adipocytes increased the expression of antiapoptosis and proliferative proteins in MM cells. Leptin treatment of myeloma cells stimulated cell growth and reduced bortezomib efficacy, whereas the administration of antileptin antibodies in myeloma/adipocyte cocultures suppressed myeloma cell proliferation. These effects of leptin are mediated by the protein kinase B (AKT) and signal transducer and activator of transcription 3 pathways.52 Leptin also has suppressive effects on immune cells in MM and induces anergy of invariant natural killer T cells, decreasing invariant natural killer T–cell interferon-gamma expression, cell motility, and antitumor activity.54
Resistin is another adipokine that is associated with higher body fat mass. It stimulates the production of the proinflammatory cytokines TNF-α, IL-1β, IL-6, IL-8, and IL-12.55 Resistin upregulates the prosurvival proteins Bcl-2 and Bcl-xL and downregulates proapoptotic proteins, such as Bcl-2–associated x protein via modulation of the NF-κB and PI3K/AKT pathways. Resistin also increases the expression of the adenosine triphosphate (ATP)-binding cassette (ABC) transporters ABCC5 and ABCG2, which promote the efflux of chemotherapy drugs from MM cells, contributing to drug resistance. In in vivo models of MM, resistin administration rendered mice less sensitive to melphalan treatment and worsened disease outcomes.56 However, interestingly, lower resistin levels are associated with a higher MM risk.55 This may be due to the production of inflammatory cytokines, such as TNF-α, IL-1β, IL-6, IL-8, and IL-12, which cause a negative feedback effect on resistin.
Adipsin is an adipokine that is increased in obesity and has been shown to enhance autophagy and inhibit apoptosis in MM cells. The addition of recombinant adipsin to MM cell cultures increases the expression of autophagy-related 3 and microtubule-associated protein light chain 3 (LC3-I/II). In these experiments, both adipsin treatment and coculture with adipocytes protected MM cells against melphalan-induced cell death, whereas the addition of anti-adipsin antibodies abrogated adipocyte-induced protection from apoptosis.57
Obesity and bone metabolism
The relationship between obesity and bone health is multifaceted. Adipokines such as leptin and adiponectin are secreted by the AT and can directly act on BM stem cells, osteoblasts, and osteoclasts. Leptin, which is increased in obesity, has been shown to promote the proliferation and osteoblastic differentiation of BM stem cells,58 stimulate mineralization of the collagen bone matrix,59,60 and trigger the transcription and translation of osteoprotegerin, a secreted protein that acts as a decoy receptor and inhibits receptor activator of NF-κB ligand (RANKL; a major activator of osteoclast differentiation and function) signaling.61 Furthermore, in in vitro cultures, leptin prevents the differentiation of peripheral blood mononuclear cells into osteoclasts.61 Taken together, these studies suggest that leptin has probone-formation effects. However, results from genetic models have been somewhat mixed: in leptin-deficient mice, trabecular bone volume and bone mineral density decreased in the peripheral skeleton but increased in the axial skeleton.62 Injection of leptin into the calvarium of mice promoted bone formation and inhibited osteoclastogenesis.63 In contrast, adiponectin is an adipokine that is decreased in obesity and is thought to promote osteogenesis. It stimulates cyclooxygenase 2 expression in mesenchymal stem cells, thereby promoting bone morphogenetic protein 2 expression, causing osteoblast proliferation and maturation, increased alkaline phosphatase activity increase, and matrix mineralization.64 Adiponectin also inhibits osteoclastogenesis by suppressing AKT1, inhibiting RANKL-mediated differentiation of osteoclasts, stimulating osteoclast apoptosis, and decreasing the survival of osteoclast progenitors.65 In vitro treatment of osteoclast precursor cells with adiponectin decreased tartrate-resistant acid phosphatase–positive cells and expression of osteoclast markers such as cathepsin K, nuclear factor of activated T cells 2, and osteoclast-associated receptor. In vivo models of adiponectin-deficient mice show decreased bone mass and increased adipose mass.66
In addition to adipokines, AT and adipose-infiltrating immune cells secrete proinflammatory cytokines that affect bone metabolism. TNF-α stimulates osteoclast differentiation by promoting the formation of osteoclast precursor cells in the BM, activating double-stranded RNA-dependent protein kinases, and upregulating RANKL expression in the BM macrophages, osteocytes, and stromal cells.67,68 Concomitantly, TNF-α suppresses the expression of type 1 collagen, osteocalcin, alkaline phosphatase, and the transcription factors RUNX2, osterix, and β-catenin, which are required for osteoblast survival, differentiation, and mineralization.69 Interestingly, in mouse models of high-fat diet–induced obesity, TNF-α deletion protected the mice against trabecular bone loss, femur osteoclastogenesis, and accelerated bone turnover.70 IL-1β, another proinflammatory cytokine that is elevated in obesity, upregulates osteoclast expression of CCR7 to promote cell migration,71 stimulates osteoclast progenitor proliferation and differentiation into mature osteoclasts,72 and promotes RANKL expression in osteoclasts, osteoblasts, and stromal cells in the BM.73 Conversely, IL-1β concurrently inhibits osteoblast migration74 and promotes matrix metalloproteinase expression in osteoblasts, which results in bone tissue destruction75 and increases fibroblast growth factor 23 levels, which can dysregulate vitamin D and phosphate metabolism, thereby disrupting trabecular bone architecture.76,77
In addition to adipokines and cytokines, AT can secrete extracellular vesicles and noncoding RNAs that regulate bone metabolism. For example, the microRNA MiR-34a-5p, which is increased in obesity, is associated with decreased bone density in the spine78 and inhibits osteogenic differentiation of mesenchymal stem cells.79 The microRNA MiR-155 is found in exosomes from obese AT and inhibits osteoblast differentiation by suppressing the expression of SMAD5, a protein required for osteogenesis.80 MiR-142-5p, which is upregulated in obesity, promotes osteoclast differentiation from BM macrophages by upregulating the PTEN/PI3K/AKT/FoxO1 pathway.81 In contrast, the microRNA MiR-503 is downregulated in obesity and has been shown to enhance osteoblast differentiation by suppressing Smurf1 expression.82
Finally, obesity-associated hyperglycemia, hyperlipidemia, and endothelial dysfunction increase oxidative stress within the BM microenvironment, which can damage bone tissues and suppress osteoblast differentiation and function. In in vitro cultures of mesenchymal stem cells, increased levels of intracellular reactive oxygen species promote adipogenesis during osteoblastogenesis.83 Free fatty acids (FAs) released by adipocytes in adipocyte/osteoblast cocultures have a lipotoxic effect on osteoblasts, causing cell death and decreasing osteoblast expression of RUNX1, osteocalcin, and collagen 1, which impairs mineralization capacity.84 Reactive oxygen species production by adipose or BM immune cells also promotes osteoclast differentiation and bone resorption through the upregulation of nuclear factor of activated T cells.85
Importantly, there is significant cross talk between MM cells, osteoclasts, and osteoblasts. MM cells express RANKL and secrete cytokines such as macrophage inflammatory protein 1α (MIP-1α),86 stromal derived factor 1,87 and IL-3,88 which promote osteoclast differentiation and inhibit osteoblast function. In return, osteoclasts can enhance the growth and survival of MM cells by secreting osteopontin, vascular endothelial growth factor, and IL-6, which protect MM cells from apoptosis, promote MM cell expansion, and provide a favorable vascular microenvironment for MM cell growth.89 Furthermore, osteoclasts secrete IL-10 and TGF-β, which promote regulatory T–cell differentiation and create an immunosuppressive space in which MM cells are protected from immune clearance.90 Osteoclasts may also accelerate disease relapse by remodeling the endosteal niche and reactivating dormant myeloma cells.91 Thus, the effects of obesity on bone metabolism and BM microenvironment can amplify the vicious cycle between osteoclasts and MM cells, leading to disease progression.
Obesity and BM adiposity
White AT (WAT) and brown AT (BAT) play critical roles in energy homeostasis. In recent years, BM AT (BMAT) has been increasingly recognized as a distinct AT.92 Although the local and systemic effects of WAT and BAT have been extensively investigated, very little is known about the functions of BMAT. BM adiposity increases with age and accounts for up to 70% of the BM volume in the older population.93 Although originally considered an inert space-filling component of the BM, BMAT is increasingly being recognized as an important contributor to energy homeostasis and a source of adipokines.92 BMAT comprises >10% of the total AT mass in the body and expresses a distinct genetic and protein profile compared with WAT, BAT, and beige adipocytes.92,94 BMAT is both an endocrine target and an endocrine organ that responds to hormones such as insulin and secretes cytokines and interleukins, such as IL-1, IL-6, TNF-α, and adipokines, such as leptin and adiponectin.95 BMAT can be further subdivided into constitutive and regulated (cBMAT and rBMAT, respectively) components that reside at distinct anatomical locations in the bone.96 cBMAT is mainly found at the bone distal ends and resembles densely packed WAT, whereas rBMAT is scattered throughout the BM cavity and is in close proximity to metabolically active bone regions.96 Their lipid content seems to differ, with cBMAT storing unsaturated fats and rBMAT preferentially storing saturated fats, suggesting that the 2 fat depots have distinct functions and secretomes through which they regulate BM and bone homeostasis.96
Like WAT, BMAT volume is highly responsive to pathophysiological changes, including aging, obesity, and type 2 diabetes. Mice fed a high-fat diet showed increased BMAT, which led to persistent long-term changes in bone quality.96 Therefore, BMAT volume can be modified through environmental changes, such as diet, and when coupled with the effects of aging, it can have profound effects on the functionality of neighboring cells. The recruitment of immune cells at sites of inflammation in the BM can also enhance lipolysis and secretion of proinflammatory proteins and lipids. This, in turn, can have local and systemic negative impacts, such as the induction of insulin resistance, hyperglycemia, and hyperlipidemia, which are associated with oxidative stress and cancer development and/or progression. These changes activate signaling pathways that enhance the survival, proliferation, migration, and chemoresistance of cancerous cells.97
BM adiposity and MM
Investigations in solid tumor models have shown that adipocytes are a key component of the tumor microenvironment (TME) and are critical regulators of tumor cell metabolism. In these models, cancer-associated adipocytes support tumor progression via the secretion of adipokines, reprogramming metabolic pathways between cancer cells and cancer-associated adipocytes, and modulation of the TME, such as T-cell suppression through the overexpression of programmed death ligand-1 (PD-L1).98,99 MM originates in the BM TME, and MM cells are in close contact with BM adipocytes (BMAds).100 There is evidence that MM cells may adhere to BMAds and that this action may protect MM cells from apoptosis and, moreover, promote MM cell migration and proliferation.51 The pathophysiological factors that increase BM adiposity, including aging, obesity, osteoporosis, radiotherapy, and glucocorticoid treatment, are all highly applicable to patients with MM.
MM cells, in turn, affect BM function. BM mesenchymal stem cells can differentiate into adipocytes and osteoblasts. Extensive single-cell sequencing, in vitro cocultures, and in vivo studies have demonstrated that MM cells inhibit osteoblastogenesis and promote adipogenesis of mesenchymal stem cells through integrin-mediated cell-cell contact and stabilization of peroxisome proliferator activated receptor γ 2.101 Thus, MM cells directly contribute to the suppression of bone formation and promote myeloma bone disease, which is a critical hallmark of MM. A study by Lwin et al showed that a high-fat diet promoted the development of a myeloma-like condition in C57/Bl6 mice when compared with control diet mice.25 Trotter et al also showed an increased expression of the preadipocyte marker Pref-1 and increased size of BMAds via histological evaluation of BM biopsy specimens from patients newly diagnosed with MM.102 These findings support the notion that increased BM adiposity is protumorigenic and increased adiposity caused by excessive calorie intake can further exacerbate disease progression.
An increase in BMAT can potentially promote cancer progression through both direct and indirect mechanisms. Directly, interleukins and adipokines secreted by BMAds have been shown to promote MM cell survival and migration and protect MM cells from chemotherapy through the activation of autophagy pathways.57,95,103 Moreover, lipids and FAs from adipocytes can be directly taken up by tumor cells and used to fuel their increasing energy demand and metabolic reprogramming.104 Indirectly, BMAT acts as an energy supply to fuel bone metabolism and maintain bone homeostasis.105 BMAds are mainly found near more metabolically active bone areas, and an increase in BMAT and proinflammatory cytokines may disrupt the bone remodeling balance to favor bone resorption. Increased osteoclast activity and bone destruction are advantageous for MM cell growth and survival. In turn, MM cells release and stimulate neighboring stromal cells to secrete factors that additionally enhance osteoclast activity, such as IL-1β, IL-6, MIP-1, TGF-β, TNF-α, and metalloproteinases, resulting in a vicious feed-forward cycle that ultimately leads to myeloma bone disease.
Although BMAT-derived proteins have been well studied, little is known about BMAT-derived lipids in patients with MM. BMAT stores triglycerides and releases FAs that can subsequently be used for ATP generation. When MM cells were cocultured with BMAds, a decreased lipid content was noted in BMAds.106 Interestingly, there is a 37-fold increased risk of MM among patients with Gaucher disease, which is associated with lipid accumulation but not obesity.107 Nair et al showed that both patients with MGUS and Gaucher disease and mouse models of Gaucher-related monoclonal gammopathies displayed an increased proportion of immunoglobulins that are specific to a particular class of lipids called lysolipids.108 The authors proposed that long-term immune activation by lysolipids such as lyso-glucosylceramide and lysophosphatidylcholine may be an underlying cause of the pathogenesis of monoclonal gammopathies. Indeed, reducing the levels of these lipids in mice decreases the risk of Gaucher-related monoclonal gammopathy.108 Gonsalves et al used untargeted metabolite and targeted complex lipid profiling of BM plasma to identify significant differences in the amino acid profiles and total levels of complex lipids in patients with MGUS and MM,109 suggesting that lipids may play an important antigenic role in driving clonal plasma cell proliferation. Although this work provided novel results and some insight into the possible differences between the intracellular metabolic pathways involved in plasma cell clonal evolution, more studies are needed to fully understand the role of BMAT and its lipidome in the progression from an asymptomatic to a mature MM disease state.
FAs as regulators of cancer metabolism
Adipocytes have been shown to support cancer cell survival and proliferation by influencing mitochondrial activity and lipid metabolism.110,111 B lymphocytes exhibit metabolic regulation during mitochondrial biogenesis and glucose uptake.112,113 Activated B lymphocytes upregulate oxidative phosphorylation, tricarboxylic acid cycle, and nucleotide biosynthesis, but not glycolysis. Blocking oxidative phosphorylation inhibits B-cell differentiation and proliferation. Activated B lymphocytes also upregulate ATP-citrate lyase levels and activity, generating cytosolic acetylcoenzyme A (acetyl-CoA),114 which is the substrate for de novo FA synthesis. Inhibition of ATP-citrate lyase activity inhibits B-cell proliferation and decreases the expression of CD138, a plasma cell marker, and Blimp1, a transcription factor responsible for differentiation into terminal plasma cells.114 FAs are essential for the biosynthesis of membranes, organelles, and signal molecules and function as substrates for energy production. FAs secreted from adipocytes are considered signaling molecules that regulate energy metabolism, and as the nature of their composition is very heterogenous, so are their effects and actions on different target tissues.
FAs have long been considered to pass through membranes via passive diffusion; however, recent studies have shown that various FA transporters present on cell membranes can increase FA uptake in cells. CD36 is a multifunctional glycoprotein receptor on cell membranes, is responsible for long-chain FA and lipoprotein uptake, and has been implicated in cancer development and metastasis.104 Other protein families involved in FA transport that have been implicated in cancer development and progression are FA-binding proteins and FA transport proteins (FABPs and FATPs, respectively). FABPs facilitate the transport of long-chain FAs intracellularly and regulate lipid synthesis and oxidation.115 Alteration of FABP expression has been observed in many diseases, including cancers of the prostate,116 breast,117 and other tissues.118 FABPs have been shown to increase FA traffic and storage in malignant cells119 and enhance prostate and ovarian tumor cell proliferation by increasing lipid availability and FA oxidation,120,121 altering glucose metabolism, and mediating signal transduction and apoptosis in both healthy and cancerous cells.122 Patients with MM have been found to have significantly higher serum FABP4 levels than healthy individuals.123
Another group of FA transporter proteins comprises of FATPs, a family of 6 proteins involved in FA uptake and activation. FATPs function as both FA transporters and acyl-CoA synthetases and are abundantly expressed in different tissues. In the recent years, studies have described the role of FATPs in tumor metabolism. Blask et al showed that the upregulation of FATPs increased FA uptake in rat hepatomas.124 Zhang et al demonstrated that melanoma cells express FATPs that act as transporters of adipocyte-released FAs to the melanoma cells to support their increased metabolic demand.125 Blocking FATPs decreased tumor cell growth and lipid content in melanoma cells. In line with this study, we have also recently shown that MM cells induce lipolysis in adipocytes and then take up the released FAs to their advantage. We found that both MM cell lines and CD138+ cells from patients express high levels of FATPs, which modulate, at least in part, the internalization of FAs.126 We demonstrated that samples from patients with MM showed an altered lipidome profile, with a specific reduction in arachidonic acid (and downstream ω-6 FAs) in the BM plasma compared with healthy donor samples. Interestingly, arachidonic acid displayed a dual effect on MM cells, with lower doses increasing proliferation and higher doses inducing ferroptosis. The arachidonic acid–induced ferroptosis in MM cells was also recapitulated in an in vivo plasmacytoma model.126 Therefore, inhibiting either FATPs in MM cells or lipolysis in BMAds could represent a potential novel strategy to limit MM progression. However, because it is not yet clear how FATPs mediate FAs uptake, exactly which FATPs can act as transporters, and whether some of the FATPs function in a dual role as both a FA carrier and enzyme with acyl-CoA activity, more studies on the roles of FATPs in MM are needed.
Therapeutics
Metformin
Given the altered insulin axis and proinflammatory state observed in obesity, MM, and MGUS, metformin has become an increasingly attractive therapeutic target that may help impede the progression from MGUS to MM. Several potential mechanisms may be implicated. Firstly, metformin attenuates proinflammatory mediators such as IL-6 and IGF-1,127 which are culprits associated with carcinogenesis.128 Secondly, metformin has also been independently associated with weight loss,129 so curbing obesity may independently reduce the risk of malignant transformation. Thirdly, metformin may activate AMPK signaling and inhibit mammalian target of rapamycin, both of which may directly inhibit carcinogenesis.24,130 Fourthly, metformin has been shown to improve effector T–cell function and memory formation as well as inhibit the induction and activity of immunosuppressive regulatory T cells, which may improve immune system recognition and activity against MM cells.131,132 Finally, metformin has been hypothesized to modulate the gut microbiota in such a manner that it may increase the efficacy of immunotherapy treatments.133
Initial supporting evidence for metformin use came from a study that found that although patients with MM and had diabetes had worse overall survival compared with patients with MM without diabetes (hazard ratio [HR], 1.62; 95% confidence interval [CI], 1.33-1.97), patients with MM and diabetes treated with metformin compared with other treatments such as insulin or GLP-1 agonists had improved overall survival (HR, 0.66; 95% CI, 0.48-0.90).134 Subsequently, in a retrospective study of patients with MGUS and diabetes, a comparison of metformin-using with -nonusing patients showed reduced risk of progression to MM observed among metformin users (HR, 0.47; 95% CI, 0.25-0.87).23 In another study in which transformed MGUS cases were compared with nontransformed cases, among all patients with diabetes, >2-year use of metformin resulted in almost a 50% decreased risk of developing active MM (odds ratio [OR], 0.40; 95% CI, 0.08-2.04).24 However, metformin use for <2 years was not associated with this reduced risk. A follow-up question is whether a similar effect might be observed with metformin use in patients who were overweight/obese with MGUS or smoldering MM but without diabetes, which is currently being explored.135
Statins
Given that a proinflammatory state promotes carcinogenesis, as described earlier, hydroxymethylglutaryl-CoA reductase inhibitors (statins) have become of increasing interest because of their potential to decrease C-reactive protein (CRP), a core tenet of the proinflammatory storm. In vitro studies have shown significant antimyeloma activity in MM cells exposed to statins, with higher rates of apoptosis and increased intracytoplasmic calcium levels.136 Furthermore, patients with MM with t(4;14) disease, which portends a worse prognosis, display a unique vulnerability to statins because t(4;14)-positive cells are metabolically dependent on the mevalonate pathway, which is inhibited by statins. Upon treatment with statins, t(4;14)-positive cells activate an integrated stress response and undergo apoptosis; this effect works particularly well in synergy with bortezomib treatment.137 Additionally, statins have been shown to inhibit the expression of MIP-1α, an osteoclast-activating factor that also promotes MM cell growth, migration, and adhesion via downregulation of the Ras/extracellular signal–regulated kinase and Ras/Akt pathways.138
Retrospective epidemiologic studies have found mixed results. In the Veterans Administration Central Cancer Registry, statin use was associated with a 24% decrease in MM-specific mortality (n = 4757; HR, 0.76; 95% CI, 0.67-0.86) and a 31% decrease in the risk of developing skeletal-related events.139 A recent meta-analysis concluded that there was a 20% reduced risk of MM development among statin users as compared with that among nonusers (n = 19 827 646; OR, 0.8; 95% CI, 0.68-0.93).140 In contrast, a case-control study of patients with MGUS that progressed to MM (as compared with patients with MGUS that did not progress), found no protective effect associated with statins (n = 200; OR, 1.18; 95% CI, 0.53-2.63).36 More well-powered prospective studies are needed to be done to answer whether statins affect MM development and progression.
Diet
Diet appears to play a crucial role in MGUS and myeloma pathogenesis. In a mouse model in which 5T myeloma cells were injected into C57/Bl6 mice, only the mice fed a high-fat diet developed features of myeloma, including increase in serum IgG2 paraprotein levels, accumulation of myeloma cells in the BM and spleen, and loss of bone volume. In contrast, the mice maintained on the control diet did not display these features. Additionally, in the same mouse model, mice that were initially fed a high-fat diet during tumor inoculation but were then returned to a normal diet displayed a reduced tumor burden compared with mice maintained on a high-fat diet, suggesting that dietary interventions after the disease has been established can still have a therapeutic impact.25
Diet also critically affects the microbiome, which has been found to be an important component of MM disease pathogenesis. Increased consumption of vegetables and whole grains is associated with a decreased risk of MM, whereas intake of refined sugars is associated with an increased risk.141 Calcinotto et al showed that the gut bacteria Prevotella heparinolytica promotes proinflammatory Th17 differentiation of intestinal T cells that then migrate to the BM to secrete IL-17, which directly accelerates MM cell proliferation and also modulates eosinophils to secrete TNF-α and IL-6, which further stimulate MM cell growth.142 Other studies have shown that diets rich in fiber lead to increased production of short-chain FAs (SCFAs) by intestinal microbiota, which suppress the NF-κB pathway, inhibit proinflammatory cytokine signaling,143 and promote CD8 T-cell function and differentiation by downregulating histone deacetylases,144 which may sensitize patients with MM to immunotherapy. Indeed, the levels of SCFA-producing microorganisms, such as Clostridium butyricum, Clostridium saccharobutylicum, and Anaerostipes hadrus are markedly reduced in patients with MM.145 Additionally, MM cells have been shown to be metabolically dependent on L-glutamine (Gln), a nutrient that acts as a nitrogen donor for nucleotide biosynthesis and supports biomass production in proliferating MM cells.146 Patients with MM have increased gut bacteria levels of Klebsiella and Streptococcus species, which are bacteria associated with nitrogen recycling. Fecal transplantation of Klebsiella pneumoniae into mice with MM causes disease progression due to increased glutamine synthesis.145 Finally, after treatment, sustained minimal residual disease negativity was been shown to be associated with healthier plant-based diets and increased intestinal levels of Eubacterium hallii and Faecalibacterium prausnitzii, which produce the SCFA butyrate.141,147 Thus, modulation of the gut microbiome composition through diet modification may be an attractive therapeutic target.
Epidemiologic studies evaluating the role of diet on MGUS and MM development have found protective effects of fish,148 fresh fruit,27,149 whole grains, vegetables,150 and plant-based diets.151 In an analysis from the Nurses’ Health Study, Health Professionals Follow-up Study, healthier prediagnosis diet patterns led to longer overall survival rates than less healthy diets (HR, 1.24; 95% CI, 1.07-1.44).152 In contrast, unhealthy dietary patterns (eg, diets that promote inflammation and hyperinsulinemia, associated with increases in IL-6, CRP, and TNF-αR2) have been associated with an elevated risk of MM development in men but not in women (HR, 1.96; 95% CI, 1.22-3.13). Interestingly, stratified analyses demonstrate that this risk appears to be higher in lean individuals (BMI <25) than in overweight or obese individuals (BMI ≥25), indicating that diet pattern may be a more important risk factor in individuals with lower adiposity.153 Preliminary results from ongoing clinical trials suggest that patients with MGUS consuming a plant-based diet have improved disease biomarkers, such as decreased levels of the proinflammatory cytokines IL-8, IL-12β, and TNF-β and increased stool butyrate concentration.154,155 Finally, observational studies of dietary intake suggest that consuming fruit at least 3 times a week has been associated with decreased risk of progression from MGUS to MM (HR, 0.34; 95% CI, 0.13-0.89).149 These studies suggest that the modulation of diet or nutrition may play a complementary and therapeutically targetable role in MM.
Conclusions
Although increasing evidence suggests a role for obesity as a risk factor for the development of MGUS and progression to MM, the results of individual epidemiological studies are heterogeneous. Several reasons are implicated. Firstly, adiposity may be confounded by sociodemographic factors, such as race and SES, and current methodological approaches cannot capture the true impact of adiposity on disease burden. A second related concern is that MGUS and MM are associated with older adults, and BMI is a suboptimal measure of adiposity in this group; therefore, studies using other measures of adiposity may lead to more conclusive results. The most promising studies highlighted the role of visceral fat using FDG/PET and its association with progression of disease. This demonstrated not only more visceral fat in those whose condition progressed but also more metabolic activity in the AT of these subjects, even though both groups had statistically similar BMIs. Finally, there is the issue of power: a few of the highlighted studies, especially case-control studies, failed to achieve the statistical power needed to detect a difference between the 2 groups.
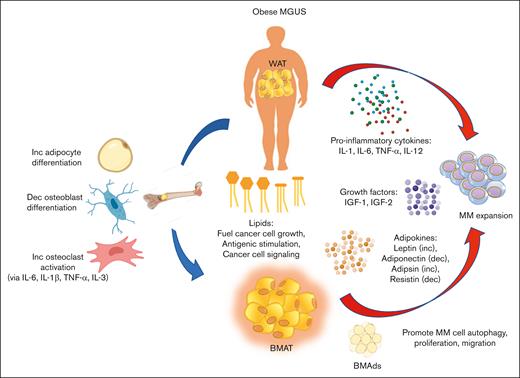
Biologically, there are numerous pathways by which AT (both WAT and BMAT) may promote MGUS to MM progression (summarized in Figure 1). Obesity is a state of chronic inflammation; therefore, stressed adipocytes and fat-infiltrating immune cells secrete proinflammatory cytokines, growth factors, adipokines, and FAs, which promote MM expansion and growth. Cell-cell interactions between MM cells and BMAds in the BM also stimulate MM cell trafficking, autophagy, chemoresistance, and proliferation. Finally, obesity may predispose the BM environment to bone disease development by promoting osteoclast differentiation and resorptive activity, which in turn stimulates MM cell proliferation and growth in a positive feed-forward cycle.
Model of how obesity promotes the progression of MGUS to MM. Fat depots such as WAT and BMAT secrete proinflammatory cytokines, growth factors, and adipokines that promote MM proliferation and cell growth. Lipids released from adipocytes act not only as fuel for cancer cell growth but also promote the formation of lysolipids that antigenically stimulate plasma cell proliferation. FAs are also transported across membranes by various FA transporters and affect cell signaling to promote MM cell growth. MM cells closely interact with and adhere to BMAds in the BM, which allows MM cell autophagy, trafficking, and proliferation. Within the BM microenvironment, increased adiposity promotes osteoclast differentiation and activity while repressing osteoblast differentiation and activity. Furthermore, proinflammatory cytokines also promote osteoclast activity, which contributes to bone fragility in patients with MM and promotes a prooncogenic niche for MM cell expansion. Dec, decrease; Inc, increase.
Model of how obesity promotes the progression of MGUS to MM. Fat depots such as WAT and BMAT secrete proinflammatory cytokines, growth factors, and adipokines that promote MM proliferation and cell growth. Lipids released from adipocytes act not only as fuel for cancer cell growth but also promote the formation of lysolipids that antigenically stimulate plasma cell proliferation. FAs are also transported across membranes by various FA transporters and affect cell signaling to promote MM cell growth. MM cells closely interact with and adhere to BMAds in the BM, which allows MM cell autophagy, trafficking, and proliferation. Within the BM microenvironment, increased adiposity promotes osteoclast differentiation and activity while repressing osteoblast differentiation and activity. Furthermore, proinflammatory cytokines also promote osteoclast activity, which contributes to bone fragility in patients with MM and promotes a prooncogenic niche for MM cell expansion. Dec, decrease; Inc, increase.
Delineation of these obesity-related pathogenic pathways in MGUS and MM has led to further research on pharmacologic and lifestyle interventions that could be targeted in overweight/obese patients, thereby reducing MGUS to MM progression. Metformin, statins, and dietary modifications may be potential therapeutics that could complement or work synergistically with more traditional treatments like chemotherapy, immunotherapy, and radiation. As obesity affects an increasing number of patients, there will be increasingly more patients with cancer who have concomitant metabolic disease, and this is an area that is ripe for future investigative work.
Acknowledgment
N.R. and C.P. were funded by the Paula and Rodger Riney Foundation.
Authorship
Contribution: R.C., C.P., and R.B. performed the literature review and wrote the manuscript, and N.R. provided guidance and input and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Noopur Raje, Medical Oncology, Massachusetts General Hospital Cancer Center, Harvard Medical School, 55 Fruit St, Boston, MA 02114; e-mail: nraje@mgh.harvard.edu.
References
Author notes
∗R.C. and C.P. contributed equally to this work.