Key Points
Busulfan exposure is associated with OS; 5-year survival with an exposure of ≥59.5 vs <59.5 mg × h/L was 67% (95% CI, 59-76) vs 40%.
In 25% of the patients, busulfan levels were outside the optimal range.
Abstract
Busulfan is an alkylating drug routinely used in conditioning regimens for allogeneic hematopoietic cell transplantation (allo-HCT). A myeloablative conditioning regimen, including busulfan, is commonly used in patients undergoing T-cell depletion (TCD) and allo-HCT, but data on optimal busulfan pharmacokinetic (PK) exposure in this setting are limited. Between 2012 and 2019, busulfan PK was performed to target an area under the curve exposure between 55 and 66 mg × h/L over 3 days using a noncompartmental analysis model. We retrospectively re-estimated busulfan exposure following the published population PK (popPK) model (2021) and correlated it with outcomes. To define optimal exposure, univariable models were performed with P splines, wherein hazard ratio (HR) plots were drawn, and thresholds were found graphically as the points at which the confidence interval crossed 1. Cox proportional hazard and competing risk models were used for analyses. 176 patients were included, with a median age of 59 years (range, 2-71). Using the popPK model, the median cumulative busulfan exposure was 63.4 mg × h/L (range, 46.3-90.7). The optimal threshold was at the upper limit of the lowest quartile (59.5 mg × h/L). 5-year overall survival (OS) with busulfan exposure ≥59.5 vs <59.5 mg × h/L was 67% (95% CI, 59-76) vs 40% (95% CI, 53-68), respectively (P = .02), and this association remained in a multivariate analyses (HR, 0.5; 95% CI, 0.29; 0.88; P = .02). In patients undergoing TCD allo-HCT, busulfan exposure is significantly associated with OS. The use of a published popPK model to optimize exposure may significantly improve the OS.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is the only curative option for patients with certain hematologic malignancies, including most patients with acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS).1,2 Despite major improvements in HCT outcomes, owing mostly to decreases in transplant-related mortality (TRM),3 graft-versus-host disease (GVHD) remains a major challenge, affecting patients' quality of life and survival after HCT.4 Acute GVHD (aGVHD) and chronic GVHD (cGVHD) are significant post-HCT complications, with a cumulative incidence ranging from 30% to 60%.4,5 An effective method of preventing GVHD is via the depletion of T lymphocytes from the donor allograft before infusion, which, in retrospective and prospective studies,6-9 resulted in a significantly lower incidence of GVHD yet without improved survival. Moreover, a myeloablative regimen is the preferred conditioning regimen for patients with AML and fit patients with MDS, with data supporting improved relapse-free survival (RFS).10,11 Accordingly, a myeloablative, chemotherapy-only conditioning regimen that combines busulfan, melphalan, and fludarabine is commonly used in adult and pediatric patients undergoing T-cell-depleted (TCD) allo-HCT.
Busulfan is an alkylating drug routinely used in conditioning regimens in preparation for allo-HCT.12,13 Considerable interpatient variability exists in the effectiveness, and toxicity of busulfan-containing regimens when dosing is based on either body weight (mg/kg) or body surface area (mg/m2).14 The variability in clinical outcomes is partly due to interpatient differences in busulfan pharmacokinetic (PK) and the narrow therapeutic window of systemic busulfan exposure.15 Higher exposure has been reported to be associated with an increased risk of toxicity, such as mucositis, GVHD, veno-occlusive disease of the liver, and TRM,13,16,17 whereas a low busulfan exposure has been associated with a higher probability of graft rejection, poor graft function, and disease relapse.18-20 Therefore, the use of therapeutic drug monitoring (TDM) to target a specific area under the curve (AUC) in the more recent period after consensus21 and/or concentration at steady state has historically provided a personalized approach to dosing to better ensure exposure within the narrow therapeutic window of efficacy and toxicity for each patient.12 IV busulfan can be administered once daily or divided every 6 or 12 hours22 without a difference in toxicities.13 Historically, various units have been used to report busulfan AUC goals, which led to misinterpretation and was a barrier to data capture by HCT registries. This led to efforts to harmonize busulfan plasma exposure unit reporting and resulted in agreement to report exposure as an AUC with the units mg × h/L.21 In addition, the use of different models to estimate exposure may result in different exposure reports, as shown in a study conducted by Bartelink et al.13
In adult and pediatric patients undergoing TCD allo-HCT, data regarding the optimal busulfan exposure in this setting are limited. Therefore, we aimed to assess the relationship between busulfan exposure estimation following the published population PK (popPK)15,23 model and clinical outcomes in a retrospective analysis of prospectively collected TDM data.
Methods
Study design and patients
We performed a retrospective, PK, and pharmacodynamic analysis of data from consecutive pediatric and adult patients with MDS and AML who underwent their first allo-HCT with ex vivo TCD allografts using the CliniMACS CD34 Reagent System (Miltenyi Biotech, Gladbach, Germany) for calcineurin inhibitor-free GVHD prophylaxis at the Memorial Sloan Kettering Cancer Center (MSKCC) between 2012 and 2019. The patients received supportive care, growth factors, and antimicrobial prophylaxis according to the MSKCC institutional protocol. All the patients received a chemotherapy-based myeloablative conditioning regimen with IV busulfan (from days −9 to −7), melphalan 70 mg/m2 (from days −6 to −5), and fludarabine 25 mg/m2 (from days −6 to −2). Additionally, all patients received rabbit anti–thymocyte globulin (Thymoglobulin) to prevent graft rejection on days −3, −2, and ±1.24 All patients provided written informed consent for transplantation in accordance with the principles of the Declaration of Helsinki, and transplantation outcome analysis was approved by the MSKCC Institutional Review and Privacy Board.
Busulfan was dosed at 3.2 mg/kg per day, divided either every 6 hours or as a single daily dose; patients aged < 4 years received 1 mg/kg every 6 hours. The first-dose AUC was estimated with 6 concentration-time samples using a noncompartmental analysis model (Phoenix WinNonLin, Certara USA, Princeton, NJ). The 2 subsequent doses were adjusted to target an exposure from 18 to 22 mg × h/L (every 24 hours) or from 4.5 to 5.5 mg × h/L (every 6 hours), with goal cumulative AUC (cAUC) over 3 days between 55 and 66 mg × h/L, with all units converted to reporting per the latest consensus recommendations.21 The PK sampling and analysis were not repeated at doses 2 and 3. Busulfan samples were obtained from a Clinical Laboratory Improvement Amendments (CLIA)-approved clinical laboratory. In this retrospective analysis, the initial dosing, the same 6 measured time-concentration samples, and any subsequent dose changes, if made, were entered into the InsightRX Nova Bayesian dosing platform (Insight Rx Inc, 2021, San Francisco, CA) to re-estimate the cumulative busulfan exposure using popPK. The estimated exposures were then related to clinical outcome measures.
Outcomes of interest
The primary outcome of interest was overall survival (OS) at 2 and 5 years. OS was defined as the time from HCT to death from any cause. Data of surviving patients were censored on the date of their last contact. Other outcomes of interest included relapse, nonrelapse mortality, RFS, TRM, and acute GVHD on day 100. Time to relapse was defined as the time from HCT to disease relapse. Data of patients without relapse were censored at the last follow-up, whereas deaths before relapse were treated as competing events. RFS was defined as survival without any evidence of relapse. TRM was defined as the time from HCT to death due to causes other than the relapse of hematologic malignancies. Patients who were still alive were censored at the date of the last follow-up and relapse was treated as a competing event. Acute GVHD was graded and staged according to national and international guidelines (staging based on the Center for International Blood and Marrow Transplant Research guidelines, with grading using modified Keystone criteria).25,26
Statistical analyses
Survival rates were estimated using the Kaplan-Meier method. To define optimal busulfan exposure, univariate models were performed with P splines, wherein hazard ratio (HR) plots were drawn. Thresholds were found visually at the points (exposure) at which the spline exceeded the HR of 1. If this point was around a quartile, exposure was used as a cutoff for further analysis. Survival rates were estimated using the Kaplan-Meier method. The impact of busulfan exposure and other variables, including sex, age (adult vs pediatric), disease (MDS vs AML), donor type, patient and donor cytomegalovirus serostatus, HCT-specific comorbidity index,27 and disease risk index (DRI),28 were assessed using univariate Cox proportional models. Busulfan was examined both when broken into quartiles and as a binary variable, with a cutoff point determined from optimal exposure figures using a penalized spline. In the presence of competing risks, cumulative incidence analysis was used, and cause-specific HRs were reported. The results are presented as HRs, 95% confidence intervals (95% CIs), and Wald test P values. Firstly, factors were assessed in univariable models and then entered in the multivariable model if P ≤ .10. A backward variable selection was performed, and variables with P ≤ .05 were retained in the final model.
Results
Patients and busulfan exposure
A total of 176 patients were included: 144 adults and 32 pediatric patients, with a median age of 59 years and median follow-up among survivors was 53.6 months (interquartile range, 38.3-79.5 months). The patients and HCT characteristics are shown in Table 1. Thirty-four patients (19.3%) received daily busulfan dosing, and 142 (80.7%) were treated with a 6-hour regimen. In 70 patients (39.7%), the target AUC was attained with the first dose, and no subsequent dose adjustments were required; in 77 patients (43.7%), a dose increase was required, and in 29 patients (16%), a dose reduction was required. Using the popPK model, the median cAUC was 63.4 mg × h/L (range, 46.3-90.7). The lowest quartile distribution ranged from 46.3 to 59.4 mg × h/L, second quartile from 59.5 to 63.4 mg × h/L, third quartile from 63.5 to 68.8 mg × h/L, and the fourth quartile ranged from 68.9 to 90.7 mg × h/L.
Patient characteristics
| Characteristic . | N = 176 . |
|---|---|
| Age, median (range) | 59 (2-71) |
| Gender (Female) | 71 (40%) |
| Adults/children | 144 (82%)/32 (18%) |
| AML/MDS | 104 (59%)/72 (41%) |
| DRI | |
| Low | 13 (7.6%) |
| High | 25 (15%) |
| Intermediate | 134 (78%) |
| Unknown | 4 |
| CMV status before HCT (positive) | 78 (55%) |
| HCT-CI | |
| Low | 10 (6.0%) |
| High | 85 (51%) |
| Intermediate | 73 (43%) |
| Unknown | 8 |
| Donor | |
| MRD | 44 (25%) |
| MURD | 100 (57%) |
| MMRD/MMURD | 32 (18%) |
| Characteristic . | N = 176 . |
|---|---|
| Age, median (range) | 59 (2-71) |
| Gender (Female) | 71 (40%) |
| Adults/children | 144 (82%)/32 (18%) |
| AML/MDS | 104 (59%)/72 (41%) |
| DRI | |
| Low | 13 (7.6%) |
| High | 25 (15%) |
| Intermediate | 134 (78%) |
| Unknown | 4 |
| CMV status before HCT (positive) | 78 (55%) |
| HCT-CI | |
| Low | 10 (6.0%) |
| High | 85 (51%) |
| Intermediate | 73 (43%) |
| Unknown | 8 |
| Donor | |
| MRD | 44 (25%) |
| MURD | 100 (57%) |
| MMRD/MMURD | 32 (18%) |
CMV, cytomegalovirus; HCT-CI, HCT cormobidity index; MMRD, mismatched related donor; MMURD, mismatched unrelated donor; MRD, matched related donor; MURD, matched unrelated donor.
Busulfan exposure and outcomes
Survival
Using the splines, the thresholds found graphically for OS, nonrelapse mortality, and RFS were around the upper limit of the lowest quartile (Figure 1). Therefore, we subsequently analyzed outcomes using 59.5 mg × h/L as a threshold. A cAUC <59.5 mg × h/L was associated with worse OS compared with higher busulfan exposure; the 5-year OS in patients with busulfan exposure <59.5 mg × h/L was 40% (95% CI, 27-60) compared with patients with exposure ≥59.5 mg × h/L, whose 5-year OS was 67% (95% CI, 59-76; P = .02; Table 2; Figure 2A). Similarly, the 5-year RFS in patients with busulfan exposure <59.5 mg × h/L was 36% (95% CI, 23-56) vs 60% (95% CI, 52-69; P = .02) in patients with an exposure ≥59.5 mg × h/L (Table 2; Figure 2B).
P splines were used to define an optimal busulfan exposure. Busulfan exposure < 59.5 mg × h/L was associated with worse OS (A) and worse RFS (B).
P splines were used to define an optimal busulfan exposure. Busulfan exposure < 59.5 mg × h/L was associated with worse OS (A) and worse RFS (B).
Univariate analysis for OS, RFS, and TRM
| Characteristic . | N . | OS . | RFS . | TRM . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | ||
| Age | 176 | 1.02 | 1.00-1.04 | .005 | 1.02 | 1.01-1.04 | .003 | 1.05 | 1.02-1.09 | < .001 |
| Gender | 176 | > .9 | .5 | .7 | ||||||
| Female | — | — | — | — | — | — | ||||
| Male | 1.02 | 0.62-1.66 | 1.15 | 0.73-1.82 | 0.89 | 0.47-1.69 | ||||
| Disease | 176 | > .9 | .5 | > .9 | ||||||
| AML | — | — | — | — | — | — | ||||
| MDS | 0.99 | 0.61-1.60 | 1.17 | 0.75-1.83 | 1.04 | 0.55-1.98 | ||||
| DRI∗ | 172 | .017 | .005 | |||||||
| Low | — | — | — | — | — | — | ||||
| High | 5.30 | 1.22-23.1 | 4.76 | 1.40-16.2 | ||||||
| Intermediate | 2.77 | 0.67-11.4 | 2.18 | 0.68-6.95 | ||||||
| CMV status before HCT | 143 | .11 | .4 | .2 | ||||||
| Negative | — | — | — | — | — | — | ||||
| Positive | 1.52 | 0.90-2.56 | 1.24 | 0.77-1.99 | 1.48 | 0.76-2.87 | ||||
| HCT-CI∗ | 168 | > .9 | .8 | |||||||
| Low | — | — | — | — | — | — | ||||
| High | 1.10 | 0.34-3.60 | 1.35 | 0.42-4.37 | ||||||
| Intermediate | 1.09 | 0.33-3.58 | 1.24 | 0.38-4.04 | ||||||
| Donor | 176 | .038 | .077 | .3 | ||||||
| MRD | — | — | — | — | — | — | ||||
| MMRD/MMURD | 1.96 | 1.00-3.85 | 2.10 | 1.08-4.08 | 1.92 | 0.76-4.86 | ||||
| MURD | 0.88 | 0.49-1.58 | 1.18 | 0.67-2.06 | 1.02 | 0.47-2.25 | ||||
| PopPK cAUC (InsightRX Nova) | 171 | 0.97 | 0.93-1.01 | .085 | 0.98 | 0.94-1.01 | .14 | 0.99 | 0.94-1.03 | .6 |
| PopPK cAUC (InsightRX Nova), binary | 171 | .020 | .020 | |||||||
| <59.5 | — | — | — | — | — | — | .057 | |||
| ≥59.5 | 0.53 | 0.31-0.89 | 0.55 | 0.33-0.89 | 0.50 | 0.26, 0.99 | ||||
| NCA cAUC (WinNonLin, Certara) | 176 | 1.00 | 0.96-1.04 | > .9 | 0.99 | 0.95-1.03 | .7 | |||
| Characteristic . | N . | OS . | RFS . | TRM . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | ||
| Age | 176 | 1.02 | 1.00-1.04 | .005 | 1.02 | 1.01-1.04 | .003 | 1.05 | 1.02-1.09 | < .001 |
| Gender | 176 | > .9 | .5 | .7 | ||||||
| Female | — | — | — | — | — | — | ||||
| Male | 1.02 | 0.62-1.66 | 1.15 | 0.73-1.82 | 0.89 | 0.47-1.69 | ||||
| Disease | 176 | > .9 | .5 | > .9 | ||||||
| AML | — | — | — | — | — | — | ||||
| MDS | 0.99 | 0.61-1.60 | 1.17 | 0.75-1.83 | 1.04 | 0.55-1.98 | ||||
| DRI∗ | 172 | .017 | .005 | |||||||
| Low | — | — | — | — | — | — | ||||
| High | 5.30 | 1.22-23.1 | 4.76 | 1.40-16.2 | ||||||
| Intermediate | 2.77 | 0.67-11.4 | 2.18 | 0.68-6.95 | ||||||
| CMV status before HCT | 143 | .11 | .4 | .2 | ||||||
| Negative | — | — | — | — | — | — | ||||
| Positive | 1.52 | 0.90-2.56 | 1.24 | 0.77-1.99 | 1.48 | 0.76-2.87 | ||||
| HCT-CI∗ | 168 | > .9 | .8 | |||||||
| Low | — | — | — | — | — | — | ||||
| High | 1.10 | 0.34-3.60 | 1.35 | 0.42-4.37 | ||||||
| Intermediate | 1.09 | 0.33-3.58 | 1.24 | 0.38-4.04 | ||||||
| Donor | 176 | .038 | .077 | .3 | ||||||
| MRD | — | — | — | — | — | — | ||||
| MMRD/MMURD | 1.96 | 1.00-3.85 | 2.10 | 1.08-4.08 | 1.92 | 0.76-4.86 | ||||
| MURD | 0.88 | 0.49-1.58 | 1.18 | 0.67-2.06 | 1.02 | 0.47-2.25 | ||||
| PopPK cAUC (InsightRX Nova) | 171 | 0.97 | 0.93-1.01 | .085 | 0.98 | 0.94-1.01 | .14 | 0.99 | 0.94-1.03 | .6 |
| PopPK cAUC (InsightRX Nova), binary | 171 | .020 | .020 | |||||||
| <59.5 | — | — | — | — | — | — | .057 | |||
| ≥59.5 | 0.53 | 0.31-0.89 | 0.55 | 0.33-0.89 | 0.50 | 0.26, 0.99 | ||||
| NCA cAUC (WinNonLin, Certara) | 176 | 1.00 | 0.96-1.04 | > .9 | 0.99 | 0.95-1.03 | .7 | |||
CMV, cytomegalovirus; HCT-CI, HCT comorbidity index; MMRD, mismatched related donor; MMURD, mismatched unrelated donor; MRD, matched related donor; MURD, matched unrelated donor.
The models for HCT-CI and DRI do not converge for the nonrelapse mortality end point.
Association between busulfan exposure and transplant outcomes. Busulfan exposure < 59.5 mg × h/L was associated with worse OS (A) and worse RFS (B); increased TRM was noted with the lowest and highest busulfan quartiles (C).
Association between busulfan exposure and transplant outcomes. Busulfan exposure < 59.5 mg × h/L was associated with worse OS (A) and worse RFS (B); increased TRM was noted with the lowest and highest busulfan quartiles (C).
In multivariate analysis, along with busulfan exposure, increasing age and higher DRI were the other 2 variables that were associated with worse OS and RFS, with an HR of 4.1 (0.93-18.1) and 3.72 (1.08-12.8), respectively, in patients with high DRI (Table 3).
Multivariate analysis for OS and RFS
| Characteristic . | OS . | RFS . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR1 . | 95% CI . | P value . | |
| Age | 1.04 | 1.02-1.06 | < .001 | 1.03 | 1.01-1.05 | < .001 |
| DRI | .045 | .012 | ||||
| Low | — | — | — | — | ||
| High | 4.10 | 0.93-18.1 | 3.72 | 1.08-12.8 | ||
| Intermediate | 2.11 | 0.51-8.72 | 1.61 | 0.50-5.18 | ||
| Donor | .002 | .008 | ||||
| MRD | — | — | — | — | ||
| MMRD/MMURD | 3.00 | 1.47-6.15 | 2.91 | 1.44-5.87 | ||
| MURD | 0.87 | 0.48-1.59 | 1.15 | 0.64-2.06 | ||
| PopPK cAUC (InsightRX Nova), binary | .020 | .045 | ||||
| <59.5 | — | — | — | — | ||
| ≥59.5 | 0.50 | 0.29-0.88 | 0.58 | 0.34-0.97 | ||
| Characteristic . | OS . | RFS . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR1 . | 95% CI . | P value . | |
| Age | 1.04 | 1.02-1.06 | < .001 | 1.03 | 1.01-1.05 | < .001 |
| DRI | .045 | .012 | ||||
| Low | — | — | — | — | ||
| High | 4.10 | 0.93-18.1 | 3.72 | 1.08-12.8 | ||
| Intermediate | 2.11 | 0.51-8.72 | 1.61 | 0.50-5.18 | ||
| Donor | .002 | .008 | ||||
| MRD | — | — | — | — | ||
| MMRD/MMURD | 3.00 | 1.47-6.15 | 2.91 | 1.44-5.87 | ||
| MURD | 0.87 | 0.48-1.59 | 1.15 | 0.64-2.06 | ||
| PopPK cAUC (InsightRX Nova), binary | .020 | .045 | ||||
| <59.5 | — | — | — | — | ||
| ≥59.5 | 0.50 | 0.29-0.88 | 0.58 | 0.34-0.97 | ||
MMRD, mismatched related donor; MMURD, mismatched unrelated donor; MRD, matched related donor; MURD, matched unrelated donor.
Engraftment
One patient died before day 30 and was, therefore, not evaluable for engraftment. Otherwise, all patients achieved engraftment with a median time to neutrophil engraftment of 10 days (range, 8-20), with similar engraftment rates in both pediatric and adult patients.
TRM
Among all patients, the 2 years cumulative incidence of TRM was 20% (95% CI, 14-26). Busulfan exposure < 59.5 mg × h/L showed a trend toward a higher risk of TRM than exposure ≥59.5 mg × h/L (HR, 0.5; 95% CI, 0.26-0.99; P = .057). Interestingly, when comparing the TRM between the 4 quartiles, the TRM rates were similar in the lowest and highest quartiles and higher than the rates observed in quartiles 2 and 3 (Figure 2C). Increasing age was the only variable that was significantly associated with TRM (HR, 1.05; 95% CI, 1.02-1.09; P ≤ .001; Table 2).
Relapse
Among all patients, the 5-year cumulative incidence of relapse was 24% (95% CI, 17-31). Although patients with the highest busulfan exposure (quartile 4 with exposure > 68.9 mg × h/L) had the lowest relapse rates when compared with the other quartiles, this result was not statistically significant (HR, 0.31; 95% CI, 0. 11-0.9; P = .1; supplemental Figure 1A). High DRI was the only variable that was associated with a higher risk of relapse (HR, 3.25; 95% CI, 0.92-11.6; P = .006)
GVHD
The cumulative incidence of acute GVHD by day 100 was 30% (95% CI, 24-37), with no difference according to busulfan exposure (supplemental Figure 1B) or any other variable. The vast majority of aGVHD cases were grades 1 and 2 (95%), and only 5% were grade 3, with no cases of grade 4 GVHD.
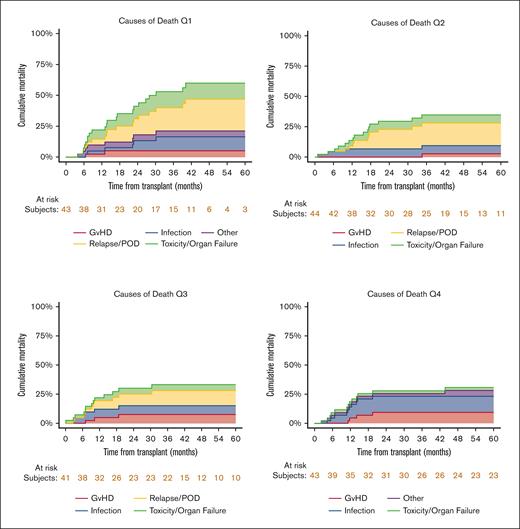
Cause of death
The causes of death distribution was different among the 4 quartiles (Figure 3), with no mortality due to relapse noted in the fourth quartile (highest busulfan exposure group).
Cause of death distribution among the 4 busulfan quartiles. Relapse did not account for any cases of mortality in the fourth quartile (highest exposure).
Cause of death distribution among the 4 busulfan quartiles. Relapse did not account for any cases of mortality in the fourth quartile (highest exposure).
Discussion
In this analysis of pediatric and adult patients with myeloid malignancies who underwent TCD allo-HCT using a homogeneous, chemotherapy-only conditioning regimen, we demonstrated that the cumulative busulfan exposure before allo-HCT was significantly associated with OS, suggesting that optimizing busulfan exposure using a popPK model may improve HCT outcomes. Optimal exposure of >59.5 mg × hL was found. Because clinically there is no reason to believe that a cutoff of 59.5 mg × h/L behaves differently from a cutoff of 60 mg × h/L, the recommendation from this analysis is to aim for busulfan levels >60 mg × h/L.
In a previous analysis, including >650 pediatric and young adult patients (up to age 34 years)13 who underwent an unmodified allo-HCT using busulfan-containing conditioning regimens, the optimal busulfan exposure, re-estimated for all patients by following the published popPK model, was found to be from 78 to 101 mg × h/L (for single- and double-alkylator conditioning). Exposures <78 mg × h/L and >101 mg × h/L were associated with significantly lower event-free survival due to relapse/graft failure (<78 mg × h/L) and toxicity (>101 mg × h/L). In this analysis as well as in a study by Bognar et al,29 the addition of an alkylator agent increased toxicity compared with single alkylator conditioning regimens, even when the total exposure was the same. In this study, using a homogenous conditioning regimen with 2 alkylator agents (busulfan and melphalan), a lower threshold (in this mainly adult cohort of patients) was identified, in which inferior OS and RFS were observed in patients with busulfan exposure <59.5 mg × h/L. In this analysis, higher levels of busulfan exposure were not associated with worse outcomes, though it is important to indicate that the highest exposure in this cohort was 90.7 mg × h/L, which is within the optimal range found by Bartelink et al. 113
Previous studies in adults who underwent unmodified allo-HCT with myeloablative conditioning regimens containing busulfan, such as BU/FLU/TBI16 or BU/Cy,30 reported worse OS with high busulfan exposure, with significantly higher TRM in patients with high busulfan exposure than in those with low exposure. However, it is important to note that different busulfan PK models were used in these studies (trapezoidal AUC estimation and not popPK estimation), which limits the ability to compare the actual levels of busulfan exposure between different studies, because these exposure estimations do not consistently match those of the popPK model.13
To the best of our knowledge, this is the first study assessing the association between busulfan exposure and key outcomes in patients undergoing TCD-selected allo-HCT. A recent prospective randomized Blood & Marrow Transplant Clinical Trials Network (BMT CTN) study comparing TCD-selected allo-HCT with an unmodified allo-HCT with either tacrolimus and methotrexate or posttransplant cyclophosphamide, tacrolimus, and mycophenolate mofetil for GVHD prophylaxis reported worse OS for patients treated in the TCD arm, mostly driven by increased TRM secondary to infectious complications and organ failure.6 The results of the current analysis suggest that targeting busulfan levels higher than 59.5 mg × h/L (60 mg × h/L) may improve OS without increasing TRM among patients undergoing a TCD allo-HCT, with results similar to those reported in the randomized CTN trial for the 2 other arms.
Despite having a homogenous study population regarding the conditioning regimen and allo-HCT platform, the relatively small size of the cohort limited our ability to define a firm optimal range beyond the threshold of 59.5 mg × h/L (60 mg × h/L). However, a higher busulfan exposure was associated with a lower incidence of relapse suggesting that an exposure target level from 59.5 or 60 mg × h/L to 68.8 mg × h/L may provide better disease control without increasing TRM in patients with AML/MDS undergoing TCD allo-HCT with a chemotherapy-only regimen. However, this needs to be confirmed prospectively. Given the variation in busulfan TDM practices across different centers, studies have found inaccuracies in up to 29% of dosing recommendations provided based on first-dose AUC calculations.31 Use of a popPK model to estimate exposure has the benefit of integrating all relevant PK information across a range of doses and populations, identifying covariates that influence exposure, and incorporating age-specific clearance parameters. Additionally, the Food and Drug Administration and EMA have recognized published popPK analysis as their preferred methodology for exposure analysis (Food and Drug Administration 202232 and EMEA 200733), and the use of these methods may optimize outcomes through a more accurate estimation of cumulative busulfan exposure. For this analysis, it was difficult to determine the exact cause of worse survival in the lower exposure group (<59 mg × h/L); TRM and relapse in the lower quartile did not reach significance, but were both visually higher in the low exposure than those in the high exposure group. Thus, the different survival was a composite of these 2 main causes of failure: increased relapsed-related death (Figure 3A) and increased TRM. Based on other reports by Bartelink et al, we speculated that lower exposure was associated with worse graft function, which can be associated with increased infections.
In summary, this analysis adds to the growing literature supporting the importance of TDM and precision drug dosing of conditioning regimen agents and their association with allo-HCT outcomes in both unmodified and TCD-selected allo-HCTs. 113,24,34,35 Moreover, our group is actively studying precision conditioning regimen drug dosing in the context of TCD allo-HCT in an actively accruing, prospective clinical trial (NCT04872595).
Acknowledgments
This research was supported, in part, by the National Institutes of Health award number P01 CA23766 and NIH/NCI Cancer Center support grant P30 CA008748. The content is the sole responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: R.T., M.S., and J.J.B. substantially contributed to the conception and design of the study; R.T., M.S., B.M.K., A.P., A.L., J.F., C.C., S.D., E.K., F.B., M.I.C., K.J.C., A.A.J., N.A.K., A.L.K., R.J.O., E.B.P., S.P., A.S., B.C.S., G.S., B.S., B.G., S.A.G., M.-A.P., and J.J.B. helped acquire, analyze, and interpret data; R.T., M.S., B.M.K., A.P., A.L., J.F., C.C., S.D., E.K., F.B., M.I.C., K.J.C., A.A.J., N.A.K., A.L.K., R.J.O., E.B.P., S.P., A.S., B.C.S., G.S., B.S., B.G., S.A.G., M.-A.P., and J.J.B. helped draft, critically review, and revise the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: R.T. serves as a BMT CTN medical monitor for Angiocrine Bioscience and Omeros; M.S. received research support from Angiocrine Bioscience and Omeros; served as a consultant for Angiocrine Bioscience, Omeros, and McKinsey; was a member of an ad hoc advisory board for Kite Pharma; and had a one-time speaking commitment for i3Health. A.P has Roche stock. K.J.C. served as a consultant for Mesoblast; served as a consultant and received research funding from Novartis; and received research funding from Celgene. N.A.K. holds equity in Amgen, Johnson & Johnson, Pfizer, and Merck. R.J.O. served as a consultant for, holds patents with, and receives royalties from an EBV-specific T-cell bank and research funding from Atara Biotherapeutics. S.P. served on the advisory board of CellEvolve, ADMA, and Smart Immune; received honoraria from Regeneron; received support for conducting clinical trials from Atara Biotherapeutics and Jasper Pharmaceuticals; and is listed as an inventor of intellectual property licensed to Atara by MSKCC, with all her rights assigned to MSKCC. A.S. served as a member of the board of directors and advisory committees for Excellthera. G.S. received research funding from Janssen, Amgen, Bristol Myers Squibb (BMS), and Beyond Spring; and serves as a data safety monitoring board member of Arcellx. S.A.G. served as a consultant for and received honoraria and research funding from Celgene and Novartis; served as a consultant for and received research funding from Amgen, Actinuum, and Miltenyi Biotech; served as a consultant for and received honoraria from Jazz Pharmaceuticals and Omeros; received research funding from Takeda; and served as a consultant for Kite Pharma. M.-A.P. has received honoraria from AbbVie, Astellas, BMS, Celgene, Equilium, Incyte, Karyopharm, Kite Pharma/Gilead, Merck, Miltenyi Biotec, MorphoSys, Novartis, Nektar Therapeutics, Omeros, Takeda, VectivBio AG, and Vor Biopharma; served on data and safety monitoring boards for Cidara Therapeutics, Medigene, Sellas Life Sciences, and Servier; served on a scientific advisory board for NexImmune; has ownership interests in NexImmune and Omeros; has received research support for clinical trials from Incyte, Kite Pharma/Gilead, Miltenyi Biotec, and Novartis; serves as a volunteer member of the board of directors of the American Society for Transplantation and Cellular Therapy and BeTheMatch (National Marrow Donor Program); and serves on the Cellular Immunotherapy Data Resource Executive Committee for the Center for International Blood and Marrow Transplant Research. J.J.B. served as a consultant for Race Oncology, Omeros, Avrobio, Takeda, Advanced Clinical, and Bluerock. The remaining authors declare no competing financial interests.
Correspondence: Roni Tamari, Department of Medicine, Bone Marrow Transplant Service, Memorial Sloan Kettering Cancer Center, Department of Medicine, Weill Cornell Medical College, David H. Koch Center, 530 E 74th St, New York, NY 10021; e-mail: tamarir@mskcc.org.
References
Author notes
∗R.T. and M.S. are joint first authors.
Original data are available on request from the corresponding author, Roni Tamari (tamarir@mskcc.org).
The full-text version of this article contains a data supplement.