TO THE EDITOR:
The B-cell leukemia/lymphoma-2 selective inhibitor venetoclax in combination with cytarabine or hypomethylating agents has been reported to be safe and efficacious in treating adult patients with newly diagnosed acute myeloid leukemia (AML).1-3 Its role in pediatric myeloid neoplasms is still under evaluation. A phase 1/2 study of venetoclax in combination with cytarabine, with or without idarubicin, for the treatment of pediatric relapsed or refractory (r/r) AML showed an overall response rate of 80%, with generally manageable toxicities.4 More recent retrospective studies support the use of venetoclax-containing regimens in AML or myelodysplastic syndrome (MDS), particularly as a bridge therapy for allogeneic hematopoietic stem cell transplantation (HSCT).5,6 Here, we report a multicenter retrospective analysis of pediatric patients (0-18 years) with r/r AML, postcytotoxic therapy (chemotherapy or radiation therapy) MDS/AML, or advanced MDS who received venetoclax-based combination therapies.
Clinical data were collected using an electronic case report form compiled by treating physicians at the participating centers. Advanced MDS was defined as MDS with excess blasts per the International Consensus Classification.7,8 The response was classified as complete (CR) when blasts were ≤5% in the bone marrow (BM), with absence of extramedullary disease; partial (PR) when the BM blast percentage decreased to 5% or 20% or there was a decrease in pretreatment BM blast percentage >50%; and nonresponse (NR) when there was an increase or persistence of BM blasts ≥20% or a reduction of blast percentage <50%.9 CR with incomplete count recovery was defined as CR with a platelet count < 100 × 109/L and/or absolute neutrophil count < 1 × 109/L. Treatment failure (TF) was defined as the need for treatment interruption because of toxicities, making the response inevaluable. Adverse events were reported using the Common Terminology Criteria for Adverse Events version 5.0. Approval was obtained from the institutional review board of each institution, and parental informed consent was obtained in accordance with the Declaration of Helsinki.
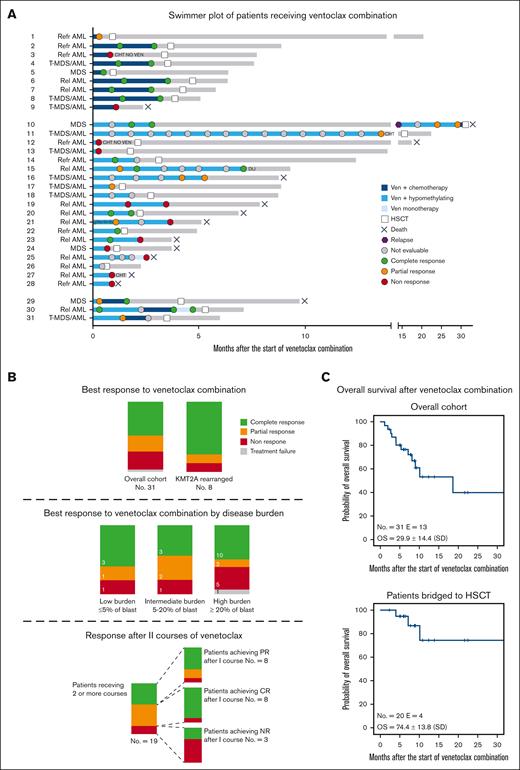
Thirty-one patients were included in the analysis; their clinical characteristics and summary of treatments are reported in Table 1 and Figure 1A. Detailed information of the patients is reported in supplemental Tables 1, 2, and 3. Median age was 10.2 years (range, 1.3-17.4 years). Diagnoses included advanced MDS (n = 4), r/r AML (n = 18), and postcytotoxic MDS/AML (n = 9). Patients had received a median of 3 previous lines of therapy (range, 1-7) before being exposed to venetoclax. Thirteen (31.2%) patients relapsed after a previous allogeneic HSCT. Relevant molecular/cytogenetic alterations included KMT2A-rearrangements (n = 8), monosomy 7 or deletion of 7q (n = 6), FLT3-ITD mutations (n = 5), and a structurally complex karyotype (n = 2). Patients received a median of 2 cycles of venetoclax (range, 1-15), which was administered at a median dose of 350 mg/m2 daily (range, 125-500). Nineteen patients received hypomethylating agents (azacitidine or decitabine) in combination with venetoclax. Nine patients were treated with venetoclax and cytotoxic agents, including cytarabine, fludarabine, idarubicin, or liposomal doxorubicin. Three patients received both hypomethylating and chemotherapy courses. Dose escalation of venetoclax at the beginning of the first cycle was performed in 25 of 31 patients. Venetoclax was administered for a median duration of 28 days (range, 9-28 days), with 21 patients adopting a 28-day and 10 adopting a 14- to 21-day cycle. Adverse events occurring during venetoclax therapies included grade 4 pancytopenia (n = 1), grade 3 prolonged neutropenia (n = 3), grade 3 fungal infection (n = 3), grade 3 acute kidney injury after azacitidine (n = 1), grade 2 diarrhea (n = 2), grade 2 sensitive neuropathy (n = 1), and grade 2 hypokalemia (n = 1). Patient 28 discontinued venetoclax because of severe pancytopenia after 9 days, and this was recorded as a TF. The median percentage of blasts at the start of venetoclax treatment was 20% (range, 0%-80%). Extramedullary disease was present in 2 patients with AML, 1 each with central nervous system and skin involvement. The median number of cycles required to achieve the best response was 1 (range, 1-7). In the whole cohort, the best response achieved was CR, PR, and NR in 16 (51.6%), 6 (19.4%), and 8 (25.8%) children, respectively. Of the 16 patients who achieved CR, 4 were tested flow cytometry-minimal residual disease-negative and 2 were tested polymerase chain reaction-minimal residual disease-negative, whereas 5 presented a CR with incomplete count recovery. Patients who received a combination of venetoclax with cytotoxic agents achieved CR, PR, and NR in 6 (66.7%), 1 (11.1%), and 2 (22.2%) cases, respectively, whereas patients receiving venetoclax with hypomethylating agents achieved CR, PR, NR, and TF in 7 (36.8%), 5 (26.3%), 6 (31.6%), and 1 (5.3%) case, respectively. Patients with KMT2A rearrangements achieved CR or PR in 6 (75.0%) and 1 (12.5%) cases, respectively. Of the 4 patients with advanced MDS, 3 achieved CR, whereas 1 did not respond. The analysis of response based on the burden of diseases is shown in Figure 1B. Eight patients experienced PR after the first cycle and received a second cycle, achieving CR, PR, and NR in 5 (62.5%), 2 (25.0%), and 1 (12.5%) case (Figure 1B), respectively. One patient with isolated extramedullary skin disease achieved CR after 7 cycles of venetoclax and azacitidine, as documented via a fluorodeoxyglucose-positron emission tomography and skin biopsy. Twenty patients received allogeneic HSCT after venetoclax bridging therapy; transplantation was performed at a median time of 3.3 months (range, 0.6-31.1 months) from the start of venetoclax. These patients received transplantation from an HLA-haploidentical relative (n = 6), an HLA-matched unrelated volunteer (n = 12), or a compatible sibling (n = 2) after myeloablative conditioning. Median follow-up of the whole cohort was 7.7 months (range, 1.1-32.3 months). The estimated overall survival (OS) at 30 months after the start of venetoclax treatment was 29.9% ± 14.4 (standard error) for the whole cohort and 74.4% ± 13.8 (standard error) for patients undergoing HSCT (Figure 1C). Thirteen patients died by the end of follow-up, with a median time to death from the start of venetoclax of 5.3 months (range, 1.1-32.3 months). Causes of death included disease progression before HSCT in 8 patients, transplantation-related mortality in 3, and disease relapse after HSCT in 2.
Patient characteristics
| Patients (n = 31) . | n (% or range) . |
|---|---|
| Sex | |
| Male | 18 (58.1) |
| Female | 13 (41.9) |
| Diagnosis | |
| Advanced MDS | 4 (23.5) |
| Relapsed AML | 11 (35.5) |
| Refractory AML | 7 (22.6) |
| Postcytotoxic therapy MDS/AML | 9 (29.0) |
| Age, y, median (range) | 10.2 (1.3-17.4) |
| Extramedullary disease | |
| No | 29 (93.5) |
| Yes | 2 (6.5) |
| BM blast at diagnosis, median (range) | 25% (0-95) |
| BM blasts before the first venetoclax therapy, median (range) | 20% (0-80) |
| Previous lines of therapies since last complete response, median (range) | 3 (1-7) |
| 0 | 4 (23.5) |
| 1 | 3 (17.6) |
| 2 | 2 (11.8) |
| ≥ 2 | 8 (45.1) |
| Previous HSCT | |
| 0 | 18 (58.1) |
| 1 | 9 (29.0) |
| 2 | 4 (12.9) |
| Unfavorable genetics | |
| KMT2A rearrangements | 8 (25.8) |
| - 7/del7q | 6 (19.3) |
| FLT3-ITD | 5 (16.1) |
| Complex karyotype | 2 (6.5) |
| KRAS | 2 (6.5) |
| PTPN11 | 1 (3.2) |
| No. of venetoclax cycles per patient, median (range) | 2 (1-15) |
| Best response achieved | |
| CR∗ | 16 (51.6) |
| PR | 6 (19.4) |
| ORR | 22 (71.0) |
| NR | 8 (25.8) |
| TF | 1 (3.2) |
| No. of cycles to best response (in 22 responders), median, range | 1 (1-7) |
| Patients (n = 31) . | n (% or range) . |
|---|---|
| Sex | |
| Male | 18 (58.1) |
| Female | 13 (41.9) |
| Diagnosis | |
| Advanced MDS | 4 (23.5) |
| Relapsed AML | 11 (35.5) |
| Refractory AML | 7 (22.6) |
| Postcytotoxic therapy MDS/AML | 9 (29.0) |
| Age, y, median (range) | 10.2 (1.3-17.4) |
| Extramedullary disease | |
| No | 29 (93.5) |
| Yes | 2 (6.5) |
| BM blast at diagnosis, median (range) | 25% (0-95) |
| BM blasts before the first venetoclax therapy, median (range) | 20% (0-80) |
| Previous lines of therapies since last complete response, median (range) | 3 (1-7) |
| 0 | 4 (23.5) |
| 1 | 3 (17.6) |
| 2 | 2 (11.8) |
| ≥ 2 | 8 (45.1) |
| Previous HSCT | |
| 0 | 18 (58.1) |
| 1 | 9 (29.0) |
| 2 | 4 (12.9) |
| Unfavorable genetics | |
| KMT2A rearrangements | 8 (25.8) |
| - 7/del7q | 6 (19.3) |
| FLT3-ITD | 5 (16.1) |
| Complex karyotype | 2 (6.5) |
| KRAS | 2 (6.5) |
| PTPN11 | 1 (3.2) |
| No. of venetoclax cycles per patient, median (range) | 2 (1-15) |
| Best response achieved | |
| CR∗ | 16 (51.6) |
| PR | 6 (19.4) |
| ORR | 22 (71.0) |
| NR | 8 (25.8) |
| TF | 1 (3.2) |
| No. of cycles to best response (in 22 responders), median, range | 1 (1-7) |
ORR, overall response rate.
Five with morphological CR; 4 who tested flow cytometry-minimal residual disease negative; 2 who tested polymerase chain reaction-minimal residual disease negative (with extramedullary complete remission detected upon fluorodeoxyglucose-positron emission tomography and skin biopsy in 1 case); and 5 with CR with incomplete recovery.
Treatments and responses of patients treated with venetoclax-based combinations. (A) Swimmer plot of patients receiving venetoclax-based combinations. Patients are divided into 3 groups based on having received venetoclax plus chemotherapy (top), venetoclax plus hypomethylating agents (middle), and both (bottom). Dots represent disease evaluation, and response is specified in the legend. (B) Summary of response for the overall cohort, for KMT2A-rearranged patients (top), and for patients with low (≤5% of blast), intermediate (5%-20% of blast), and high disease burden (≥ 20% of blast) (middle) before venetoclax start. (Bottom) The larger bar represents the response after the first cycle for patients receiving 2 or more cycles of venetoclax-based combination. The smaller bars represent the response after the second cycle for patients achieving CR, PR, and NR after the first cycle. (C) Kaplan-Meier estimates of the OS for the entire cohort (top) and patients bridged to HSCT (bottom). Rel, relapsed; Refr, refractory; Ven, venetoclax.
Treatments and responses of patients treated with venetoclax-based combinations. (A) Swimmer plot of patients receiving venetoclax-based combinations. Patients are divided into 3 groups based on having received venetoclax plus chemotherapy (top), venetoclax plus hypomethylating agents (middle), and both (bottom). Dots represent disease evaluation, and response is specified in the legend. (B) Summary of response for the overall cohort, for KMT2A-rearranged patients (top), and for patients with low (≤5% of blast), intermediate (5%-20% of blast), and high disease burden (≥ 20% of blast) (middle) before venetoclax start. (Bottom) The larger bar represents the response after the first cycle for patients receiving 2 or more cycles of venetoclax-based combination. The smaller bars represent the response after the second cycle for patients achieving CR, PR, and NR after the first cycle. (C) Kaplan-Meier estimates of the OS for the entire cohort (top) and patients bridged to HSCT (bottom). Rel, relapsed; Refr, refractory; Ven, venetoclax.
In this study, we reported the largest pediatric cohort of high-risk myeloid malignancies treated with different venetoclax-based therapies. Drug administration was variable, with a median daily dose of 350 mg/m2, consistent with the phase 2 study that recommended 360 mg/m2.4 The optimal duration of venetoclax treatment in children remains to be fully elucidated, and current trials are using different schemes. In our cohort, venetoclax-based therapies were safe, with only 1 TF due to adverse events, confirming a generally favorable toxicity profile in pediatric patients.4-6 The response rate was also remarkable, with an overall response rate of 71% and a CR of 51.6%, which are comparable with those in previous reports.4,5 CR was achieved in 3 of the 4 patients with MDS. Considering the variability of diagnosis and disease stage in our cohort, further studies are needed to more precisely assess the efficacy of different venetoclax-based approaches in diverse settings. Notably, of the 8 patients with KMT2A rearrangements, 6 achieved CR and 1 achieved PR. Preclinical data suggest a high antiapoptotic dependence and sensitivity to venetoclax in in vitro models of KMT2A-rearranged B-cell lymphoblastic and myeloid leukemia.10,11 Recently, preliminary results from a retrospective adult KMT2A-rearranged cohort showed a high response rate when venetoclax was combined with hypomethylating agents.12 Five patients presented FLT3-internal tandem duplication (ITD) mutations, previously reported to be associated with venetoclax resistance in some experimental models.13 Among them, 3 achieved PR, 1 did not respond, and 1 patient with FLT3-mutated MDS achieved CR but relapsed after HSCT. Further studies are needed to assess the possible roles of different genetic lesions in venetoclax resistance.14,15 In our cohort, venetoclax combinations induced responses, even in patients with high disease burdens. Thus, considering the heavily pretreated population, venetoclax seems to be an effective option as a bridge to transplantation. We recorded favorable OS in patients receiving HSCT for consolidation despite the fact that no conclusion can be made considering the limitations of a relatively short follow-up and a small cohort. Notably, all patients who achieved CR without complete recovery of blood counts received HSCT and were alive at the end of the follow-up. The feasibility of venetoclax-based therapy as a bridge to HSCT was also recently investigated by Pfeiffer et al in pediatric patients, showing no significant impact on the transplantation-related mortality, graft-versus-host diease, and engraftement.6 Interestingly, all patients with postcytotoxic MDS/AML bridged to HSCT were alive at the last follow-up, similar to what was recently described in a pediatric cohort with secondary myeloid neoplasms treated with CPX-351 and bridged to HSCT.16
Although the heterogeneity in venetoclax dosage, the combination with either cytotoxic agents or hypomethylating agents, and the length of treatment represent limitations of this study, our findings confirm the activity and safety of venetoclax combinations in pediatric high-risk myeloid neoplasms, with durable responses and promising OS in patients bridged to HSCT. Larger prospective, possibly randomized, studies are needed to confirm the efficacy of venetoclax and its role in the different phases of AML/MDS treatment.
Acknowledgments: This study was supported by research funding from Italian Fondazione AIRC (AIRC IG 26039) (R.M.). This work was supported in part by the Fondazione Umberto Veronesi (Milan) (to F.L., PALM project).
Contribution: R.M. and F.B. designed the study; A.D.G., F.V., M.B., M.L., M.T., L.V., M.E., and B.S. collected the patient data; D.L., F.B., and F.G. designed the figures and performed the analysis; and R.M., C.M.N., and F.L. critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Davide Leardini, Pediatric Oncology and Hematology “Lalla Seràgnoli,” IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy; e-mail: davide.leardini3@studio.unibo.it.
References
Author notes
∗R.M. and F.B. contributed equally to this study and are joint first authors.
Data are available on request from the corresponding author, Davide Leardini (davide.leardini3@studio.unibo.it).
The full-text version of this article contains a data supplement.