Key Points
Higher day 0 alemtuzumab levels predict MC and secondary GF after RIC HCT with T-cell–replete grafts.
Day 0 alemtuzumab and post-HCT CXCL9 levels correlated inversely, suggesting less graft-versus-marrow effect with more lymphodepletion.
Abstract
Overall survival after reduced-intensity conditioning (RIC) allogeneic hematopoietic cell transplantation (HCT) using alemtuzumab, fludarabine, and melphalan is associated with high rates of mixed chimerism (MC) and secondary graft failure (GF). We hypothesized that peritransplantation alemtuzumab levels or specific patterns of inflammation would predict these risks. We assessed samples from the Bone Marrow Transplant Clinical Trials Network 1204 (NCT01998633) to study the impact of alemtuzumab levels and cytokine patterns on MC and impending or established secondary GF (defined as donor chimerism <5% after initial engraftment and/or requirement of cellular intervention). Thirty-three patients with hemophagocytic lymphohistiocytosis (n = 25) and other IEIs (n = 8) who underwent HCTs with T-cell–replete grafts were included. Patients with day 0 alemtuzumab levels ≤0.32 μg/mL had a markedly lower incidence of MC, 14.3%, vs 90.9% in patients with levels >0.32 μg/mL (P = .008). Impending or established secondary GF was only observed in patients with day 0 alemtuzumab levels >0.32 μg/mL (P = .08). Unexpectedly, patients with impending or established secondary GF had lower CXCL9 levels. The cumulative incidence of impending or established secondary GF in patients with a day 14+ CXCL9 level ≤2394 pg/mL (day 14+ median) was 73.6% vs 0% in patients with a level >2394 pg/mL (P = .002). CXCL9 levels inversely correlated with alemtuzumab levels. These data suggest a model in which higher levels of alemtuzumab at day 0 deplete donor T cells, inhibit the graft-versus-marrow reaction (thereby suppressing CXCL9 levels), and adversely affect sustained engraftment in the nonmyeloablative HCT setting. This trial was registered at www.clinicaltrials.gov as #NCT01998633
Introduction
Allogeneic hematopoietic cell transplantation (HCT) offers curative therapy for patients with genetic hemophagocytic lymphohistiocytosis (HLH) disorders and other inborn errors of immunity (IEIs). Reduced-intensity conditioning (RIC) regimens containing alemtuzumab, fludarabine, and melphalan have been studied as an alternative to myeloablative conditioning (MAC) for these patients because of the lower risk of treatment-related toxicities and early mortality. Another potential benefit of a RIC approach is decreased pathologic inflammation during conditioning and the peritransplantation period.1,2 Patients with uncontrolled HLH at the time of HCT have worse outcomes than those without active inflammation.3 Alemtuzumab, which targets the CD52 antigen present on most mature blood mononuclear cells, efficiently depletes these cells, reduces inflammation, and has been used successfully to treat some patients with highly refractory HLH.4
Although alemtuzumab, fludarabine, and melphalan RIC HCT has been associated with improved survival, it is also associated with high rates of mixed chimerism (MC) and a lack of sustained donor engraftment, which often results in secondary graft failure (GF).1,5,6 The Bone Marrow Transplant Clinical Trials Network (BMT CTN) prospectively assessed the safety and efficacy of alemtuzumab, fludarabine, and melphalan RIC HCT in a prospective, multicenter, phase 2 study (BMT CTN 1204, Reduced-Intensity Conditioning for Hemophagocytic Syndromes or Selected Primary Immune Deficiencies [RICHI]; NCT01998633) for patients with HLH and other IEIs. The 1-year overall survival was favorable at 80.4%, but survival with intervention-free sustained engraftment was only 39.1%.2 To better understand the mechanisms responsible for poor intervention-free sustained engraftment, we studied serial peripheral blood samples prospectively banked as part of the BMT CTN 1204 RICHI study. We hypothesized that peritransplantation alemtuzumab levels and specific patterns of inflammation influence the likelihood of sustained donor engraftment. The primary objective of this study was to systematically evaluate alemtuzumab levels and cytokine patterns and their association with sustained high-level engraftment after RIC HCT.
Methods
Patients and samples
Patients enrolled in the BMT CTN 1204 RICHI study (NCT01998633) (n = 47) were eligible for this correlative biology study. Consenting patients provided peripheral blood samples on predefined days [day −14 (window, from day −28 to −14), day −7 (±1 day), day −1 (±1 day), day +1 (window, from day +1 to +3), day +14 (±2 days), day +28 (± 2 days), day +42 (±3 days), day +70 (±10 days), and day +100 (±10 days)]. Day 0 was the planned day for stem cell infusion. Blood samples were shipped to the National Heart, Lung, and Blood Institute Biologic Specimen Repository, operated by the National Marrow Donor Program, for processing and storage and were later released to the investigators. This manuscript was submitted on behalf of the BMT CTN.
Alemtuzumab and cytokine measurements
Alemtuzumab levels were measured using a flow cytometric assay modified from the methodology of Rebello and Hale,7 as previously reported.8 Samples were available for most patients: day −14 (n = 29; 88%), day −7 (n = 28; 85%), day −1 (n = 30; 91%), day +1 (n = 27; 82%), day +14 (n = 26; 79%), day +28 (n = 30; 91%), day +42 (n = 26; 79%), and day +70 (n = 24; 73%). Day 0 alemtuzumab levels were estimated by calculating the mean of day −1 and day +1 levels, unless only a single, either day −1 or +1, sample was available.
Comprehensive cytokine analysis was performed for 140 analytes using the MagPix platform (supplemental Table 1). Samples were available for most patients: day −14 (n = 27; 82%), day −1 (n = 28; 85%), day +14 (n = 26; 79%), day +28 (n = 29; 88%), day +42 (n = 26; 79%), and day +100 (n = 22; 67%). CXCL9 levels from healthy controls and patients with HLH, previously described by Lin et al, were used for comparison.9
Transplantation and outcomes
The RICHI study was a BMT CTN multicenter, phase 2 trial; the study design and inclusion criteria were previously published.2 The pre-HCT conditioning regimen included administering alemtuzumab from days −14 to −10 (total of 1 mg/kg, subcutaneous route with a maximum cumulative dose of 90 mg), fludarabine from days −8 to −4 (total of 150 mg/m2, or 5 mg/kg in patients <10 kg), and melphalan from day −3 (140 mg/m2, or 4.7 mg/kg in patients <10 kg). Details regarding HCT outcomes were previously reported.2 For this study, primary GF was defined as whole blood donor chimerism <5% before day +42. MC was defined as whole blood or sorted T-cell donor chimerism <95%. Impending secondary GF was defined as falling donor chimerism requiring donor lymphocyte infusion (DLI) and/or second HCT (with or without conditioning and including CD34+ selected stem cell boosts). Established secondary GF was defined as whole blood donor chimerism <5% after initial engraftment. Survival with intervention-free sustained engraftment was defined as survival without primary GF, impending secondary GF, or established secondary GF.
Statistical analysis
For analysis of cytokine levels, patients were grouped based on the occurrence of GF and box plots produced using BRB-Arraytools.10 Significant analytes were determined by analyzing the log-transformed concentrations using multivariate permutation tests with a maximum false discovery proportion of 0.1 at an 80% confidence level. Patient, disease, and transplantation characteristics were compared using Fischer exact test. Peritransplantation alemtuzumab levels were compared using a mixed-effects model and Sidak multiple comparisons test. Survival and cumulative incidence curves were created using the Kaplan-Meier method, and groups were compared with the log-rank test. For the probability of survival with intervention-free sustained engraftment, an event was defined as primary GF, impending secondary GF, established secondary GF, or death. For cumulative incidence curves, patients without MC, GF, or graft-versus-host disease (GVHD) were censored at last follow-up (up to 365 days) or death, as appropriate. Analyses were performed using GraphPad Prism version 9.0.0 for Windows, GraphPad Software, San Diego, CA (www.graphpad.com). Receiver operating characteristic (ROC) analyses were performed with XLSTAT (Addinsoft, Paris, France).
Results
Patient and transplantation characteristics
Thirty-three patients treated at 17 sites were included with underlying diagnoses of HLH (n = 25; 76%) or other IEIs (n = 8; 24%) (Table 1). Indications for HCT in patients with other IEIs were chronic active Epstein-Barr virus infection (n = 3), chronic granulomatous disease (n = 2), hyperimmunoglobulin M syndrome (n = 2), and immune dysregulation, polyendocrinopathy, and enteropathy, X-linked syndrome (n = 1). Twenty-two patients (67%) were male, and 27 (82%) were White. Ten patients (30%) were <1 year old at time of HCT, 18 (55%) were aged from 1 to 17 years old, and 5 patients (15%) were aged from 17 to 22 years old. All patients received unmanipulated bone marrow. Twenty-seven patients (82%) received a graft from an unrelated donor. Twenty-one patients (64%) received a graft from an 8/8 or 6/6 HLA-matched donor, and 12 (36%) received a graft from a 7/8 HLA-mismatched donor. Patients with HLH and other IEIs had similar demographics and transplantation characteristics except for their age at transplantation. No patients with other IEIs were <1 year old at the time of HCT, whereas 10 patients with HLH were <1 year old (P = .03).
Patient and transplantation characteristics
| . | All patients, n (%)∗ . | HLH, n (%)∗ . | Other IEIs, n (%)∗ . |
|---|---|---|---|
| Total patients | 33 (100%) | 25 (100%) | 8 (100%) |
| Diagnosis | |||
| HLH | 25 (76%) | 25 (100%) | 0 |
| CAEBV | 3 (9%) | 0 | 3 (38%) |
| CGD | 2 (6%) | 0 | 2 (25%) |
| HIGM | 2 (6%) | 0 | 2 (25%) |
| IPEX | 1 (3%) | 0 | 1 (12%) |
| Sex | |||
| Male | 22 (67%) | 17 (68%) | 5 (62%) |
| Female | 11 (33%) | 8 (32%) | 3 (38%) |
| Race | |||
| White | 27 (82%) | 20 (80%) | 7 (88%) |
| Non-White or not reported | 6 (18%) | 5 (20%) | 1 (12%) |
| Age at transplantation, y | |||
| <1 | 10 (30%) | 10 (40%) | 0 |
| 1-16 | 18 (55%) | 13 (52%) | 5 (63%) |
| 17-22 | 5 (15%) | 2 (8%) | 3 (37%) |
| Stem cell source | |||
| Marrow | 33 (100%) | 25 (100%) | 8 (100%) |
| Donor relation | |||
| Sibling | 5 (15%) | 5 (20%) | 0 |
| Other related | 1 (3%) | 0 | 1 (12%) |
| Unrelated | 27 (82%) | 20 (80%) | 7 (88%) |
| Patient/Donor HLA match | |||
| 8/8 or 6/6 | 21 (64%) | 16 (64%) | 5 (63%) |
| 7/8 | 12 (36%) | 9 (36%) | 3 (37%) |
| . | All patients, n (%)∗ . | HLH, n (%)∗ . | Other IEIs, n (%)∗ . |
|---|---|---|---|
| Total patients | 33 (100%) | 25 (100%) | 8 (100%) |
| Diagnosis | |||
| HLH | 25 (76%) | 25 (100%) | 0 |
| CAEBV | 3 (9%) | 0 | 3 (38%) |
| CGD | 2 (6%) | 0 | 2 (25%) |
| HIGM | 2 (6%) | 0 | 2 (25%) |
| IPEX | 1 (3%) | 0 | 1 (12%) |
| Sex | |||
| Male | 22 (67%) | 17 (68%) | 5 (62%) |
| Female | 11 (33%) | 8 (32%) | 3 (38%) |
| Race | |||
| White | 27 (82%) | 20 (80%) | 7 (88%) |
| Non-White or not reported | 6 (18%) | 5 (20%) | 1 (12%) |
| Age at transplantation, y | |||
| <1 | 10 (30%) | 10 (40%) | 0 |
| 1-16 | 18 (55%) | 13 (52%) | 5 (63%) |
| 17-22 | 5 (15%) | 2 (8%) | 3 (37%) |
| Stem cell source | |||
| Marrow | 33 (100%) | 25 (100%) | 8 (100%) |
| Donor relation | |||
| Sibling | 5 (15%) | 5 (20%) | 0 |
| Other related | 1 (3%) | 0 | 1 (12%) |
| Unrelated | 27 (82%) | 20 (80%) | 7 (88%) |
| Patient/Donor HLA match | |||
| 8/8 or 6/6 | 21 (64%) | 16 (64%) | 5 (63%) |
| 7/8 | 12 (36%) | 9 (36%) | 3 (37%) |
CAEBV, chronic active Epstein-Barr virus disease; CGD, chronic granulomatous disease; HIGM, hyperimmunoglobulin M syndrome; IPEX, immune dysregulation, polyendocrinopathy, and enteropathy, X-linked syndrome.
Column percentage given.
Primary GF, MC, and impending or established secondary GF
Information regarding sustained engraftment is summarized in supplemental Table 2. One patient (3%) with HLH experienced primary GF on day +28. Ten patients (30%), 7 with HLH and 3 with IEIs, had sustained full donor chimerism. Twenty-two patients (67%), 17 with HLH and 5 with IEIs, developed MC after HCT. Eleven patients (33%), 8 with HLH and 3 with IEIs, with MC did not require cell-product intervention. Eleven patients (33%) received cell-product intervention(s) for impending secondary GF. Seven patients (21%), 6 with HLH and 1 with IEI, were treated with cell-product intervention but did not develop graft loss. Four patients (12%), 3 with HLH and 1 with IEI, were diagnosed with established secondary GF due to graft loss despite cell-product intervention (supplemental Table 2). Among patients with impending or established secondary GF, 8 patients received DLI, 1 underwent a second HCT, and 2 received both DLI and a second HCT. The median time to the first post-HCT intervention was 90 days (range, 53-126 days).
Peritransplantation alemtuzumab levels
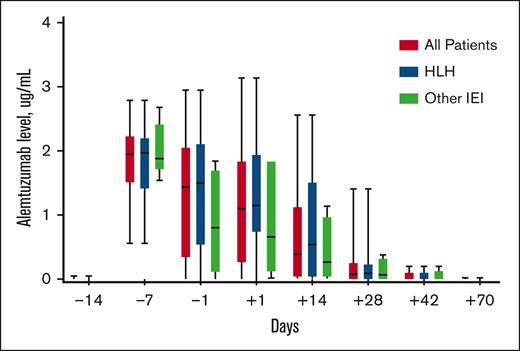
Peri-HCT alemtuzumab levels were similar in patients with HLH when compared with those with other IEIs (Figure 1). Levels peaked on day −7 in most cases, and all patients cleared alemtuzumab by day +70. The median day −1 alemtuzumab level was 1.44 μg/mL (interquartile range [IQR], 0.33-2.06), and the median day +1 level was 1.10 μg/mL (IQR, 0.25-1.85). Thirty patients (91%) had day −1 and/or day +1 samples, and the estimated day 0 alemtuzumab level (mean of day −1 and day +1) was median 1.29 μg/mL (IQR, 0.32-1.95).
Peritransplantation alemtuzumab levels. Box and whisker plots outline alemtuzumab levels measured on specific days in relation to HCT day 0. The boxes represent the 25th percentile, median, and 75th percentile on each specified day. The lines represent the minimum and maximum alemtuzumab levels on each specified day.
Peritransplantation alemtuzumab levels. Box and whisker plots outline alemtuzumab levels measured on specific days in relation to HCT day 0. The boxes represent the 25th percentile, median, and 75th percentile on each specified day. The lines represent the minimum and maximum alemtuzumab levels on each specified day.
Impact of alemtuzumab levels on MC and impending or established secondary GF
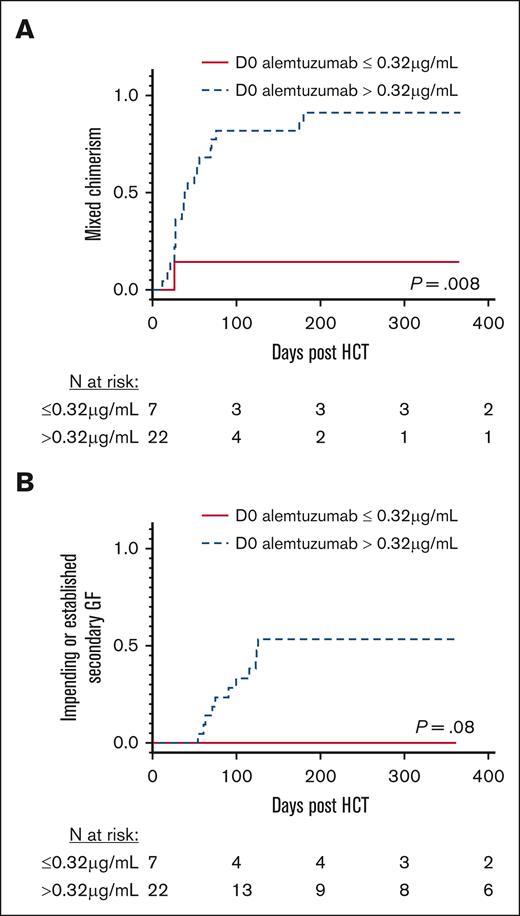
The in vitro and in vivo lytic levels of alemtuzumab are approximately from 0.1 to 0.15 μg/mL.11,12 Only 4 patients had a day 0 alemtuzumab level less than 0.15 μg/mL. None of these 4 patients developed MC or GF. Because of the small sample size, we compared patients who had day 0 alemtuzumab levels above and below the lowest cohort quartile level of 0.32 μg/mL as a surrogate for the lytic threshold. The 1 patient with primary GF (who had a day 0 alemtuzumab level of 1.63 μg/mL) was excluded from these analyses. Patients with day 0 alemtuzumab levels ≤0.32 μg/mL had a markedly lower cumulative incidence of MC compared with patients with levels >0.32 μg/mL (14.3% vs 90.9%, respectively; P = .008, Figure 2A). The cumulative incidence of MC increased with each rise in the alemtuzumab level range quartile (P = .014; supplemental Figure 1), and every patient with a level of 1.3 μg/mL or higher developed MC. None of the patients with alemtuzumab levels ≤0.32 μg/mL developed an impending or established secondary GF. In contrast, the incidence of impending or established secondary GF within 1 year after HCT was 54.3% in patients with alemtuzumab levels >0.32 μg/mL (P = .08; Figure 2B), and it increased with each rise in the alemtuzumab level range quartile (P = .082; supplemental Figure 1). Patients separated based on HLH or other IEI diagnoses had similar incidences of MC and impending or established secondary GF (supplemental Figure 2).
MC and impending or established secondary GF stratified based on day 0 alemtuzumab levels. (A) Cumulative incidence of MC, defined as donor chimerism <95%. (B) Cumulative incidence of impending or established secondary GF. Stratification using the first quartile day 0 alemtuzumab levels (level ≤ 0.32 μg/mL). D0, day 0.
MC and impending or established secondary GF stratified based on day 0 alemtuzumab levels. (A) Cumulative incidence of MC, defined as donor chimerism <95%. (B) Cumulative incidence of impending or established secondary GF. Stratification using the first quartile day 0 alemtuzumab levels (level ≤ 0.32 μg/mL). D0, day 0.
Impact of alemtuzumab levels on GVHD
The incidence of de novo acute GVHD (arising from the stem cell graft and not the result of a DLI) was also analyzed because day 0 alemtuzumab levels are known to affect the risk of acute GVHD. The cumulative incidence of grade II-IV acute GVHD before any DLI was 31.4% in patients with day 0 alemtuzumab levels ≤0.32 μg/mL, compared with 13.2% in patients with levels >0.32 μg/mL (P = .17). The cumulative incidence of grade III-IV acute GVHD before DLI was 31.4% in patients with day 0 alemtuzumab levels ≤0.32 μg/mL, compared with 9.1% in patients with levels >0.32 μg/mL (P = .05). The total cumulative incidence of acute GVHD grades II-IV (31.4% vs 33.0% [P = .67]) and III-IV (35.7% vs 23.6% [P = .32]) were equivalent between groups because of the frequent use of DLI in patients with day 0 alemtuzumab levels >0.32 μg/mL (supplemental Figure 3). There was no difference in the cumulative incidence of chronic GVHD (25.0% vs 37.7% [P = .99]).
Peritransplantation cytokine patterns in patients with and without sustained intervention-free engraftment
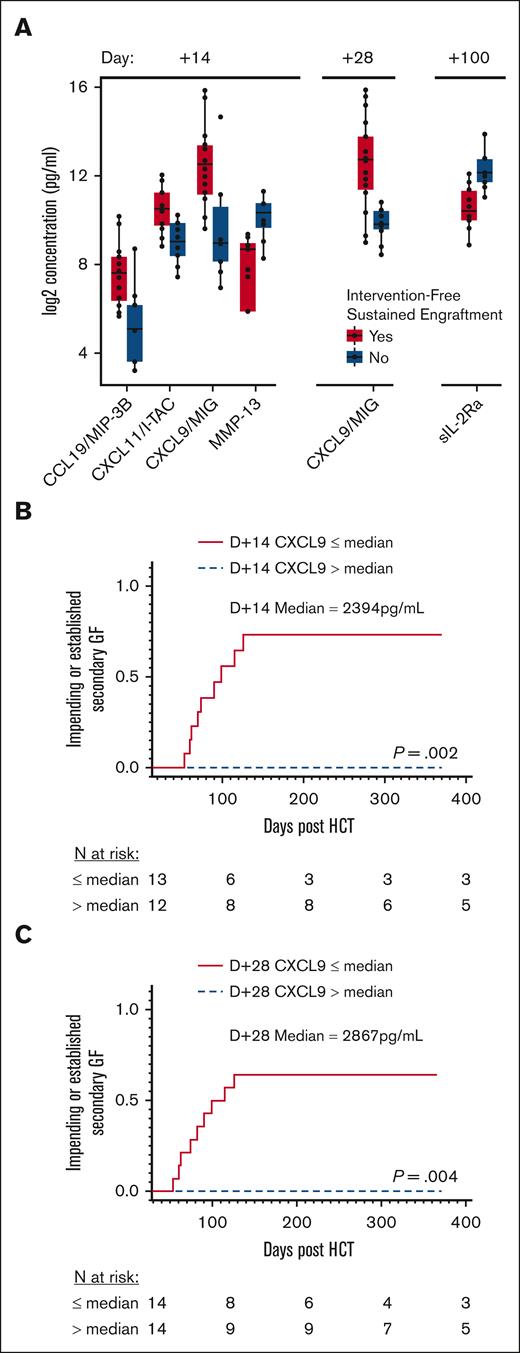
We initially performed an unbiased, comprehensive cytokine and other biomarker analysis. We compared 140 analytes between all patients with and without intervention-free sustained engraftment at different collection times (Figure 3A). There were no significant differences in cytokine levels on day −14 or day −1, with a maximum false discovery proportion of 0.1. On day +14, the group that did not experience intervention-free sustained engraftment had significantly lower CXCL9, CXCL11, and CCL19 levels compared with patients with intervention-free sustained engraftment. On day +28, CXCL9 was still significantly lower in patients who did not experience intervention-free sustained engraftment. There were no other significant differences in cytokine levels at days +28, +42, or +100 other than a difference in day +100 levels of soluble interleukin-2 receptor alpha (sIL-2Rα) of unclear significance, given the clinical heterogeneity present at that time after HCT.
MC and impending or established secondary GF stratified based on CXCL9 levels. (A) Grouped plasma proteomic expression in patients with and without intervention-free sustained engraftment. Five analytes were identified to be statistically significant (maximum false discovery proportion of 0.1 at an 80% confidence level). (B) Cumulative incidence of secondary GF, impending or established, stratified by median D+14 CXCL9 level, 2394 pg/mL. (C) Cumulative incidence of secondary GF, impending or established, stratified based on median D+28 CXCL9 level, 2867 pg/mL. D+14, day +14 after HCT; D+28, day +28 after HCT.
MC and impending or established secondary GF stratified based on CXCL9 levels. (A) Grouped plasma proteomic expression in patients with and without intervention-free sustained engraftment. Five analytes were identified to be statistically significant (maximum false discovery proportion of 0.1 at an 80% confidence level). (B) Cumulative incidence of secondary GF, impending or established, stratified by median D+14 CXCL9 level, 2394 pg/mL. (C) Cumulative incidence of secondary GF, impending or established, stratified based on median D+28 CXCL9 level, 2867 pg/mL. D+14, day +14 after HCT; D+28, day +28 after HCT.
Correlation of CXCL9 levels with primary GF and impending or established secondary GF
Next, we performed more detailed analyses of CXCL9 levels and engraftment outcomes, given that CXCL9 was the only biomarker identified to significantly associate with intervention-free sustained engraftment at 2 time points after HCT. Firstly, we individually examined the CXCL9 levels of the single patient who experienced primary GF and compared these with the levels in patients who experienced impending or established secondary GF. This was performed because it was recently reported that early immune-mediated GF in the T-cell–depleted partially-matched family donor transplantation setting is associated with significant elevations in CXCL9 between days +3 and +14.13 Indeed, the one patient in our study with primary GF had a day +14 CXCL9 level that was markedly elevated (26604 pg/mL) compared with lower levels in patients with impending or established secondary GF (range, 127-2378 pg/mL). These results suggest that although early primary GF after alemtuzumab, fludarabine, and melphalan may share the same proposed pathophysiology as reported in the T-cell–depleted, mismatched, related donor setting, secondary GF (impending or established) results from a different mechanism.
We subsequently examined the impact of lower CXCL9 levels on the risk of impending or established secondary GF. The median CXCL9 level on day +14 for all study patients was 2394 pg/mL (IQR, 716-10189). The cumulative incidence of impending or established secondary GF in patients with a lower day +14 CXCL9 level (≤2394 pg/mL) was 73.6% vs 0.0% in patients with higher levels (>2394 pg/mL; P = .002; Figure 3B). The cumulative incidence of impending or established secondary GF in patients with HLH with lower day +14 CXCL9 levels (≤2394 pg/mL) was 85.2% and 50.0% for those with other IEIs (supplemental Figure 4). The same analysis was done based on the median day +28 CXCL9 level, which was 2867 pg/mL (IQR, 868-8378). The cumulative incidence of impending or established secondary GF was again higher in patients with lower day +28 CXCL9 levels (≤2867 pg/mL), 64.3% compared with 0.0% in patients with higher levels (>2867 pg/mL; P = .004; Figure 3C). The incidence of impending or established secondary GF in patients with HLHwith lower day +28 CXCL9 levels (≤2867 pg/mL) was 63.6% and 66.7% for patients with other IEIs (supplemental Figure 4).
Relationship between alemtuzumab and CXCL9 levels
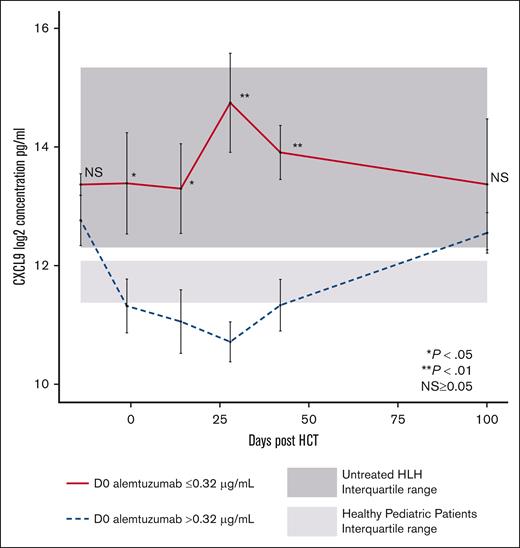
We next explored the relationship between CXCL9 levels and day 0 alemtuzumab levels. Higher peri-HCT alemtuzumab levels may be expected to deplete CD52-expressing T cells more efficiently and other lymphocytes that produce interferon-gamma (IFN-γ), which induces subsequent CXCL9 production by other types of cells. In patients with day 0 alemtuzumab levels ≤0.32 μg/mL, the median CXCL9 levels on days −1, +14, +28, and +42 were significantly higher than in patients with day 0 alemtuzumab levels >0.32 μg/mL (Figure 4) and controls. In contrast, the peri-HCT CXCL9 levels in patients with alemtuzumab levels >0.32 μg/mL were lower and similar to those of controls. Patients separated by HLH or other IEI diagnoses had a similar pattern of peri-HCT CXCL9 levels (supplemental Figure 5). These data establish an inverse relationship between peritransplantation CXCL9 levels and alemtuzumab levels. This relationship may result from higher alemtuzumab levels causing increased leukocyte depletion of donor grafts, including donor T cells. Efficient depletion results in fewer T cells being available to be stimulated by allogeneic or other antigens, less IFN-γ production, and less IFN-γ mediated induction of CXCL9.
Peritransplantation CXCL9 levels stratified based on day 0 alemtuzumab levels. Peritransplantation CXCL9 median levels for all patients. Stratification using first quartile day 0 alemtuzumab levels (level ≤0.32 μg/mL). Light gray shading represents range (25th-75th percentile) of CXCL9 values in healthy pediatric controls. Dark gray shading represents range (25th-75th percentile) of CXCL9 values in pediatric patients with untreated HLH. D0, day 0 after HCT.
Peritransplantation CXCL9 levels stratified based on day 0 alemtuzumab levels. Peritransplantation CXCL9 median levels for all patients. Stratification using first quartile day 0 alemtuzumab levels (level ≤0.32 μg/mL). Light gray shading represents range (25th-75th percentile) of CXCL9 values in healthy pediatric controls. Dark gray shading represents range (25th-75th percentile) of CXCL9 values in pediatric patients with untreated HLH. D0, day 0 after HCT.
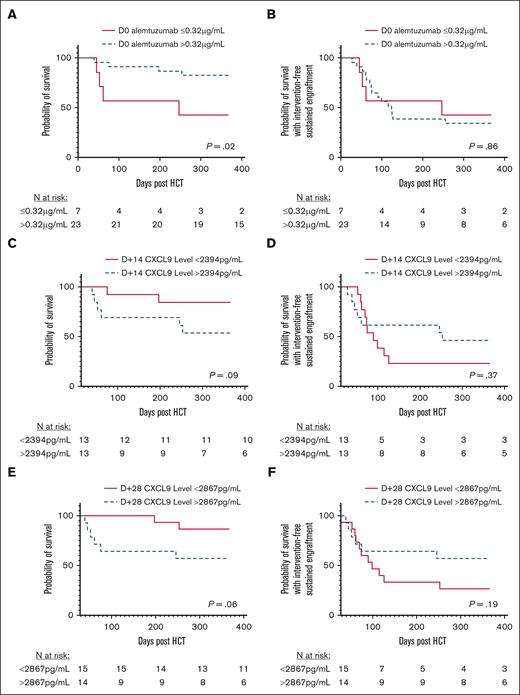
Survival
The overall survival was higher in patients with day 0 alemtuzumab levels >0.32 μg/mL at 1 year (Figure 5A). However, survival in that group decreased soon thereafter because of 2 additional deaths at day +394 and day +482 related to DLI-associated GVHD (supplemental Figure 6). Deaths within 1 year in patients with day 0 alemtuzumab levels >0.32 μg/mL were due to infection (n = 2; day +39 and +75), organ failure (n = 1; day +197), and post-HCT lymphoproliferative disease (n = 1; day +253). Deaths in patients with day 0 alemtuzumab levels ≤0.32 μg/mL were due to acute GVHD (n = 2; day +52 and +246), organ failure (n = 1; day +45), and primary disease recurrence (n = 1; day +61). Overall survival analyses stratified based on day +14 and day +28 CXCL9 levels demonstrated a trend toward increased survival in patients with relatively lower CXCL9 levels (P = .06-.09; Figure 5C,E). We did not observe an impact of day 0 alemtuzumab levels on survival with intervention-free sustained engraftment in this cohort. The 1-year survival with intervention-free sustained engraftment for patients with day 0 alemtuzumab levels >0.32 μg/mL was 34.8%, and for patients with levels ≤0.32 μg/mL, it was 42.9% (P = .86; Figure 5B). The 1-year survival with intervention-free sustained engraftment also displayed nonsignificant differences between patients stratified by CXCL9 levels (Figure 5D,F).
Overall survival and survival with intervention-free sustained engraftment stratified based on alemtuzumab and CXCL9 levels. Overall survival stratified based on day 0 alemtuzumab levels (A), day +14 CXCL9 levels (C), and day +28 CXCL9 levels (E). Probability of survival with intervention-free sustained engraftment stratified based on day 0 alemtuzumab levels (B), day +14 CXCL9 levels (D), and day +28 CXCL9 levels (F).
Overall survival and survival with intervention-free sustained engraftment stratified based on alemtuzumab and CXCL9 levels. Overall survival stratified based on day 0 alemtuzumab levels (A), day +14 CXCL9 levels (C), and day +28 CXCL9 levels (E). Probability of survival with intervention-free sustained engraftment stratified based on day 0 alemtuzumab levels (B), day +14 CXCL9 levels (D), and day +28 CXCL9 levels (F).
ROC analyses to determine optimal risk thresholds
Lastly, we performed ROC analyses to determine optimal day 0 alemtuzumab and day +14 CXCL9 level thresholds that could be used to predict outcomes and guide future transplantation strategies aimed to reduce rates of MC and GF. As shown in supplemental Figure 7, the optimal cut-off point of the day 0 alemtuzumab level to predict MC was ≥0.9 μg/mL, and the area under the curve (AUC) was 0.894. The optimal cut-off point to predict impending or established secondary GF was ≥1.6 μg/mL, and the AUC was 0.818. Optimal thresholds of CXCL9 to predict MC or impending or established secondary GF were ≤2409 pg/mL and ≤2378 pg/mL, respectively, and the AUC for each analysis was 0.882 and 0.924, respectively. Neither alemtuzumab nor CXCL9 levels predicted survival.
Discussion
RIC with alemtuzumab, fludarabine, and melphalan was implemented to improve survival for patients with HLH and other IEIs but was found to be associated with high rates of MC and poor sustained engraftment in the BMT CTN 1204 RICHI study. Understanding the mechanism may present opportunities to improve RIC HCT strategies for patients with HLH and immune regulatory disorders, who are historically challenging patients.3,14 Our findings demonstrate a strong relationship between alemtuzumab levels and sustained engraftment and, through an unbiased approach of 140 analytes, a strong correlation of sustained engraftment with periengraftment CXCL9 levels.
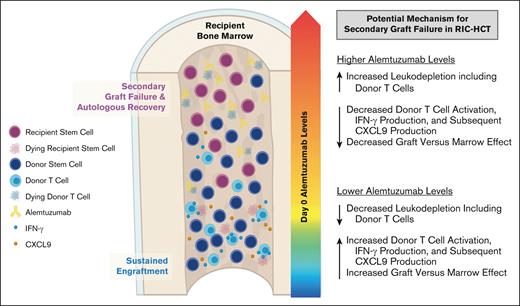
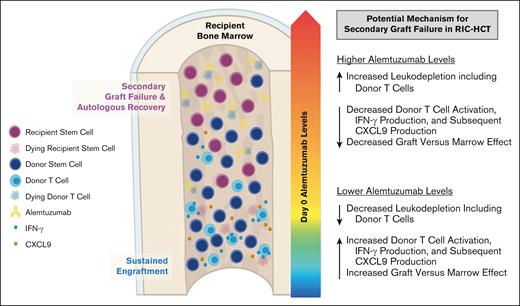
Firstly, we reported an increased risk of MC after RIC HCT with proximal timing and high dosing of alemtuzumab6 and subsequently demonstrated the risk of high peritransplantation alemtuzumab levels.12 In this study using samples collected in a prospective multicenter study, we further validated that patients with higher peritransplantation alemtuzumab levels had an increased risk of MC and impending or established secondary GF; although the latter did not reach statistical significance likely because of the small sample size. We postulate that higher alemtuzumab levels at the time of HCT likely result in more efficient and profound depletion of donor hematopoietic cell graft lymphocytes, including donor T cells, which results in decreased graft-versus-marrow effect and increased risk of MC and poor sustained engraftment (Figure 6). This is especially relevant to the RIC/nonMAC fludarabine and melphalan regimens, in which autologous recovery can occur because of a lack of myeloablation.
Proposed model in which higher peritransplantation alemtuzumab levels drive T-cell depletion of the stem cell graft, inhibit graft-versus-marrow effect, and facilitate secondary GF after RIC HCT.
Proposed model in which higher peritransplantation alemtuzumab levels drive T-cell depletion of the stem cell graft, inhibit graft-versus-marrow effect, and facilitate secondary GF after RIC HCT.
Higher alemtuzumab levels also likely result in more efficient depletion of recipient and/or donor antigen-presenting cells which express CD52. Antigen-presenting cells are important for T-cell stimulation and may contribute to some beneficial graft-versus-marrow effect. CXCL9 is produced by several cell types, including macrophages after the induction by IFN-γ, which is secreted by TH1 CD4+ T cells, CD8+ cytotoxic T cells, and natural killer cells.15,16 Levels of CXCL9 were significantly lower on multiple days after HCT in patients with higher levels of alemtuzumab, and CXCL9 already appeared to be decreasing by day 0, suggesting that alemtuzumab-mediated depletion of both host and graft leukocytes may contribute to lower CXCL9 levels.
GF is a complex immune-mediated process influenced by donor T cells, host T cells, and donor-specific antibodies.17 Previous studies have observed expansion of host T cells in patients who experience primary GF.18,19 Merli et al demonstrated significantly higher CXCL9 levels on days +3, +7, +10, and +14 after HCT in patients with early GF who received haploidentical HCT using TCRαβ T-cell–depleted grafts and MAC.13 Their data suggested early immunogenic GF after HCT is associated with host T-cell activation and IFN-γ pathway activation and may be driven, at least in part, by this cytokine. Here, only 1 patient (n = 33) experienced primary GF. This patient had notably elevated day +14 CXCL9, which supports the findings by Merli et al.
Notably, we also observed that patients with lower alemtuzumab levels displayed higher levels of CXCL9 at days +14 and +28 after transplantation. These patients had much lower risks of MC and secondary GF. This correlation suggests that CXCL9 elevations can also reflect activation of donor T cells, IFN-γ production, and subsequent CXCL9 production by cells in the bone marrow environment, thus signifying graft-vs-hematopoiesis (or graft vs host) effect in the T-cell–replete transplantation setting.
Monitoring CXCL9 at day +14/+28 after RIC HCT may be an effective way to screen for lack of graft-versus-hematopoiesis and increased risk of secondary GF. We demonstrate that lower CXCL9 levels between days +14 and +28 predict risk of MC and possible later risk of secondary GF. A CXCL9 level below ∼2400 pg/mL when quantified with the MagPix platform was identified as an optimal threshold to predict MC. It is possible that reducing or withdrawing immunosuppressive GVHD prophylaxis medications in patients at high risk of developing MC might encourage donor T-cell allogeneic responses and reduce the risk of MC, though there may be an increased risk of triggering acute GVHD. Carefully planned trials to test this hypothesis may be indicated, and studies are needed to determine comparable ranges with standardized clinical CXCL9 assays.
Our data also support the testing of precision alemtuzumab dosing strategies to target patient day 0 alemtuzumab levels to an ideal therapeutic concentration window. Ideally, levels should be above the lytic threshold (0.1-0.15 μg/mL)11,12 to minimize the risk of acute GVHD. Our current study ROC analyses suggest that an upper limit goal of day 0 alemtuzumab should be <0.9 μg/mL to reduce the risk of MC and subsequent possible secondary GF. A narrower therapeutic concentration window between 0.2 and 0.6 μg/mL was previously proposed to optimize early immune recovery.12
The impact of alemtuzumab levels on the risks of MC and secondary GF may be less profound with conditioning regimens that achieve myeloablation. Reduced-toxicity MAC regimens that include alemtuzumab are associated with lower rates of secondary GF.20-24 One may infer from these studies that increasing the intensity of the fludarabine and melphalan conditioning regimen such as by adding thiotepa may be one approach to overcoming the risks of MC and secondary GF while still limiting treatment-related morbidity/mortality associated with fully myeloablative strategies.24 Indeed, a recent CIBMTR study reported superior sustained engraftment and event-free survival with the addition of thiotepa.25
Despite the observed benefit of lower alemtuzumab levels and higher CXCL9 levels on sustained engraftment, we did not observe a benefit on survival with intervention-free sustained engraftment. We observed higher overall survival in patients with higher day 0 alemtuzumab levels, though this effect decreased beyond 1 year. This was surprising as a larger retrospective study describing HCT outcomes and peri-HCT alemtuzumab levels, including 105 patients with various nonmalignant disorders, did not find a difference in overall survival.12 However, the heterogeneity of the population may have prevented the detection of any early difference specifically among patients with HLH. Here, alemtuzumab levels greater than the study cohort quartile cut-off of 0.32 μg/mL were associated with lower CXCL9 levels, which, although detrimental to sustained engraftment, may have benefit in patients with HLH who may have lingering disease activity at the time of HCT. Given this observation, targeted agents, such as emapalumab (anti–IFN-γ monoclonal antibody), may be indicated to maintain lower levels of IFN-γ activity and prevent excessive early inflammation after BMT when targeting lower day 0 alemtuzumab concentrations in patients with HLH.
Here, we were able to take advantage of prospectively collected biospecimens from a well-defined cohort of consistently treated patients to study the undercurrents at play in the complex immune interactions of allotransplantation. Despite the limited sample size, we were able to identify a relationship between peritransplantation alemtuzumab levels, CXCL9 levels, MC, and secondary GF (impending or established) after RIC HCT in patients with HLH and other IEIs. Although uncontrolled inflammation may result in pathological consequences in some circumstances, donor T-cell activity (as reflected by CXCL9 levels) may play an important role in successful engraftment following RIC. Precision alemtuzumab dosing strategies designed to limit inflammation yet facilitate sustained donor engraftment may offer an opportunity to reduce the risk of secondary GF for patients who undergo RIC HCT.
Acknowledgments
This manuscript was prepared using BMT CTN 1204 Research Materials obtained from the National Heart, Lung, and Blood Institute. Support for the BMT CTN 1204 RICHI study was provided by grant #U10HL069294 to the Blood and Marrow Transplant Clinical Trials Network from the National Heart, Lung, and Blood Institute and the National Cancer Institute. The graphical abstract was created using BioRender.com.
Support for this correlative biology study was provided by a grant from the Primary Immune Deficiency Treatment Consortium, the HistioCure Foundation, and the St. Baldrick’s Foundation Innovation Grant (C.E.A.). This work was supported in part by funding from the Intramural Research Program, National Institutes of Health, National Cancer Institute, Center for Cancer Research.The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: A.V.G., B.S., C.K., R.F., M.B.J., A.Z.-L., T.-K.M., C.E.A., and R.A.M. designed the study, reviewed data, performed data analyses, generated figures or oversaw figure generation, and drafted the manuscript; and M.A.P., M.E., J.A.C., C.M.B., S.-Y.P., C.N.D., L.S.K., K.S.B., L.M.B., J.R.A., S.S., P.R., R.H., J.-A.T., K.R.S, E.O.S, H.L., and K.L.M. contributed data and invaluable expertise and edited the manuscript.
Conflict-of-interest disclosure: C.E.A. is on the advisory board for Sobi, Atara Biotherapeutics, and Electra Therapeutics. M.B.J. is a consultant for Sobi and received research funding from Sobi. K.L.M. is on the advisory board of Sobi. J.A.C. is a Consultant for X4 Consultancy and is on the advisory board for Sobi and Horizon. P.R. is a consultant for Sobi. C.M.B. owns stock in Mana Therapeutics, Cabaletta Bio, Catamaran Bio, Repertoire Immune Medicine, and Neximmune, is a Data and Safety Monitoring Board (DSMB) member for Sobi, and is on the ad hoc advisory board for BMS and Pfizer. S.S. is on the advisory board for Janssen Pharma Inc, Graphite Bio, and Bristol Myer Squibb, is a consultant for California Institute of Regenerative Medicine and on DSMB for Aruvant. The remaining authors declare no conflicts of interest. A.Z-L received consulting fees from Sobi.
Correspondence: Rebecca A. Marsh, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: rebecca.marsh@cchmc.org; and Carl E. Allen, Texas Children’s Hospital, 1102 Bates Ave, Ste 750, Houston, TX 77030; e-mail: ceallen@texaschildrens.org.
References
Author notes
∗A.V.G., B.S., and C.K. are joint first authors.
†C.E.A. and R.A.M. are the joint last authors.
Data are available upon request to the corresponding author, Carl E. Allen (ceallen@texaschildrens.org).
The full-text version of this article contains a data supplement.