Key Points
Steroid-dependent and -refractory aGVHD are significant independent risk factors for development of chronic GVHD after allogeneic HCT.
cGVHD after steroid-dependent aGVHD has a similar prognosis to cGVHD after steroid-refractory aGVHD.
Abstract
Chronic graft-versus-host disease (cGVHD) is a major limitation to the long-term success of allogeneic hematopoietic cell transplantation (HCT). Our prior study of acute GVHD (aGVHD) defined distinct treatment-response groups based on the response to first-line corticosteroids: steroid-sensitive (SS), steroid-resistant (SR), and steroid-dependent (SD) aGVHDs. We conducted a retrospective, single-institution, cohort study to assess the incidence, risk factors, and clinical outcomes of patients with cGVHD after a previous diagnosis of SS, SD, or SR aGVHD, compared with those with no history of aGVHD. Among 784 consecutive adult and pediatric recipients of HCT for hematologic malignancies between 2008 and 2016, 347 (44%) developed aGVHD, with 13% SS, 12% SD, and 19% SR aGVHD. The 3-year cumulative incidence of cGVHD was 25%. Among those with cGVHD, 39% had no prior aGVHD diagnosis, whereas among those with a prior aGVHD diagnosis, 16% had SS, 24% had SD, and 21% had SR aGVHD. Mild or moderate cGVHD was highest among those with preceding SD aGVHD, whereas severe cGVHD was most frequent among those with previous SR aGVHD. We identified SD and SR aGVHDs as significant independent risk factors for the development of cGVHD after allogeneic HCT, whereas SS aGVHD was not a risk factor. Our study demonstrates that cGVHD after SD aGVHD did not have an intermediate prognosis between SR and SS groups as hypothesized; rather, cGVHD after both SD and SR aGVHD have similar prognoses. Our findings suggest that previous aGVHD response states are important predictors of cGVHD severity and outcomes.
Introduction
Chronic graft-versus-host disease (cGVHD) remains a major cause of morbidity and mortality in recipients of hematopoietic cell transplantation (HCT).1-3 cGVHD is most often treated with immunosuppressive therapy (IST) to modulate the immune response, control symptoms, and prevent further organ damage.4 Response to corticosteroids and other treatments can lead to extended survival and be associated with malignant disease control through the graft-versus-tumor effect.5,6 Our prior study of acute GVHD (aGVHD) defined distinct treatment-response groups based on the response to first-line corticosteroids: steroid-sensitive (SS), steroid-dependent (SD), and steroid-resistant (SR) aGVHDs.7 Because cGVHD is often, but not always, preceded by aGVHD, we hypothesized that an aGVHD precursor state may influence the presentation, organ involvement, severity, and treatment resistance of subsequent cGVHDs. The incidence and risk factors for cGVHD that occur after a previous diagnosis of SS, SD, or SR aGVHD compared with de novo cGVHD are unknown. We therefore analyzed the impact of aGVHD treatment response on the severity and clinical outcomes of cGVHD occurring after allogeneic HCT.
Methods
Study design and inclusion criteria
The objective of this retrospective, single-institution, cohort study was to assess the incidence, risk factors, and clinical outcomes of patients with cGVHD after a previous diagnosis of SS, SD, or SR aGVHD as compared with those with no history of aGVHD (ie, de novo cGVHD). The study population included 784 consecutive adult and pediatric recipients of allogeneic HCT at the University of Minnesota who underwent HCT for malignant disorders between 2008 and 2016. Only first allogeneic HCT recipients were included. Bone marrow, peripheral blood stem cell, and umbilical cord blood (UCB) graft sources and all related and unrelated donors (URDs) were included. Recipients received myeloablative conditioning (MAC) or reduced-intensity conditioning (RIC) regimens, and all GVHD prophylaxis strategies were included. Only aGVHD cases treated with systemic steroids as the first-line therapy were included.
All patients or their guardians signed a written informed consent for the use of their medical data in clinical research. This study was reviewed and approved by the University of Minnesota institutional review board. Data were retrieved from the UMN BMT database, with supplemental details extracted from the electronic health record.
Definitions
Our prior study defined distinct aGVHD treatment response groups as SS, SR, or SD aGVHD based on the response to first-line corticosteroids.7 GVHD was defined based on clinical presentation and supported using the biopsy of the involved organ when it was clinically indicated. All patients with aGVHD were treated using a standard, clearly defined steroid dose and taper schedule. All patients who developed aGVHD were reviewed individually to determine the classification of their disease as SS, SD, or SR. Detailed definitions of each response class, steroid dosing, and taper schedule were reported previously.7
The 2014 National Institutes of Health Consensus Criteria were used for cGVHD diagnosis, organ involvement, and the overall cGVHD severity at maximum grade.1 The onset of cGVHD was defined as progressive if cGVHD developed without resolution of prior aGVHD, de novo if cGVHD developed without prior aGVHD, or quiescent if cGVHD developed after complete resolution of prior aGVHD. cGVHD was classified as classic vs overlap cGVHD when concurrent aGVHD and cGVHD were present in the latter.
Systemic IST included any form of systemic treatment, including extracorporeal photopheresis to treat cGVHD. Topical IST for skin, oral, ophthalmic involvement, isolated topical gastrointestinal treatment with budesonide, and nonimmunosuppressive treatment (eg, montelukast or azithromycin) for lung cGVHD were not considered as systemic immune suppression. The start and stop dates of each IST were captured. Additional lines of systemic IST were defined as a change or addition of a new treatment not used for initial cGVHD therapy to treat an inadequate response (per the treating physician) or progression of cGVHD.
Nonrelapse mortality (NRM) was defined as death in the absence of disease relapse or progression, accounting for relapse as a competing risk. Overall survival (OS) was defined as the time from cGVHD to death from any cause. cGVHD and relapse-free survival (CRFS) and moderate-to-severe cGVHD (msCRFS) was defined as either the survival without cGVHD that required systemic therapy or relapse or death. Failure-free survival (FFS) after cGVHD was defined as survival without relapse, death, or new systemic treatment initiated >30 days after cGVHD onset.
Patient and transplantation characteristics
Clinical factors examined as potential covariates included sex, age, year of HCT, diagnosis, donor type (matched sibling donor, matched URD, partially matched or mismatched URD, haploidentical, or UCB), prior autologous transplantation, conditioning intensity (MAC vs RIC), GVHD prophylaxis (cyclosporine [CsA] with methotrexate, CsA with mycophenolate mofetil [MMF], sirolimus with MMF, and others, including T-cell depletion and 15 patients who received posttransplantation cyclophosphamide), disease-risk index (DRI; low, intermediate, or high/very high risk), HCT comorbidity index (score of 0, 1-2, or ≥3), and Karnofsky performance status (<90 or ≥90).8,9 The Minnesota aGVHD risk score (standard risk vs high risk) was also used in the assessment of patients with aGVHD.5
Statistical analysis
The OS was estimated using the Kaplan-Meier method.10 Cumulative incidences of cGVHD and relapse were estimated using the cumulative incidence function, with death but without cGVHD and NRM as competing risks, respectively.11 Single-variable null hypothesis tests were performed using either the log-rank test (for OS) or Gray test.
For CRFS, the event time was defined as either the earliest time of relapse, death, or cGVHD that required systemic therapy. If systemic therapy was initiated within 30 days of cGVHD onset, the event time was the day of cGVHD onset; otherwise, it was the day of therapy initiation. Cumulative incidences of cGVHD, death without cGVHD, CRFS, and msCRFS were analyzed in a landmark analysis starting 120 days after HCT. This was chosen because nearly all aGVHD response classifications would be known by that day. Most aGVHD cases occurred before day 50 (interquartile range, 26-49 days), and the response classification was defined within 80 days of treatment. Choosing a later landmark time would exclude more cGVHD cases. Patients with a defined event (eg, cGVHD, relapse, or death) before day 120 were excluded from the analysis of outcomes that included that event.
Fine and Gray proportional hazards regression was used to create a multivariable model for risk of cGVHD, using the day-120 landmark analysis.12 Factors considered in regression analyses were aGVHD treatment response (SS vs SD vs SR vs no aGVHD), sex (male vs female), donor type and HLA matching, DRI, HCT comorbidity index, conditioning intensity (MAC vs RIC), and age. Age was modeled as a continuous variable using restricted cubic splines,13,14 which do not assume a strictly linear relationship between age and cGVHD risk. Thus, model-estimated relative risk of cGVHD can vary depending on the chosen reference ages. We reported the hazard ratio (HR) for a 50-year-old patient relative to a 10-year-old patient; however, reporting HRs for other reference ages would not change the underlying model.
FFS and other outcomes after cGVHD (OS, relapse, and NRM) were analyzed from the date of cGVHD onset. For FFS, relapse, and NRM, patients who relapsed before cGVHD were excluded. aGVHD response was considered to be static after cGVHD onset; only 13 of 198 cGVHD cases occurred within 80 days of aGVHD onset.
Follow-up was censored after each patient’s last contact date when they were alive. Median follow-up among surviving patients was 6 years (minimum 1 year), with 75% of patients followed up for at least 4 years. Analyses were performed using R software, version 4.0.5.
Results
Patient and transplantation characteristics
Patient, disease, and transplantation characteristics are shown in Table 1. Among 784 recipients of allogeneic HCT, 347 (44%) developed aGVHD requiring systemic corticosteroid therapy, with 13% SS, 12% SD, and 19% SR aGVHD. Minnesota high-risk aGVHD was more frequent in the SR group, followed by the SD and SS groups (26%, 20%, and 16%, respectively). Among the 784 patients, 198 patients developed cGVHD, with a 3-year cumulative incidence of 25%. The 3-year cumulative incidences of mild, moderate, and severe cGVHD were 4.4% (95% confidence interval [CI], 3-6), 15.3% (95% CI, 13-18), and 5.6% (95% CI, 4-7), respectively. Among those with cGVHD, 78 (39%) had no prior aGVHD diagnosis. Of those with a prior aGVHD diagnosis, 31 patients (16%) had SS aGVHD, 48 patients (24%) had SD aGVHD, and 41 patients (21%) had SR aGVHD. CsA/MMF was the most common GVHD prophylaxis in all 4 groups that developed cGVHD. (supplemental Table 1). cGVHD that developed after SS aGVHD was predominantly of quiescent onset (81%), whereas progressive cGVHD was highest in those with prior SD and SR aGVHD (40% and 38%, respectively), which was not unexpected because the criteria for SD and SR cohorts involved disease progression and poor treatment response. All 4 groups showed relatively similar incidence of classic vs overlap cGVHD (Table 2). Among patients with cGVHD requiring systemic immune suppression, 71% in the SS group had achieved treatment discontinuation for >6 months, followed by 67% in the SD group and 66% in the SR group.
Demographic and clinical characteristics of aGVHD response groups
| . | No aGVHD (n = 437) . | SS aGVHD (n = 99) . | SD aGVHD (n = 96) . | Steroid-refractory aGVHD (n = 152) . |
|---|---|---|---|---|
| Female sex, n (%) | 181 (41%) | 36 (36%) | 37 (39%) | 56 (37%) |
| Age at transplantation, y | ||||
| 0-17 | 113 (26%) | 15 (15%) | 4 (4%) | 15 (10%) |
| ≥18 | 324(74%) | 84 (85%) | 92 (96%) | 137 (90%) |
| Diagnosis category | ||||
| Acute leukemia | 312 (72%) | 75 (75%) | 63 (65%) | 99 (65%) |
| MDS | 52 (12%) | 9 (9%) | 10 (10%) | 22 (14%) |
| Multiple myeloma | 9 (2%) | 4 (4%) | 7 (7%) | 7 (5%) |
| Lymphoma | 58 (13%) | 10 (10%) | 14 (15%) | 14 (9%) |
| Myeloproliferative disorder/other | 6 (1%) | 1 (1%) | 2 (2%) | 10 (7%) |
| Donor | ||||
| Haploidentical | 35 (8%) | 1 (1%) | 3 (3%) | 4 (3%) |
| Matched sibling | 168 (38%) | 30 (30%) | 33 (34%) | 44 (29%) |
| UCB | 214 (49%) | 60 (61%) | 50 (52%) | 75 (49%) |
| URD | 20 (5%) | 8 (8%) | 10 (10%) | 29 (19%) |
| Conditioning intensity | ||||
| MAC | 194 (44%) | 45 (45%) | 44 (46%) | 61 (40%) |
| RIC | 243 (56%) | 54 (55%) | 52 (54%) | 91 (60%) |
| GVHD prophylaxis | ||||
| CsA/MTX | 77 (18%) | 20 (20%) | 12 (12%) | 19 (12%) |
| CsA/MMF | 246 (56%) | 66 (67%) | 78 (81%) | 105 (69%) |
| Sirolimus/MMF | 55 (13%) | 11 (11%) | 1 (1%) | 12 (8%) |
| Other | 59 (14%) | 2 (2%) | 5 (5%) | 16 (11%) |
| DRI | ||||
| Very high/high risk | 111 (25%) | 17 (17%) | 11 (11%) | 26 (17%) |
| Intermediate risk | 283 (65%) | 68 (69%) | 71 (74%) | 107 (70%) |
| Low risk | 43 (10%) | 14 (14%) | 14 (15%) | 19 (12%) |
| HCT comorbidity index | ||||
| 0 | 201 (46%) | 48 (48%) | 47 (49%) | 68 (45%) |
| 1-2 | 130 (30%) | 31 (31%) | 24 (25%) | 34 (22%) |
| 3+ | 106 (24%) | 20 (20%) | 25 (26%) | 50 (33%) |
| Karnofsky performance status | ||||
| <90 | 78 (18%) | 13 (13%) | 10 (10%) | 28 (18%) |
| ≥90 | 359 (82%) | 86 (87%) | 86 (90%) | 124 (82%) |
| MN GVHD risk | ||||
| High risk | 16 (16%) | 19 (20%) | 40 (26%) | |
| Standard risk | 83 (84%) | 77 (80%) | 112 (74%) |
| . | No aGVHD (n = 437) . | SS aGVHD (n = 99) . | SD aGVHD (n = 96) . | Steroid-refractory aGVHD (n = 152) . |
|---|---|---|---|---|
| Female sex, n (%) | 181 (41%) | 36 (36%) | 37 (39%) | 56 (37%) |
| Age at transplantation, y | ||||
| 0-17 | 113 (26%) | 15 (15%) | 4 (4%) | 15 (10%) |
| ≥18 | 324(74%) | 84 (85%) | 92 (96%) | 137 (90%) |
| Diagnosis category | ||||
| Acute leukemia | 312 (72%) | 75 (75%) | 63 (65%) | 99 (65%) |
| MDS | 52 (12%) | 9 (9%) | 10 (10%) | 22 (14%) |
| Multiple myeloma | 9 (2%) | 4 (4%) | 7 (7%) | 7 (5%) |
| Lymphoma | 58 (13%) | 10 (10%) | 14 (15%) | 14 (9%) |
| Myeloproliferative disorder/other | 6 (1%) | 1 (1%) | 2 (2%) | 10 (7%) |
| Donor | ||||
| Haploidentical | 35 (8%) | 1 (1%) | 3 (3%) | 4 (3%) |
| Matched sibling | 168 (38%) | 30 (30%) | 33 (34%) | 44 (29%) |
| UCB | 214 (49%) | 60 (61%) | 50 (52%) | 75 (49%) |
| URD | 20 (5%) | 8 (8%) | 10 (10%) | 29 (19%) |
| Conditioning intensity | ||||
| MAC | 194 (44%) | 45 (45%) | 44 (46%) | 61 (40%) |
| RIC | 243 (56%) | 54 (55%) | 52 (54%) | 91 (60%) |
| GVHD prophylaxis | ||||
| CsA/MTX | 77 (18%) | 20 (20%) | 12 (12%) | 19 (12%) |
| CsA/MMF | 246 (56%) | 66 (67%) | 78 (81%) | 105 (69%) |
| Sirolimus/MMF | 55 (13%) | 11 (11%) | 1 (1%) | 12 (8%) |
| Other | 59 (14%) | 2 (2%) | 5 (5%) | 16 (11%) |
| DRI | ||||
| Very high/high risk | 111 (25%) | 17 (17%) | 11 (11%) | 26 (17%) |
| Intermediate risk | 283 (65%) | 68 (69%) | 71 (74%) | 107 (70%) |
| Low risk | 43 (10%) | 14 (14%) | 14 (15%) | 19 (12%) |
| HCT comorbidity index | ||||
| 0 | 201 (46%) | 48 (48%) | 47 (49%) | 68 (45%) |
| 1-2 | 130 (30%) | 31 (31%) | 24 (25%) | 34 (22%) |
| 3+ | 106 (24%) | 20 (20%) | 25 (26%) | 50 (33%) |
| Karnofsky performance status | ||||
| <90 | 78 (18%) | 13 (13%) | 10 (10%) | 28 (18%) |
| ≥90 | 359 (82%) | 86 (87%) | 86 (90%) | 124 (82%) |
| MN GVHD risk | ||||
| High risk | 16 (16%) | 19 (20%) | 40 (26%) | |
| Standard risk | 83 (84%) | 77 (80%) | 112 (74%) |
MDS, myelodysplastic syndrome; MN, Minnesota; MTX, methotrexate.
cGVHD characteristics based on the aGVHD response
| . | No aGVHD (n = 78) . | SS aGVHD (n = 31) . | SD aGVHD (n = 48) . | Steroid-refractory aGVHD (n = 41) . | Total (n = 198) . |
|---|---|---|---|---|---|
| cGVHD onset | |||||
| De novo | 78 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 77 (39%) |
| Quiescent | 0 (0%) | 25 (81%) | 29 (60%) | 25 (62%) | 79 (40%) |
| Progressive | 0 (0%) | 6 (19%) | 19 (40%) | 15 (38%) | 41 (21%) |
| cGVHD type | |||||
| Classic | 54 (69%) | 17 (55%) | 31 (66%) | 25 (62%) | 127 (65%) |
| Overlap | 24 (31%) | 14 (45%) | 16 (34%) | 15 (38%) | 69 (35%) |
| cGVHD severity | |||||
| Mild | 12 (15%) | 6 (19%) | 11 (23%) | 6 (15%) | 35 (18%) |
| Moderate | 50 (64%) | 18 (58%) | 27 (57%) | 22 (55%) | 117 (60%) |
| Severe | 16 (21%) | 7 (23%) | 9 (19%) | 12 (30%) | 44 (22%) |
| . | No aGVHD (n = 78) . | SS aGVHD (n = 31) . | SD aGVHD (n = 48) . | Steroid-refractory aGVHD (n = 41) . | Total (n = 198) . |
|---|---|---|---|---|---|
| cGVHD onset | |||||
| De novo | 78 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 77 (39%) |
| Quiescent | 0 (0%) | 25 (81%) | 29 (60%) | 25 (62%) | 79 (40%) |
| Progressive | 0 (0%) | 6 (19%) | 19 (40%) | 15 (38%) | 41 (21%) |
| cGVHD type | |||||
| Classic | 54 (69%) | 17 (55%) | 31 (66%) | 25 (62%) | 127 (65%) |
| Overlap | 24 (31%) | 14 (45%) | 16 (34%) | 15 (38%) | 69 (35%) |
| cGVHD severity | |||||
| Mild | 12 (15%) | 6 (19%) | 11 (23%) | 6 (15%) | 35 (18%) |
| Moderate | 50 (64%) | 18 (58%) | 27 (57%) | 22 (55%) | 117 (60%) |
| Severe | 16 (21%) | 7 (23%) | 9 (19%) | 12 (30%) | 44 (22%) |
SD has 1 unknown type and severity. Steroid refractory has 1 unknown onset, type, and severity.
Characteristics of cGVHD within the SS, SD, and SR aGVHD cohorts
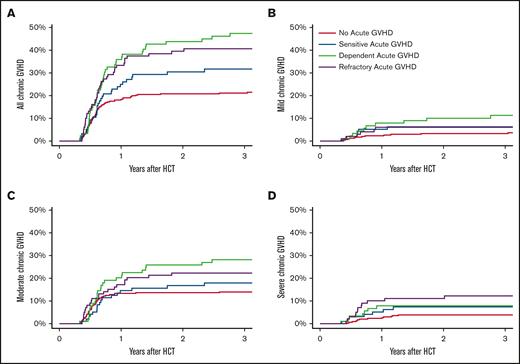
We conducted a landmark analysis within each aGVHD response group (no prior aGVHD and SS, SD, and SR aGVHD) starting on day 120 after HCT, the time point when most aGVHD responses were established with certainty. The 3-year cumulative incidence of any cGVHD was highest (47%) in the SD group, followed by the SR, SS, and no-aGVHD groups at 41%, 32%, and 21%, respectively (P < .01; Figure 1A). This finding may be influenced, in part, by a higher incidence of death without cGVHD in the SR group (43%) as compared with the SD, SS, and no-aGVHD groups at 26%, 28%, and 30%, respectively (P = .05).
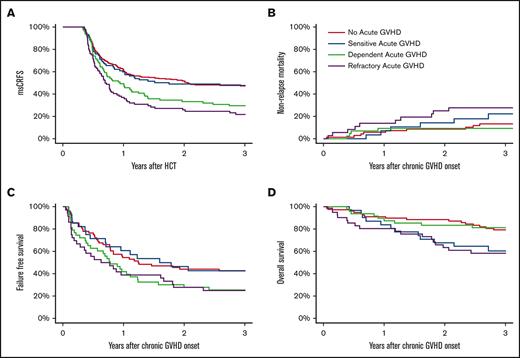
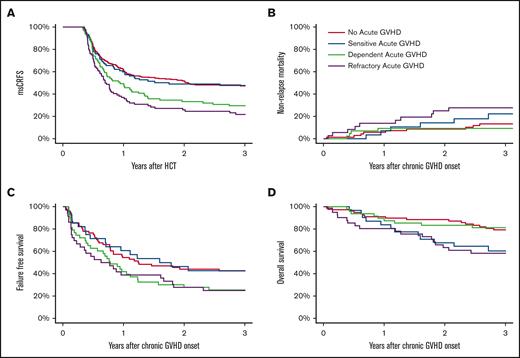
Cumulative incidence of all cGVHD and mild/moderate/severe cGVHD since day 120. Competing risks are death without cGVHD (A-D) and other severities of cGVHD (B-D).
Cumulative incidence of all cGVHD and mild/moderate/severe cGVHD since day 120. Competing risks are death without cGVHD (A-D) and other severities of cGVHD (B-D).
We examined the impact of aGVHD response on the severity of cGVHD. The 3-year cumulative incidence of mild cGVHD was highest among the SD group, at 11%, followed by the SR, SS, and no-aGVHD groups at 6%, 6%, and 3%, respectively (P = .04; Figure 1B). Moderate cGVHD was also highest among the SD group, with a cumulative incidence of 28%, followed by the SR, SS, and no-aGVHD groups at 22%, 18%, and 14%, respectively (P = .02; Figure 1C). Severe cGVHD was highest among the SR group (12%), followed by the SD, SS, and no-aGVHD groups at 8%, 7%, and 4%, respectively (P = .02; Figure 1D). Patients with no prior aGVHD consistently demonstrated the lowest cumulative incidence of cGVHD at any severity level.
Risk factors for cGVHD
We performed multivariable landmark analysis from day 120 after HCT to determine the relative risk of cGVHD among the SS, SD, and SR aGVHD cohorts compared with the no-aGVHD cohort. SD aGVHD was independently associated with the highest risk of a subsequent cGVHD (vs no aGVHD: HR, 2.2; 95% CI, 1.5-3.2; P < .01), followed by SR (HR, 1.8; 95% CI, 1.2-2.7; P < .01) and SS aGVHD (HR, 1.3; 95% CI, 0.8-2.1; P = .21; Table 3). Older age was independently associated with a higher risk of cGVHD (for age 50 vs 10 years: HR, 6.3; 95% CI, 3.1-12.5; P < .01). Factors associated with a lower risk of cGVHD included RIC (HR, 0.5; 95% CI, 0.3-0.8; P < .01), matched URD (HR, 0.5; 95% CI, 0.3-0.8; P < .01), and UCB (HR, 0.3; 95% CI, 0.2-0.5; P < .01). Sex and HCT comorbidity index were not significantly associated with an increased risk of cGVHD.
Risk factor models for cGVHD: landmark analysis from HCT day 120+
| . | c GVHD . | Death without cGVHD . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Acute GVHD response∗ | ||||||
| No acute GVHD | 1.00 | 1.00 | ||||
| SS | 1.32 | (0.85-2.05) | .21 | 0.96 | (0.62-1.50) | .86 |
| SD | 2.17 | (1.46-3.23) | <.01 | 0.85 | (0.53-1.36) | .50 |
| Steroid refractory | 1.83 | (1.22-2.74) | <.01 | 1.47 | (1.00-2.15) | .05 |
| Male sex | 1.08 | (0.80-1.46) | .62 | 1.01 | (0.76-1.35) | .93 |
| Age† (50 vs 10 y) | 6.27 | (3.15-12.5) | <.01 | 1.54 | (0.91-2.63) | .26 |
| Donor type | ||||||
| Matched sibling | 1.00 | 1.00 | ||||
| Haploidentical | 0.23 | (0.07-0.74) | .01 | 2.73 | (1.60-4.65) | <.01 |
| Matched URD | 0.46 | (0.26-0.82) | <.01 | 1.78 | (1.02-3.10) | .04 |
| Mismatched URD | 0.57 | (0.14-2.35) | .43 | 2.66 | (0.63-11.4) | .18 |
| UCB | 0.34 | (0.24-0.48) | <.01 | 1.40 | (1.00-1.95) | .05 |
| DRI | ||||||
| Low risk | 1.00 | 1.00 | ||||
| Intermediate risk | 0.58 | (0.39-0.86) | <.01 | 2.20 | (1.22-3.97) | <.01 |
| High/very high risk | 0.43 | (0.24-0.77) | <.01 | 3.45 | (1.82-6.53) | <.01 |
| HCT comorbidity index | ||||||
| 0 | 1.00 | 1.00 | ||||
| 1-2 | 1.30 | (0.91-1.86) | .15 | 0.98 | (0.68-1.40) | .90 |
| ≥3 | 1.18 | (0.81-1.71) | .40 | 1.25 | (0.88-1.77) | .22 |
| RIC | 0.50 | (0.33-0.75) | <.01 | 1.55 | (1.00-2.42) | .05 |
| . | c GVHD . | Death without cGVHD . | ||||
|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Acute GVHD response∗ | ||||||
| No acute GVHD | 1.00 | 1.00 | ||||
| SS | 1.32 | (0.85-2.05) | .21 | 0.96 | (0.62-1.50) | .86 |
| SD | 2.17 | (1.46-3.23) | <.01 | 0.85 | (0.53-1.36) | .50 |
| Steroid refractory | 1.83 | (1.22-2.74) | <.01 | 1.47 | (1.00-2.15) | .05 |
| Male sex | 1.08 | (0.80-1.46) | .62 | 1.01 | (0.76-1.35) | .93 |
| Age† (50 vs 10 y) | 6.27 | (3.15-12.5) | <.01 | 1.54 | (0.91-2.63) | .26 |
| Donor type | ||||||
| Matched sibling | 1.00 | 1.00 | ||||
| Haploidentical | 0.23 | (0.07-0.74) | .01 | 2.73 | (1.60-4.65) | <.01 |
| Matched URD | 0.46 | (0.26-0.82) | <.01 | 1.78 | (1.02-3.10) | .04 |
| Mismatched URD | 0.57 | (0.14-2.35) | .43 | 2.66 | (0.63-11.4) | .18 |
| UCB | 0.34 | (0.24-0.48) | <.01 | 1.40 | (1.00-1.95) | .05 |
| DRI | ||||||
| Low risk | 1.00 | 1.00 | ||||
| Intermediate risk | 0.58 | (0.39-0.86) | <.01 | 2.20 | (1.22-3.97) | <.01 |
| High/very high risk | 0.43 | (0.24-0.77) | <.01 | 3.45 | (1.82-6.53) | <.01 |
| HCT comorbidity index | ||||||
| 0 | 1.00 | 1.00 | ||||
| 1-2 | 1.30 | (0.91-1.86) | .15 | 0.98 | (0.68-1.40) | .90 |
| ≥3 | 1.18 | (0.81-1.71) | .40 | 1.25 | (0.88-1.77) | .22 |
| RIC | 0.50 | (0.33-0.75) | <.01 | 1.55 | (1.00-2.42) | .05 |
Other pairwise comparisons for cGVHD: SR vs SD; HR, 0.84 (95% CI, 0.54-1.31; P = .44); SS vs SD; HR, 0.61 (95% CI, 0.38-0.98; P = .04).
Age was modeled as a continuous variable using a 3-knot restricted cubic spline to model nonlinear effects. Risk of cGVHD increased proportionally with age for ages <∼50 years but was similar for ages >50 years. The reference ages of 50 and 10 years were chosen to show the total magnitude of this effect.15
Survival, CRFS, NRM, and relapse
The 3-year CRFS was highest in the no-aGVHD and SS groups (each 47%) as compared with the SD and SR groups at 26% and 20%, respectively (P < .01). Similarly, 3-year msCRFS was highest in the no-aGVHD and SS groups (each 48%) as compared with the SD and SR groups at 29% and 22%, respectively (P < .01; Figure 2A).
Cumulative incidence of msCRFS (A), NRM (B), FFS (C), and OS (D). msCRFS was analyzed starting 120 days after HCT, whereas all other outcomes were analyzed since the date of cGVHD onset.
Cumulative incidence of msCRFS (A), NRM (B), FFS (C), and OS (D). msCRFS was analyzed starting 120 days after HCT, whereas all other outcomes were analyzed since the date of cGVHD onset.
The 3-year NRM after cGVHD was highest in the SR group, at 28% (P = .05; Figure 2B). The NRM for the SD group (9%) was comparable with that of the no-aGVHD group (13%) and lower than that of the SS group (22%). Relapse risk was similar in all 4 groups (14%, 21%, 19%, and 13% in the SS, SD, SR, and no-aGVHD groups, respectively; P = .85). The no-aGVHD and SS groups, at 44% and 43%, respectively, had the highest 3-year FFS as compared with that of the SD and SR groups at 25% and 22%, respectively (P < .02; Figure 2C). As compared with the SD and no-aGVHD groups at 81% and 79%, respectively, 3-year OS after cGVHD was lowest in those with SR (58%) and SS aGVHD (60%) (P < .01; Figure 2D). We identified no obvious confounding factor explaining the lower OS in the SS group compared with the SD group, including DRI, because most patients were at intermediate risk, and this was fairly balanced across cohorts. For FFS, relapse, and NRM, patients who relapsed before cGVHD onset were excluded, unlike the OS analysis.
A total of 60 patients died within 3 years of cGHVD onset. Causes of death in the SR group were most often attributed to aGVHD or cGVHD (52%) as compared with the no-aGVHD, SS, and SD groups (29%, 17%, and 25%, respectively). There were similar rates of death from infection among all 4 groups (24%, 25%, 13%, and 11%, in the no-aGVHD, SS, SD, and SR groups, respectively). Death from disease recurrence was lowest in the SR group (21%), explained, in part, by early death, whereas it was similar in the no-aGVHD, SS, and SD groups (41%, 58%, and 50%, respectively).
Discussion
cGVHD remains a notable barrier to the long-term success of allogeneic HCT, leading to chronic tissue damage, pronounced immunodeficiency, and diminished quality of life.15 Because cGVHD often, but not always, follows aGVHD, we hypothesized that an aGVHD precursor state may influence the presentation, organ involvement, severity, and treatment responsiveness of subsequent cGVHD. This study examined the previously unreported impact of aGVHD treatment response on the severity and clinical outcomes of cGVHD occurring after allogeneic HCT.
We identified SD and SR aGVHD as independent significant risk factors for the development of cGVHD after allogeneic HCT, whereas SS aGVHD was not a risk factor. Specifically, risk of mild or moderate cGVHD was highest among those with preceding SD aGVHD. Severe cGVHD was most frequent among those with previous SR aGVHD. Although the pathophysiology is uncertain, it is possible that the prolonged need for systemic corticosteroids for aGVHD therapy leads directly, or indirectly, to the development of biologically different cGVHD states, reflecting delayed tolerance, compromised recovery of regulatory T cells or other immunomodulatory elements, and the development of clinically apparent cGVHD.
As hypothesized, cGVHD after SD aGVHD did not have an intermediate prognosis and outcome between the SR and SS groups. In fact, we observed a similar pattern of clustering of CRFS and FFS among the 4 groups, with similar trends in the no-aGVHD and SS group vs worse outcomes in the SD and SR aGVHD groups. cGVHD after SD aGVHD displayed an intermediate msCRFS and a poor FFS that were comparable with the outcome in patients in the SR group, yet a higher OS, comparable with that of the SS group. cGVHD after SR aGVHD showed the poorest survival, for both NRM and overall mortality.
There are several variables that influence treatment response classification, including aGVHD severity. However, aGVHD severity is not defined for the no-aGVHD group, which precludes adjustment for aGVHD severity independent of treatment response within the same model.
The study was limited to patients who underwent HCT for hematologic malignancies, which allowed this analysis to assess the impact of cGVHD on relapse. Outcomes might differ for those with nonmalignant disorders, particularly pediatric patients, for whom indications for HCT are quickly expanding.16
Our findings suggest that distinct cGVHD disease presentation and response is influenced by preexisting risk factors, with the finding that previous aGVHD response states (SS, SD, and SR) are important predictors of cGVHD incidence, severity, and outcome. Classification of aGVHD into these 3 response groups can guide therapeutic strategies for both acute and chronic GVHD by predicting the incidence and characteristics of future cGVHD based on prior aGVHD response state.
Acknowledgments
The authors acknowledge Michael Franklin for assistance in editing this manuscript.
Research reported in this publication was supported by National Institutes of Health grant P30CA077598 using the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota and by the National Center for Advancing Translational Sciences of the National Institutes of Health award number UL1-TR002494.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: S.H., N.E.J., and D.J.W. performed the retrospective chart reviews; N.E.J. and D.J.W. contributed to the design of the study; S.H. and N.E.J. performed the bibliographic search and wrote the first version of the manuscript; R.S. performed the statistical analysis; and all other authors edited and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shannon Herzog, Division of Hematology, Oncology and Transplantation, University of Minnesota, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: herzo171@umn.edu.
References
Author notes
Presented in abstract form at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 12 December 2022.
All data generated or analyzed during this study are included in this published article. There are no data available or eligible for access.
The full-text version of this article contains a data supplement.