TO THE EDITOR:
Chromosomal translocation t(11:14)(q13:q32) of cyclin D1 (CCND1) gene is the defining hallmark of mantle cell lymphoma (MCL), leading to cyclin D1 overexpression. This overexpression promotes aberrant cell cycle progression by forming complexes with the cyclin-dependent kinase CDK4/6, which are often overexpressed in MCL.1 Moreover, MCL exhibits high levels of genomic instability, leading to dysregulation of the cell cycle and apoptosis pathways.2-4 In particular, the antiapoptotic Bcl-2 protein is typically overexpressed in MCL.5 Although dramatic improvements in the overall survival of patients with MCL have been achieved through the development of targeted therapeutics, including venetoclax-targeting Bcl-2,6 drug resistance and treatment relapses are major challenges. Abemaciclib is a potent and selective inhibitor of CDK4/6, with a higher selectivity against CDK4 than CDK6.7 Abemaciclib demonstrated an objective response rate of 35.7% and complete response rate of 7.1% in a phase 2 clinical trial of patients with relapsed/refractory MCL.8 Therefore, a combinatorial approach is required to enhance the clinical efficacy of abemaciclib. Here, we explored the combination of abemaciclib and venetoclax as a treatment strategy for patients with relapsed/refractory MCL and discovered that the combination could exert a more robust antilymphoma effect than single agents. In addition, we discovered that HSPB1, encoding HSP27, was one of the most downregulated genes upon treatment with the abemaciclib-venetoclax combination. Overexpression or knockout (KO) of HSPB1 in MCL cells revealed its critical role in promoting tumor cell proliferation and therapeutic resistance. These results provide a strong rationale for the clinical evaluation of abemaciclib-venetoclax combination in patients with relapsed/refractory MCL.
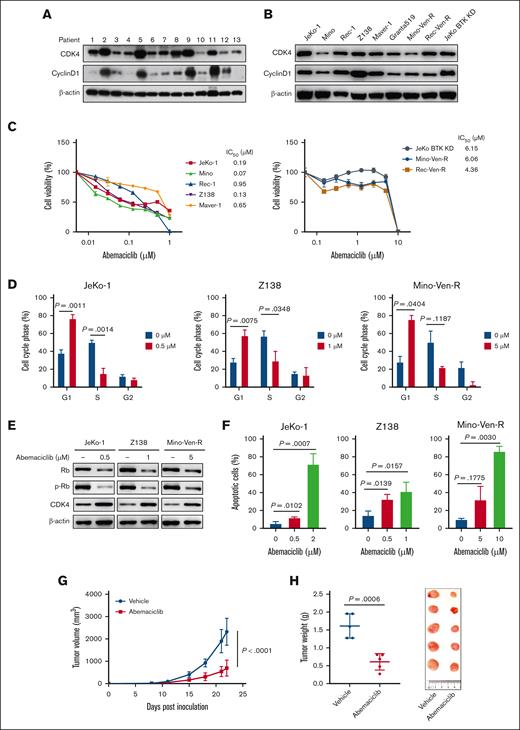
We first demonstrated that CDK4/6 and cyclin D1 were ubiquitously detected in patient samples with MCL and cell lines with cyclin D1 were more selectively expressed in a subset of patient samples (Figure 1A-B; supplemental Table 1). Abemaciclib potently induced antilymphoma activity in JeKo-1, Mino, Z138, Maver-1, and Rec-1 cells (IC50 = 0.07-0.95 μM) but not in Mino-Ven-R and Rec-Ven-R cells with acquired venetoclax resistance9 and JeKo BTK KD cells with primary resistance to both venetoclax and BTK inhibitors10 (IC50 = 4.36-6.15 μM) (Figure 1C). Functionally, abemaciclib potently induced G1-phase cell-cycle arrest in MCL cell lines, accompanied by the downregulation of phosphorylated retinoblastoma protein (p-Rb), indicating that its inhibitory effect may be because of dysregulated cell-cycle progression (Figure 1D-E; supplemental Figure 1A). Moreover, abemaciclib treatment induced apoptosis in a dose-dependent manner in all cell lines tested (Figure 1F; supplemental Figure 1B). Next, we examined the efficacy of abemaciclib in a patient-derived xenograft model derived from a patient with resistance to CD19 CAR T-cell therapy. Relative to the vehicle, abemaciclib significantly inhibited tumor growth, as indicated by tumor volume (n = 5; P < .0001) (Figure 1G) and weight (P = .0006) (Figure 1H). Abemaciclib markedly decreased the metastatic tumor load, as shown by the spleen weight and percentage of CD5-CD20 double-positive cells (P = .0004) (supplemental Figure 1C-D). Consistent with the in vitro data, abemaciclib induced apoptosis of patient-derived xenograft tumors in vivo, as evidenced by elevated PARP cleavage and caspase 7 cleavage (supplemental Figure 1E). These data suggest that targeting cell-cycle regulation is a promising therapeutic strategy in MCL. However, previous clinical data have indicated that treatment with single-agent abemaciclib in patients with relapsed/refractory MCL is suboptimal.8
Abemaciclib exerts antiMCL efficacy by inhibiting cell cycle progression and inducing apoptosis, both in vitro and in vivo. (A-B) Expression of cell cycle regulators in primary patient samples with MCL (A) and MCL cell lines (B). (C) Abemaciclib inhibits cell viability after exposure to increasing concentrations of abemaciclib for 72 hours in MCL cell lines. The IC50 values are shown on the right. Data are presented as mean ± SD. The results are representative of 3 biological replicates. (D) Abemaciclib causes cell cycle arrest at G1 phase after exposure to abemaciclib for 24 hours. Data are indicated as mean ± SD. The results are representative of 3 biological replicates. (E) Representative immunoblot shows the downregulation of p-Rb after 12 hours of treatment with the same doses of abemaciclib shown in (D). (F) Abemaciclib induces dose-dependent apoptosis in MCL cell lines after 48 hours of treatment detected by annexin V/Propidium iodide-binding assay. Results are representative of 3 biological replicates. (G-H) Abemaciclib (10 mg/kg, 5 days per week) inhibits the tumor growth in patient-derived xenograft mouse models (derived from a patient resistant to CD19 CAR T-cell therapy). Tumor volume was monitored and plotted (G). Tumor weights and images at the end of the experiment are presented (H). SD, standard deviation.
Abemaciclib exerts antiMCL efficacy by inhibiting cell cycle progression and inducing apoptosis, both in vitro and in vivo. (A-B) Expression of cell cycle regulators in primary patient samples with MCL (A) and MCL cell lines (B). (C) Abemaciclib inhibits cell viability after exposure to increasing concentrations of abemaciclib for 72 hours in MCL cell lines. The IC50 values are shown on the right. Data are presented as mean ± SD. The results are representative of 3 biological replicates. (D) Abemaciclib causes cell cycle arrest at G1 phase after exposure to abemaciclib for 24 hours. Data are indicated as mean ± SD. The results are representative of 3 biological replicates. (E) Representative immunoblot shows the downregulation of p-Rb after 12 hours of treatment with the same doses of abemaciclib shown in (D). (F) Abemaciclib induces dose-dependent apoptosis in MCL cell lines after 48 hours of treatment detected by annexin V/Propidium iodide-binding assay. Results are representative of 3 biological replicates. (G-H) Abemaciclib (10 mg/kg, 5 days per week) inhibits the tumor growth in patient-derived xenograft mouse models (derived from a patient resistant to CD19 CAR T-cell therapy). Tumor volume was monitored and plotted (G). Tumor weights and images at the end of the experiment are presented (H). SD, standard deviation.
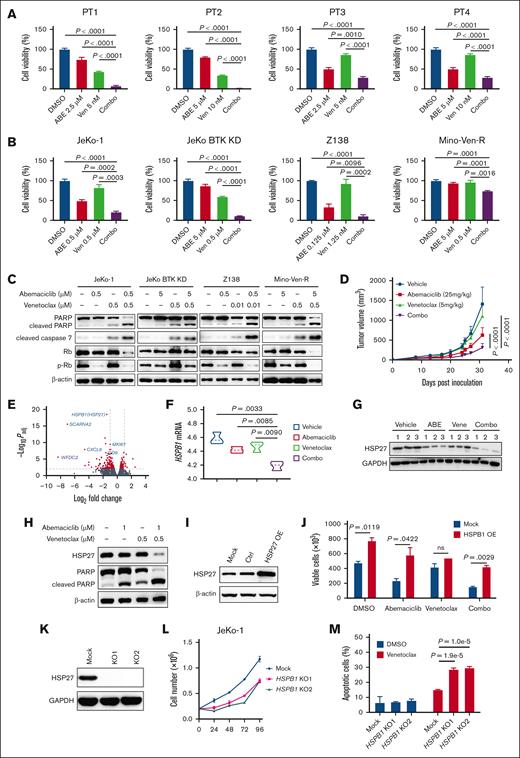
To improve the efficacy of abemaciclib, we evaluated the combination of abemaciclib and venetoclax on primary patient MCL cells. This combination synergistically exerted cytotoxic effects (combination index [CI], 0.87-0.89) in the primary patient MCL cells (Figure 2A) and in all the MCL cell lines tested (CI, 0.39-0.87) (Figure 2B). Furthermore, apoptosis in MCL cell lines was synergistically promoted by the combination treatment over single-agent treatments (CI, 0.25-0.52) (supplemental Figure 2A). Western blot analysis demonstrated enhanced cleavage of PARP and caspase 7, demonstrating that the combination induced potent antilymphoma activity via apoptosis (Figure 2C).
Abemaciclib in combination with venetoclax shows synergistically antiMCL efficacy in vitro and in vivo via downregulation of HSP27. (A) The combination of abemaciclib and venetoclax synergistically induced cytotoxicity after 24 hours of treatment in primary patient cells. (B) The combination of abemaciclib and venetoclax-induced cytotoxicity relative to a single agent after 72 hours of treatment in MCL cell lines. (C) Representative immunoblots indicate that proapoptotic markers were upregulated after treatment with single agents or in combination, as shown in panel B for 12 hours. (D) Mino-Ven-R CDX models treated with vehicle, abemaciclib (25 mg/kg per day, orally), venetoclax (5 mg/kg per day, orally), or their combination. The tumor burden indicated by the tumor volume is plotted. (E) Volcano plot indicating difference in gene expression by RNA-seq of tumors derived from mice treated with vehicle and the combination is shown. The significance with an inclusion level of >0.5 log fold change and adjusted P of <.01 is shown in red. Representative genes are labeled in blue. (F) Reduced mRNA expression of HSPB1 in the tumors of mice treated with vehicle, abemaciclib, venetoclax, or their combination. (G) Reduced HSP27 protein expression in the tumors of mice treated with vehicle, abemaciclib, venetoclax, or their combination. (H) Reduced protein levels of HSP27 and elevated proapoptotic marker cleaved PARP after combination treatment in JeKo-1 cells. (I) Ectopic overexpression of HSP27 protein in JeKo-1 with stable transduction of HSP27 cDNA. (J) Ectopic overexpression of HSP27 in JeKo-1 cells promotes cell growth and decreases sensitivity to abemaciclib and the combination of abemaciclib and venetoclax after 48 hours of treatment. (K) HSP27 KO in JeKo-1 using CRISPR/Cas9. (L-M) HSP27 KO in JeKo-1 cells leads to reduced cell growth (L) and increased sensitivity to venetoclax 48 hours after treatment (M). ABE, abemaciclib; DMSO, dimethyl sulfoxide (vehicle); Ven, venetoclax.
Abemaciclib in combination with venetoclax shows synergistically antiMCL efficacy in vitro and in vivo via downregulation of HSP27. (A) The combination of abemaciclib and venetoclax synergistically induced cytotoxicity after 24 hours of treatment in primary patient cells. (B) The combination of abemaciclib and venetoclax-induced cytotoxicity relative to a single agent after 72 hours of treatment in MCL cell lines. (C) Representative immunoblots indicate that proapoptotic markers were upregulated after treatment with single agents or in combination, as shown in panel B for 12 hours. (D) Mino-Ven-R CDX models treated with vehicle, abemaciclib (25 mg/kg per day, orally), venetoclax (5 mg/kg per day, orally), or their combination. The tumor burden indicated by the tumor volume is plotted. (E) Volcano plot indicating difference in gene expression by RNA-seq of tumors derived from mice treated with vehicle and the combination is shown. The significance with an inclusion level of >0.5 log fold change and adjusted P of <.01 is shown in red. Representative genes are labeled in blue. (F) Reduced mRNA expression of HSPB1 in the tumors of mice treated with vehicle, abemaciclib, venetoclax, or their combination. (G) Reduced HSP27 protein expression in the tumors of mice treated with vehicle, abemaciclib, venetoclax, or their combination. (H) Reduced protein levels of HSP27 and elevated proapoptotic marker cleaved PARP after combination treatment in JeKo-1 cells. (I) Ectopic overexpression of HSP27 protein in JeKo-1 with stable transduction of HSP27 cDNA. (J) Ectopic overexpression of HSP27 in JeKo-1 cells promotes cell growth and decreases sensitivity to abemaciclib and the combination of abemaciclib and venetoclax after 48 hours of treatment. (K) HSP27 KO in JeKo-1 using CRISPR/Cas9. (L-M) HSP27 KO in JeKo-1 cells leads to reduced cell growth (L) and increased sensitivity to venetoclax 48 hours after treatment (M). ABE, abemaciclib; DMSO, dimethyl sulfoxide (vehicle); Ven, venetoclax.
To further investigate the efficacy of this combination, we generated Mino-Ven-R cell line-derived xenografts (CDX). Remarkably, the abemaciclib-venetoclax combination exhibited significant antitumor efficacy beyond either single-agent treatment (n = 5; P < .0001) (Figure 2D). No apparent abnormality was observed in mice treated with abemaciclib or venetoclax alone, or in combination. No pathological changes were detected in liver or kidney function in any of the treatment group, as evidenced by serum biochemical parameters alanine aminotransferase and aspartate aminotransferase for the liver and creatinine and blood urea nitrogen for the kidney (supplemental Figure 2B).
To understand the mechanistic basis of the combinatorial antilymphoma effects, we performed bulk RNA sequencing (RNA-seq) on all groups from the Mino-Ven-R CDX models. Transcriptome analysis revealed 358 downregulated genes and 123 upregulated genes in the combination treatment group compared with those in the vehicle group (Figure 2E). Next, we identified the genes that were differentially expressed for both combination vs abemaciclib and combination vs venetoclax. These overlapping gene sets were then subjected to gene set enrichment analysis, which demonstrated significant enrichment of genes regulating hypoxia (supplemental Figure 2C). We verified the top 10 downregulated genes in the combination treatment group compared with the groups treated with abemaciclib or venetoclax. SCARNA2 and HSPB1 were the top 2 downregulated genes with the lowest P values compared with the combination with vehicle (Figure 2E). SCARNA2 was not selected for further study because of the low number of mRNA reads (supplemental Figure 2D). In contrast, HSPB1 was expressed at much higher levels and was significantly different between the combination and single treatments (Figure 2F; supplemental Figure 2E-F). WFDC2 showed the highest fold change compared with the combination with vehicle but with a much lower significance. The role of WFDC2 in cancer has not been well defined and the data appear inconsistent.11,12 Therefore, HSPB1 was selected for further analysis. HSPB1, which encodes heat shock protein 27 (HSP27), is upregulated during cell stress and is associated with protein misfolding.13 In cancer cells, HSP27 can protect cells against apoptosis and other types of cell death, promote tumor growth and metastasis, and provide a mechanism for resistance to many anticancer agents, which often stimulate HSP27 expression in cancer cells.14
The combination treatment markedly reduced HSP27 expression at both the mRNA and protein levels beyond either single agent in Mino-Ven-R CDX tumors (Figure 2F-G). This was further validated in vitro using JeKo-1 cells (Figure 2H). To understand the role of HSP27 in MCL, we ectopically overexpressed HSP27 in JeKo-1 cells (Figure 2I). HSP27 overexpression markedly promoted tumor cell growth and enhanced resistance to abemaciclib and its combination (Figure 2J). Consistent with this, HSP27 KO significantly suppressed the growth of JeKo-1 cells and primed the cells for venetoclax–induced cell apoptosis (Figure 2K-M).
In conclusion, we showed that the dual inhibition of CDK4/6 with abemaciclib and Bcl-2 with venetoclax induces synergistic antiMCL activity in a variety of in vitro and in vivo preclinical relapsed/refractory MCL models. Similar to the single agents abemaciclib and venetoclax, the combination did not induce apparent toxicity in mouse models, thus demonstrating a favorable safety profile in animals. The combination also markedly downregulated HSP27, revealing a potential mechanistic underpinning of the observed synergistic activity. Our data in MCL cells with HSP27 overexpression or KO support that HSP27 represents a novel vulnerability for therapeutic development, and targeting HSP27 may have the potential to overcome venetoclax resistance. Altogether, our study provides a strong rationale for further preclinical evaluation of this combinatorial strategy, which can hopefully be translated into a clinical setting for the treatment of patients with relapsed/refractory MCL.
Patient consent and ethical approval: Patient samples were collected after obtaining informed consent and approval from the Institutional Review Board at the MD Anderson Cancer Center. All animal procedures were approved by the Institutional Animal Care and Use Committee of the MD Anderson Cancer Center.
Acknowledgments: The authors thank Paul C. Dolber and Numsen Hail for manuscript revision and editing. This work was supported by a grant from Eli Lilly and Company.
Contribution: M.W., V.C.J., Y.C., and Y. Liu contributed to the experimental conception and design; Y.C., Y. Liu, V.C.J., Y. Li, J.M.M., A.J., and W.W. contributed to the experimental execution and data analysis; F.Y. and Y.C. contributed to the RNA-seq data analysis; and Y.C., L.N., H.-H.L., Y.Y., J.J., Z.Z., V.C.J., and M.W. contributed to manuscript draft and revision.
Conflict-of-interest disclosure: M.W. is consultant to AstraZeneca, BeiGene, BioInvent, CSTone, Deciphera, DTRM Biopharma (Cayman) Limited, Epizyme, Genentech, InnoCare, Janssen, Juno Therapeutics, Kite Pharma, Lilly, Loxo Oncology, Miltenyi Biomedicine GmbH, Oncternal, Pepromene Bio, Pharmacyclics, VelosBio. M.W. has received research support from Acerta Pharma, AstraZeneca, BeiGene, BioInvent, Celgene, Genmab, Genentech, Innocare, Janssen, Juno Therapeutics, Kite Pharma, Lilly, Loxo Oncology, Molecular Templates, Oncternal, Pharmacyclics, VelosBio, Vincerx. M.W. received speaker honoraria from Acerta Pharma, Anticancer Association, AstraZeneca, BeiGene, BGICS, BioInvent, CAHON, Clinical Care Options, Dava Oncology, Eastern Virginia Medical School, Epizyme, Hebei Cancer Prevention Federation, Imedex, Janssen, Kite Pharma, Leukemia & Lymphoma Society, LLC TS Oncology, Medscape, Meeting Minds Experts, Miltenyi Biomedicine GmbH, Moffit Cancer Center, Mumbai Hematology Group, OMI, OncLive, Pharmacyclics, Physicians Education Resources, Practice Point Communications, The First Afflicted Hospital of Zhejiang University. The remaining authors declare no competing financial interests.
Correspondence: Vivian Changying Jiang, Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, Houston, TX, 77030; e-mail: cjiang@mdanderson.org; and Michael Wang, Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, Houston, TX, 77030; e-mail: miwang@mdanderson.org.
References
Author notes
Original data and protocols are available upon request from the corresponding author, Michael Wang (miwang@mdanderson.org).
The full-text version of this article contains a data supplement.