Key Points
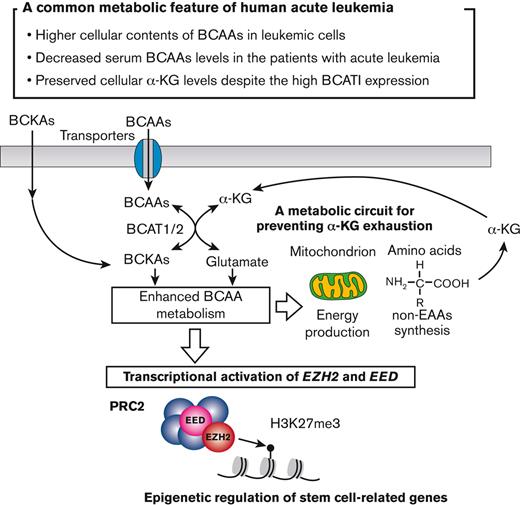
Human acute leukemia cells form a unique metabolic circuit for sustaining BCAA catabolism activity without α-KG exhaustion.
BCAA metabolism represents a novel regulator of PRC2 to maintain the stemness of acute leukemia.
Abstract
Cancer-specific metabolic activities play a crucial role in the pathogenesis of human malignancies. To investigate human acute leukemia–specific metabolic properties, we comprehensively measured the cellular metabolites within the CD34+ fraction of normal hematopoietic stem progenitor cells (HSPCs), primary human acute myelogenous leukemia (AML), and acute lymphoblastic leukemia (ALL) cells. Here, we show that human leukemia cells are addicted to the branched-chain amino acid (BCAA) metabolism to maintain their stemness, irrespective of myeloid or lymphoid types. Human primary acute leukemias had BCAA transporters for BCAA uptake, cellular BCAA, α-ketoglutarate (α-KG), and cytoplasmic BCAA transaminase-1 (BCAT1) at significantly higher levels than control HSPCs. Isotope-tracing experiments showed that in primary leukemia cells, BCAT1 actively catabolizes BCAA using α-KG into branched-chain α-ketoacids, whose metabolic processes provide leukemia cells with critical substrates for the trichloroacetic acid cycle and the synthesis of nonessential amino acids, both of which reproduce α-KG to maintain its cellular level. In xenogeneic transplantation experiments, deprivation of BCAA from daily diet strongly inhibited expansion, engraftment and self-renewal of human acute leukemia cells. Inhibition of BCAA catabolism in primary AML or ALL cells specifically inactivates the function of the polycomb repressive complex 2, an epigenetic regulator for stem cell signatures, by inhibiting the transcription of PRC components, such as zeste homolog 2 and embryonic ectoderm development. Accordingly, BCAA catabolism plays an important role in the maintenance of stemness in primary human AML and ALL, and molecules related to the BCAA metabolism pathway should be critical targets for acute leukemia treatment.
Introduction
Hematopoietic stem cells (HSCs) have the capability to self-renew, maintaining an undifferentiated status, as well as to proliferate and mature into blood cells. Similarly, cancer stem cells (CSCs) self-renew and propagate to form cancer tissues. In humans, acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) harbor such CSC-like properties, which is evidenced by the fact that a fraction of the human AML and ALL cells reconstitute leukemias serially in xenogeneic transplantation using immunodeficient mice. To obtain a “cure,” it is critical to understand molecular machinery as to how such leukemia cells self-renew and expand. Epigenetic regulation is one of the critical machineries for maintaining stem cell properties in leukemia as well as normal HSCs.1 Polycomb repressive complex 2 (PRC2) is a general epigenetic regulator of stem cells, which mediates the epigenetic gene silencing through histone modification of trimethylation of histone H3 at lysine 27 (H3K27me3).2 Disruption of PRC2 components disturbs maintenance of both normal HSCs and AML in murine models.3 Suppression of the enhancer of zeste homolog 1 (EZH1) and EZH2, catalytic subunits of PRC2, also inhibits AML propagation.4
It has been shown that specific metabolites play a critical role in the regulation of key epigenetic enzymes. A variety of metabolites, including α-ketoglutarate (α-KG),5 2-hydroxyglutarate,6 S-adenosylmethionine,7 and acetyl coenzyme A8 are substrates for epigenetic machinery and can alter the function of key epigenetic enzymes. Recent studies have shown that branched-chain amino acid (BCAA), such as Leu, Ile, and Val, plays a critical role in cancer development9,10 and the maintenance of self-renewing murine HSCs.11
The BCAA metabolism starts with the transfer of an α-amino group to α-KG, resulting in the production of Glu and branched-chain α-ketoacids (BCKAs). This BCAA catabolic process is dependent upon cytoplasmic BCAA transaminase-1 (BCAT1), which is mainly expressed in neurons or on ubiquitous mitochondrial BCAT2. Interestingly, the ectopic BCAT1 expression is associated with aggressiveness in several types of cancers, including murine myeloid malignancy models12,13 and human myeloid malignancies.14 It has been reported that murine chronic myeloid leukemia (CML) cells use BCAT1 to reversely synthesize BCAA from BCKA to maintain cellular BCAA at high levels, which play a role in leukemia propagation by activating mTOR,12 and this phenomenon was also observed in murine leukemia models with EZH2 inactivation plus oncogenic N-Ras activation.13 By contrast, another study showed that BCAA to BCKA catabolism is enhanced by the overexpressed BCAT1, resulting in a shortage of cellular α-KG, which eventually causes TET2 dysfunction in the demethylation of genes.14 Thus, although the significance of the ectopic expression of BCAT1 in myeloid malignancies has been recognized, its functional outcome is still controversial. It remains unclear whether BCAT1 catabolizes or synthesizes BCAA or whether ectopic BCAT1 affects epigenetic regulation through cellular α-KG concentration in a malignant state. It is also important to elucidate downstream molecular effectors of the BCAA metabolism in malignant cells.
In this study, using differential analysis of metabolites in normal and malignant CD34+ AML/ALL human cells, we found that activated BCAA catabolism is a common metabolic feature of human acute leukemia cells, irrespective of their lineage origin, and plays an important role in self-renewal and propagation properties of leukemia cells by regulating PRC2 function.
Material and methods
Clinical samples
Bone marrow, peripheral blood, and serum samples from patients with hematologic malignancies diagnosed according to the World Health Organization criteria were enrolled in this study. Supplemental Tables 1 and 2 summarize the characteristics of the patients with primary AML and patients with primary ALL, respectively, analyzed in the metabolome analysis. Supplemental Table 3 summarizes the information of the cellular samples used in in vitro experiments. Informed consent was obtained from all patients and control participants following the Declaration of Helsinki (1975, revised in 1983). The Institutional Review Board of Kyushu University approved all research on human participants.
Metabolome analysis
Metabolome analysis was conducted using the C-SCOPE package by Human Metabolome Technologies. Details of the metabolome analysis methods are provided in the supplemental data.
Results
Human acute leukemia cells uptake and use BCAA, irrespective of their lineage type
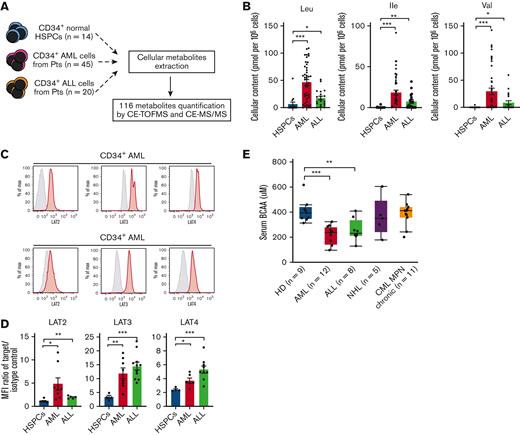
To identify the human acute leukemia–specific metabolic signature, we extracted the metabolites from purified CD34+ AML cells (n = 45), CD34+ ALL cells of B cell type (n = 20), and CD34+ normal HSPCs (n = 14) from healthy donors (HDs) and quantified 116 metabolites (supplemental Table 4) using a highly sensitive capillary electrophoresis-time-of-flight mass spectrometry (CE-TOFMS) and a capillary electrophoresis-tandem mass spectrometry (CE-MS/MS) (Figure 1A). Among the 116 metabolites, 29 in AML (supplemental Figure 1A) and 23 in ALL (supplemental Figure 1B) were identified as cellular metabolites with concentrations that were significantly different from those in HSPCs. Strikingly, all 3 BCAAs (Leu, Ile, and Val) were listed in the analysis of either type of acute leukemia. Figure 1B summarizes the concentration of BCAAs in all samples, demonstrating that CD34+ AML and ALL cells possess significantly higher BCAA contents than CD34+ HSPCs. This was also observed when the data were normalized using the total observed metabolites concentration method15 (supplemental Figure 1C).
Human acute leukemia cells actively transport and use BCAA. (A) The schema for the cellular metabolite quantification. (B) Cellular content of each BCAA in normal CD34+ HSPCs, AML cells, and ALL cells. (C) Representative fluorescence-activated cell sorting (FACS) data for the expression of BCAA transporters, including large neutral amino acid transporter 2 (LAT2), LAT3, and LAT4 in primary CD34+ AML (upper panels) and CD34+ ALL cells (lower panels). Gray and red lines represent isotype control and anti-LAT antibodies, respectively. (D) Quantification of LAT2, LAT3, and LAT4 proteins in normal and leukemic CD34+ cells using the mean fluorescent intensity (MFI) ratio between the target and the isotype control. (E) Quantification of serum BCAA concentration in HDs and patients with hematologic malignancies, including AML, ALL, non-Hodgkin lymphoma, and CML/myeloproliferative neoplasms chronic phase. Notably, patients with AML and patients with ALL had significantly decreased levels. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, panels B and D, mean ± standard error of the mean (SEM). Max, maximum; pt, patient.
Human acute leukemia cells actively transport and use BCAA. (A) The schema for the cellular metabolite quantification. (B) Cellular content of each BCAA in normal CD34+ HSPCs, AML cells, and ALL cells. (C) Representative fluorescence-activated cell sorting (FACS) data for the expression of BCAA transporters, including large neutral amino acid transporter 2 (LAT2), LAT3, and LAT4 in primary CD34+ AML (upper panels) and CD34+ ALL cells (lower panels). Gray and red lines represent isotype control and anti-LAT antibodies, respectively. (D) Quantification of LAT2, LAT3, and LAT4 proteins in normal and leukemic CD34+ cells using the mean fluorescent intensity (MFI) ratio between the target and the isotype control. (E) Quantification of serum BCAA concentration in HDs and patients with hematologic malignancies, including AML, ALL, non-Hodgkin lymphoma, and CML/myeloproliferative neoplasms chronic phase. Notably, patients with AML and patients with ALL had significantly decreased levels. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, panels B and D, mean ± standard error of the mean (SEM). Max, maximum; pt, patient.
We next evaluated LAT family proteins (LAT1, LAT2, LAT3, and LAT4) required for the BCAA transportation.16 In all the AML or ALL samples analyzed, CD34+ leukemia cells expressed higher levels of LAT2, LAT3, and LAT4, but not LAT1 (data not shown) relative to CD34+ HSPCs (Figures 1C-D), and in AML, the CD34+ CD38– leukemia stem cell fraction had further higher levels of LAT3 and LAT4 protein than normal CD34+ CD38− HSCs (supplemental Figure 1D). In both AML and ALL, BCAA levels in patients’ sera were significantly lower than those in HDs, whereas this was not observed in patients with non-Hodgkin lymphoma, CML, or myeloproliferative neoplasms (Figure 1E). These results suggest that human acute leukemia cells express BCAA transporters and actively uptake BCAA.
Human acute leukemia cells contain abundant BCAT1 and catabolize BCAA to maintain cellular α-KG
The BCAA catabolism begins with the transfer of an α-amino group to α-KG via cytosolic BCAT1 or mitochondrial BCAT2, which yields Glu and its respective BCKAs17 (Figure 2A). Intracellular flow cytometry (FCM) analysis revealed that CD34+ AML and ALL cells expressed higher levels of BCAT1 protein than normal CD34+ HSPCs (Figure 2B; supplemental Figure 2A). Quantitative real-time polymerase chain reaction (PCR) showed that the expression of BCAT1 was higher in leukemia CD34+ cells, whereas BCAT2 expression was not elevated (supplemental Figure 2B). The BCAT1 levels in CD34+ CD38– AML cells were even higher than those in CD34+ CD38− HSCs (supplemental Figure 2C). The PRECOG database18 showed that a higher level of BCAT1 is associated with poor survival in patients with AML and patients with ALL (supplemental Figure 2D), with survival z-scores representing the statistical significance of the association between each gene and survival, and those of BCAT1 in AML and ALL were 4.67 and 2.06, respectively.
Acute leukemia cells catabolize BCAA to maintain mitochondrial OXPHOS and non-EAA synthesis, sustaining cellular α-KG levels. (A) The schema for the BCAA metabolism pathway. (B) Comparison of BCAT1 expression levels in normal CD34+ HSPCs (n = 6), CD34+ AML cells (n = 9), and CD34+ ALL cells (n = 11). The MFI ratio of the target and isotype control for each sample is shown. (C) Cellular contents of α-KG in CD34+ HSPCs- (n = 14), AML cells (n = 45), and ALL cells (n = 20) in our metabolome analysis. (D) The alteration of cellular α-KG levels in primary AML cells (n = 5) and ALL cells (n = 9) by the addition of gabapentin (20 mM) and L-asparaginase (1.0 IU/mL) for 24 hours are shown. The α-KG levels under control conditions are set to 1.0. (E) The alteration of cellular α-KG levels in primary AML cells (n = 7) and ALL cells (n = 8) after 24-hour culture in control and BCAA- or Thr/Phe/Lys-free DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS) are shown. The α-KG levels in control conditions are set to 1.0. (F) The schema and results for the isotope-tracing experiments using primary AML (n = 2) and ALL (n = 2) samples cultured in HPLM containing 160 uM of [13C6,15N1] Leu are shown. Percentages of [13C6,15N1] Leu–derived citrate (M+1 and M+2) and non-EAA (M+1) including Glu, Asp, Ala, and Ser among the total amount of corresponding metabolites quantified at the indicated time points are shown. Results from at least 3 independent experiments are shown. (G) The schema and results for isotope-tracing experiments using primary AML (n = 2) and ALL (n = 1) samples cultured in HPLM containing 30 uM of [13C6] α-KIC are shown. Results from at least 3 independent experiments are shown. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, panels B, C, D, E, F, and G, mean ± SEM. α-KIC, α-ketoisocaproate; EAA, essential amino acids; HPLM, human plasma-like medium; OXPHOS, oxidative phosphorylation; TCA, trichloroacetic acid.
Acute leukemia cells catabolize BCAA to maintain mitochondrial OXPHOS and non-EAA synthesis, sustaining cellular α-KG levels. (A) The schema for the BCAA metabolism pathway. (B) Comparison of BCAT1 expression levels in normal CD34+ HSPCs (n = 6), CD34+ AML cells (n = 9), and CD34+ ALL cells (n = 11). The MFI ratio of the target and isotype control for each sample is shown. (C) Cellular contents of α-KG in CD34+ HSPCs- (n = 14), AML cells (n = 45), and ALL cells (n = 20) in our metabolome analysis. (D) The alteration of cellular α-KG levels in primary AML cells (n = 5) and ALL cells (n = 9) by the addition of gabapentin (20 mM) and L-asparaginase (1.0 IU/mL) for 24 hours are shown. The α-KG levels under control conditions are set to 1.0. (E) The alteration of cellular α-KG levels in primary AML cells (n = 7) and ALL cells (n = 8) after 24-hour culture in control and BCAA- or Thr/Phe/Lys-free DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS) are shown. The α-KG levels in control conditions are set to 1.0. (F) The schema and results for the isotope-tracing experiments using primary AML (n = 2) and ALL (n = 2) samples cultured in HPLM containing 160 uM of [13C6,15N1] Leu are shown. Percentages of [13C6,15N1] Leu–derived citrate (M+1 and M+2) and non-EAA (M+1) including Glu, Asp, Ala, and Ser among the total amount of corresponding metabolites quantified at the indicated time points are shown. Results from at least 3 independent experiments are shown. (G) The schema and results for isotope-tracing experiments using primary AML (n = 2) and ALL (n = 1) samples cultured in HPLM containing 30 uM of [13C6] α-KIC are shown. Results from at least 3 independent experiments are shown. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, panels B, C, D, E, F, and G, mean ± SEM. α-KIC, α-ketoisocaproate; EAA, essential amino acids; HPLM, human plasma-like medium; OXPHOS, oxidative phosphorylation; TCA, trichloroacetic acid.
Primary CD34+ AML and ALL cells had cellular α-KG (Figure 2C) and BCAT1 protein at higher levels than normal HSPCs. After pharmacological inhibition of BCAT1 by gabapentin, a Leu analog,19 cellular α-KG levels of primary AML and ALL significantly decreased, whereas this phenomenon was reversed by the addition of a high concentration of BCAA (Figure 2D). By contrast, L-asparaginase, a key drug that targets the amino acid metabolism by inducing the depletion of Asn and Gln,20 did not affect the α-KG level. Furthermore, when primary leukemia cells were cultured in a BCAA-free medium, the concentration of cellular α-KG was significantly decreased, whereas restriction of other major EAAs, such as Thr, Lys, and Phe did not affect α-KG levels (Figure 2E). Notably, gabapentin or BCAA restriction did not affect the survival or cell cycle status of leukemia cells, at least for 24 hours (supplemental Figure 2E-H). Short hairpin RNA–based knock down of BCAT1 (supplemental Figure 2I) reduced α-KG in the cell lines (supplemental Figure 2J). These data suggest that BCAT1 actively catabolizes BCAA-producing α-KG in human acute leukemia.
BCAA catabolism supports mitochondrial oxidative phosphorylation and non-EAA synthesis in primary human leukemia cells
To track the metabolic fate of BCAA, we performed [13C6,15N1] Leu stable-isotope tracing experiments. Two cases each of primary AML and ALL cells were transferred into HPLM21 (supplemental Table 5) containing 160 μM of [13C6,15N1] Leu. After 2 hours of culture, cellular metabolites were extracted and quantified. Figure 2F shows carbon and nitrogen atom transitions. The nitrogen atoms of [13C6,15N1] Leu were transferred to α-KG by BCATs, resulting in the production of α-KIC and M+1 Glu. α-KIC is catabolized by branched-chain α-ketoacid dehydrogenases (BCKDHs), such as BCKDH E1 subunit alpha (BCKDHA) and BCKDH E1 subunit beta (BCKDHB) to yield citrate in the TCA cycle,22 in which α-KG is one of the consequent metabolites (Figure 2F). CD34+ AML and ALL cells had BCKDHA at a higher level than CD34+ HSPCs (supplemental Figure 2K). The percentages of M+1 and M+2 citrate significantly increased during tracing, suggesting potentiation of mitochondrial OXPHOS) (Figure 2F lower panels). Consistently, BCAT1 inhibition by gabapentin reduced adenosine triphosphate–linked respiration, which represents OXPHOS activity in human leukemia cells (supplemental Figure 3A).
M+1 Glu was used to synthesize Asp, Ala, and Ser using aspartate transaminase, alanine transaminase, and phosphoserine aminotransferase, respectively, to produce α-KG (Figure 2F). During the tracing experiments, the frequencies of M+1 Glu, Asp, and Ala were significantly elevated (Figure 2F, right panels), whereas gabapentin suppressed the increase in non-EAAs and citrate from Leu in primary leukemia cells (supplemental Figure 3B-C).
BCAT1 catalyzes the reversible transamination of BCAA in murine leukemia models.12,13 We tested whether the reamination of α-KIC by BCATs contributes to BCAA synthesis12,13 in primary leukemia. When we cultured human leukemia cells in media containing 30μM of [13C6] α-KIC,13 the frequency of M+6 Leu significantly increased (Figure 2G); however, this was suppressed by gabapentin (supplemental Figure 3D), suggesting that BCAT-dependent reamination of BCKAs can occur in primary human leukemias.
Suppression of BCAA metabolism attenuates the engraftment and propagation of human leukemias in a xenogeneic transplantation model
We found that deprivation of BCAA from cultures inhibited the growth of cell lines in a dose-dependent manner. As shown in supplemental Figure 4A-B, restriction of BCAA significantly inhibited their growth in vitro, whereas deprivation of other general EAAs such as Thr, Lys, and Phe did not.
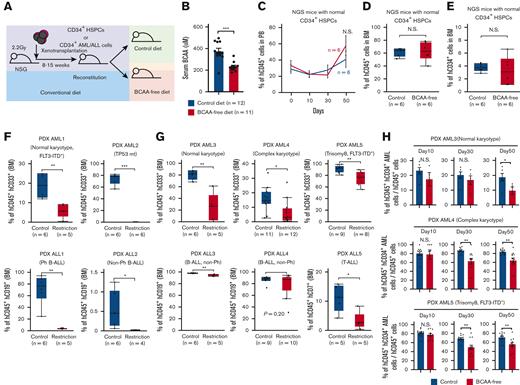
We then tested the effect of BCAA deprivation on primary human acute leukemia cells in patient-derived xenograft (PDX) models. Purified CD34+ leukemic cells from patients with AML or patients with ALL were transplanted into the irradiated NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice.23 Details of PDX experiments are summarized in supplemental Table 6. Eight to 15 weeks after transplantation, when reconstitution was confirmed, we started a BCAA-free diet that was designed to deliver the calories, minerals, and vitamins equal to the control diet but did not contain any BCAA (Figure 3A) (supplemental Table 7). NSG mice tolerated the BCAA-free diet well, except for mild weight loss during 2 the months of treatment. The serum concentration of BCAAs significantly decreased in mice fed the BCAA-free diet by up to 50% of that in mice fed with the control diet on day 14 (Figure 3B). This in vivo BCAA restriction did not affect the reconstitution of normal hematopoiesis in the blood or the bone marrow of mice transplanted with CD34+ cord blood cells (Figure 3C-D). The percentage of immature hCD34+ HSPCs remained unchanged (Figure 3E).
The BCAA-free diet suppresses human acute leukemia development in xenotransplantation models. (A) The schema for xenogeneic transplantation experiments to evaluate the effects of a BCAA-free diet on leukemia progression. (B) Changes in the concentration of serum BCAA in NSG mice 2 weeks after commencement of the BCAA-free diet. (C) Sequential changes in percentages of human cell chimerism in the blood of NSG mice reconstituted with normal CD34+ HSPCs after starting the BCAA-free diet (blue line, control group and red line, BCAA-free group). (D) Human cell chimerism in the bone marrow of NSG mice reconstituted with normal CD34+ HSPCs at 7 weeks after starting the BCAA-free diet. (E) Frequencies of hCD45+ hCD34+ HSPCs in the bone marrow of the NSG mice reconstituted with normal CD34+ HSPCs are shown. (F) Evaluation of engraftment and reconstitution potential of human AML and ALL cells in NSG mice fed the BCAA-free or control diet. Recipient mice were fed either diet, 10 days before transplantation. Six to 8 weeks after transplantation, tumor burdens were evaluated in the bone marrow. Results from 4 independent experiments are shown. (G) Percentages of human acute leukemia cells in the bone marrow of each group. (H) The changes in the frequency of CD34+ immature AML fraction during dietary restriction of BCAA in vivo. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, panels B, C, and H, mean ± SEM. BM, bone marrow; PB, peripheral blood; Ph, Philadelphia chromosome.
The BCAA-free diet suppresses human acute leukemia development in xenotransplantation models. (A) The schema for xenogeneic transplantation experiments to evaluate the effects of a BCAA-free diet on leukemia progression. (B) Changes in the concentration of serum BCAA in NSG mice 2 weeks after commencement of the BCAA-free diet. (C) Sequential changes in percentages of human cell chimerism in the blood of NSG mice reconstituted with normal CD34+ HSPCs after starting the BCAA-free diet (blue line, control group and red line, BCAA-free group). (D) Human cell chimerism in the bone marrow of NSG mice reconstituted with normal CD34+ HSPCs at 7 weeks after starting the BCAA-free diet. (E) Frequencies of hCD45+ hCD34+ HSPCs in the bone marrow of the NSG mice reconstituted with normal CD34+ HSPCs are shown. (F) Evaluation of engraftment and reconstitution potential of human AML and ALL cells in NSG mice fed the BCAA-free or control diet. Recipient mice were fed either diet, 10 days before transplantation. Six to 8 weeks after transplantation, tumor burdens were evaluated in the bone marrow. Results from 4 independent experiments are shown. (G) Percentages of human acute leukemia cells in the bone marrow of each group. (H) The changes in the frequency of CD34+ immature AML fraction during dietary restriction of BCAA in vivo. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, panels B, C, and H, mean ± SEM. BM, bone marrow; PB, peripheral blood; Ph, Philadelphia chromosome.
Next, we evaluated the effect of BCAA restriction on leukemia engraftment. CD34+ leukemic cells were transplanted into irradiated NSG mice fed a control or BCAA-free diet, 10 days before transplantation. The recipient mice were fed their respective diet for 6 to 8 weeks after transplantation. The reconstitution of leukemia was severely impaired only in mice fed the BCAA-free diet (Figure 3F). Furthermore, in mice reconstituted with primary AML and ALL, their expansion in the blood was progressively inhibited by the BCAA-free diet (supplemental Figure 4C). Mice were sacrificed on day 30 or 50, when mice fed the BCAA-free diet had significantly reduced percentages of leukemia cells in the bone marrow (Figure 3G) or spleen (supplemental Figure 4D) in all 3 AML and ALL cases tested. In this experiment, the frequency of hCD45+ hCD34+ immature AML fractions gradually declined during BCAA restriction in all tested AML cases (Figure 3H; supplemental Figure 4E), suggesting that BCAA metabolism plays a role in the maintenance of human leukemia stemness. We also evaluated the effect of gabapentin treatment on engrafted human leukemia in mice fed the control diet. After starting daily injection of gabapentin, human AML and ALL burdens were significantly reduced on day 14 (supplemental Figure 4F). These data suggest that the engraftment and propagation of human AML and ALL cells are dependent on BCAA metabolism.
Deprivation of BCAA significantly inhibits the self-renewal potential of human acute leukemia in a cell-intrinsic manner
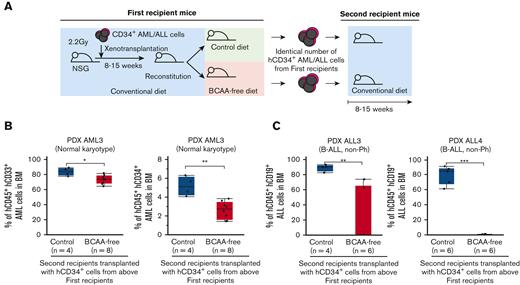
We then performed serial transplantation experiments (Figure 4A). CD34+ human AML cells from a patient (PDX AML3) were harvested from first AML recipients receiving control or BCAA-free diets, and 0.5 × 106 cells per mouse were retransplanted into secondary recipients. All the secondary recipients were fed a conventional diet. Twelve weeks after the second transplantation, cells from the first recipients fed the BCAA-free diet showed significantly less reconstitution, which was represented by lower percentages of total or hCD34+ AML cells, compared with those receiving the control diet, in the bone marrow (Figure 4B). In ALL, CD34+ human ALL cells from 2 patients (PDX ALL3 and PDX ALL4) were harvested from first ALL recipients with control or BCAA-free diet, and 0.8 ×106 or 2.8×106 cells per mouse were re-transplanted into the secondary recipients that were fed with the conventional diet. Eight and 15 weeks after the serial transplantations, hCD34+ ALL cells from mice on BCAA-free diet exhibited an impaired reconstitution of human ALL in secondary recipients, whereas those from mice fed the control diet again developed ALL with a high leukemia burden in the bone marrow (Figure 4C). Because the reconstitution potential of cells harvested from first recipients with a BCAA-free diet continued to be suppressed in secondary recipients with a normal diet, we hypothesized that BCAA insufficiency epigenetically inhibits the self-renewal of human leukemia in vivo.
Restriction of BCAA inhibits self-renewal of human AML and ALL cells. (A) The schema for serial xenotransplantation experiments. The primary recipients were fed the BCAA-free or control diet for 5 to 7 weeks, and all secondary recipients were fed the control diet for 8 to 15 weeks. (B) Frequencies of human CD45+ AML cells and CD34+ immature leukemia cells in the bone marrow of secondary recipients that were transplanted with hCD34+ AML (PDX AML3) harvested from the first recipients with or without BCAA restriction (C) Frequencies of human CD45+ ALL cells in the bone marrow of secondary recipients transplanted with hCD34+ ALL cells harvested from the first recipients with or without the dietary restriction of BCAA (PDX ALL3 and PDX ALL4). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001.
Restriction of BCAA inhibits self-renewal of human AML and ALL cells. (A) The schema for serial xenotransplantation experiments. The primary recipients were fed the BCAA-free or control diet for 5 to 7 weeks, and all secondary recipients were fed the control diet for 8 to 15 weeks. (B) Frequencies of human CD45+ AML cells and CD34+ immature leukemia cells in the bone marrow of secondary recipients that were transplanted with hCD34+ AML (PDX AML3) harvested from the first recipients with or without BCAA restriction (C) Frequencies of human CD45+ ALL cells in the bone marrow of secondary recipients transplanted with hCD34+ ALL cells harvested from the first recipients with or without the dietary restriction of BCAA (PDX ALL3 and PDX ALL4). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001.
BCAA metabolism regulates PRC2 activity to maintain the stem cell–related gene expression in human acute leukemia cells
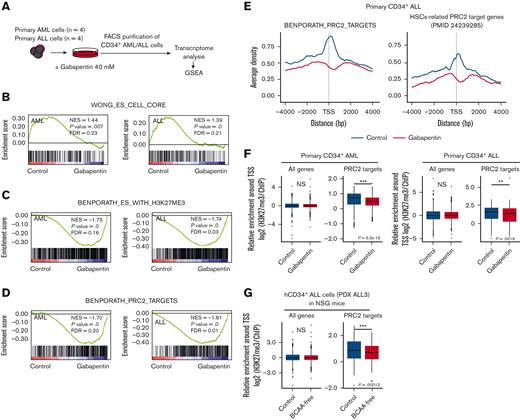
To clarify how the BCAA metabolism pathway regulates acute leukemia–initiating activities, primary CD34+ AML cells (n = 4) and CD34+ ALL cells (n = 4) were cultured with or without 40mM of gabapentin for 16 hours, and CD34+ AML and ALL cells were then purified and analyzed for global gene expression signatures (Figure 5A). We performed GSEA24 and extracted the sets significantly affected by gabapentin.
Inhibition of BCAA metabolism suppresses the PRC2 activity in human acute leukemia. (A) The schema for transcriptome assays in primary human CD34+ AML and ALL cells with or without gabapentin treatment. Global changes in expression profiles were analyzed by the GSEA method. (B) Enrichment plots of embryonic stem (ES) core genes (WONG_ES_CELL_CORE). (C) Enrichment plots of genes marked with H3K27me3 modification in their promoters in human ES cells (BENPORATH_ES_WITH_H3K27ME3). (D) Enrichment plots of PRC2-target genes in human ES cells. (E) Aggregation plots of H3K27me3 ChIP-seq signals centered at TSSs in the levels of ES PRC2 target genes (left panel) and HSCs-related PRC2 target genes (right panel). The blue and red lines show the control and the gabapentin-treated (40 mM for 16 hours) leukemia cells, respectively. (F) Box plots showing H3K27me3 ChIP-seq signal intensities for the whole or the ES PRC2 target genes at the TSS+5 kilobasepairs (kbp) in primary CD34+ AML cells (left) and CD34+ ALL cells (right). (G) Box plots showing H3K27me3 ChIP-seq signal intensities for all genes and ES PRC2 target genes at the TSS+5 kbp in hCD34+ ALL cells harvested from NSG mice treated with or without the dietary restriction of BCAA in vivo. The Wilcoxon test was used for statistical analysis. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. FDR, false discovery rate; GSEA, gene set enrichment analysis; NES, normalized enriched score; NS, not significant; TSS, transcription start sites.
Inhibition of BCAA metabolism suppresses the PRC2 activity in human acute leukemia. (A) The schema for transcriptome assays in primary human CD34+ AML and ALL cells with or without gabapentin treatment. Global changes in expression profiles were analyzed by the GSEA method. (B) Enrichment plots of embryonic stem (ES) core genes (WONG_ES_CELL_CORE). (C) Enrichment plots of genes marked with H3K27me3 modification in their promoters in human ES cells (BENPORATH_ES_WITH_H3K27ME3). (D) Enrichment plots of PRC2-target genes in human ES cells. (E) Aggregation plots of H3K27me3 ChIP-seq signals centered at TSSs in the levels of ES PRC2 target genes (left panel) and HSCs-related PRC2 target genes (right panel). The blue and red lines show the control and the gabapentin-treated (40 mM for 16 hours) leukemia cells, respectively. (F) Box plots showing H3K27me3 ChIP-seq signal intensities for the whole or the ES PRC2 target genes at the TSS+5 kilobasepairs (kbp) in primary CD34+ AML cells (left) and CD34+ ALL cells (right). (G) Box plots showing H3K27me3 ChIP-seq signal intensities for all genes and ES PRC2 target genes at the TSS+5 kbp in hCD34+ ALL cells harvested from NSG mice treated with or without the dietary restriction of BCAA in vivo. The Wilcoxon test was used for statistical analysis. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. FDR, false discovery rate; GSEA, gene set enrichment analysis; NES, normalized enriched score; NS, not significant; TSS, transcription start sites.
GSEA revealed that both ES core genes critical for the development of CSCs25 and hematopoietic stemness–related genes26 were suppressed by gabapentin treatment (Figure 5B; supplemental Figure 5A). Because ES-related gene expression signatures are associated with the properties of leukemia propagation,25,27 we attempted to clarify how BCAA metabolism regulates the expression of ES-related genes. Interestingly, 5 out of the 26 gene sets positively regulated by gabapentin were commonly related to H3K27me3 histone modification in AML and ALL experiments (supplemental Table 8). As shown in Figure 5C and supplemental Figure 5B, the expression of genes marked with H3K27me3 histone modification in their promoter regions in human ES cells28 and mouse embryonic fibroblast–derived reprogrammed MCV6 cells29 was positively regulated after gabapentin treatment, suggesting that the inhibition of BCAA metabolism induces the expression of genes that are usually suppressed by H3K27me3 histone modifications in stem cells.
PRC2 is a general epigenetic regulator of stem cells2 including ES cells,30,31 HSCs,32 and AML3,33,34 and mediates epigenetic gene silencing through H3K27me3 modification.35 We evaluated the cellular content of metabolites such as α-KG5 (Figure 2C), 2-hydroxyglutarate,6 S-adenosylmethionine,7 succinic acid,36 fumaric acid,37 and acetyl coenzyme A,8 which have been shown to regulate the function of key epigenetic enzymes; however, none of these metabolites were commonly altered in primary AML and ALL cells as compared with those in normal HSPCs (supplemental Figure 5C). We, therefore, hypothesized that BCAAs are specific metabolites that regulate H3K27me3 histone modifications in human acute leukemia. ES-related PRC2-target genes also showed a significant negative correlation with gabapentin treatment in both AML and ALL (Figure 5D). The chromatin IP followed by sequencing (ChIP-seq) analysis revealed that repressive H3K27me3 histone modifications decreased around the TSS of PRC2 target genes28 in THP-1 cells (supplemental Figure 5D) and primary CD34+ AML and CD34+ ALL cells treated with gabapentin (Figure 5E-F). H3K27me3 enrichment was significantly decreased in ES-related PRC2 target genes (Figure 5E, left), as well as in HSCs-related PRC2 target genes32 (Figure 5E, right), whereas this was not found in the analysis targeting global genes. Representative H3K27me3 modification levels of HSCs-related target genes32 such as CDKN1A and SOX7 are shown in supplemental Figure 5E. Consistent with PRC2-specific histone modification, BCAA restriction did not affect the global H3K27me3 levels (supplemental Figure 5F).
We then evaluated the H3K27me3 histone modification at PRC2 target genes in leukemia cells harvested from NSG mice transplanted with human ALL and fed a BCAA-free or a control diet for 30 days. CD34+ ALL cells from a patient with ALL (PDX ALL3) from BCAA-restricted mice, whose self-renewal capacity was strongly impaired in serial transplantation experiments (Figure 4C), showed that H3K27me3 modification was significantly reduced compared with that in mice receiving the control diet (Figure 5G). Collectively, the inhibition of BCAA metabolism induces suppression of the H3K27me3 histone modification at PRC2 target genes in human acute leukemia.
BCAA metabolism is required to maintain the expression of PRC2 components in acute leukemia cells
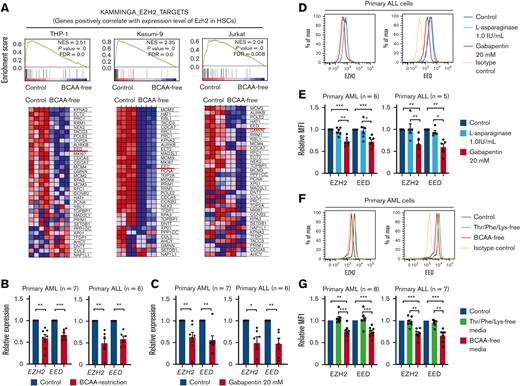
Among PRC2 components, EZH2 plays a critical role in H3K27me3 histone modification as well as in the recruitment of DNA methyltransferases to target genes for DNA hypermethylation.38 THP-1, Kasumi-9, and Jurkat cells were cultured in BCAA-free or control DMEM/F12 medium and were subjected to transcriptome analysis. The ES core genes were again suppressed under BCAA-free conditions (supplemental Figure 6A). GSEA showed that the genes positively correlated with Ezh2 expression in HSCs39 were significantly suppressed under BCAA-free conditions (Figure 6A). The list of such genes included patient EED, which is another component of PRC2 (Figure 6A). We then quantified the expression of PRC2 components, including EZH2 and EED, after culture with gabapentin (20 mM) for 24 hours or BCAA-free media for 48 hours by quantitative PCR. Under both conditions, EZH2 and EED expressions were significantly reduced in the cell lines (supplemental Figure 6B-C) and primary samples (Figure 6B-C), whereas rapamycin, a specific inhibitor of the mTOR pathway, did not suppress EZH2 and EED expression (supplemental Figure 6D). Furthermore, the knock down of BCAT1 suppressed EZH2 and EED expression (supplemental Figure 6E). BCAA restriction significantly reduced the expression levels of EZH2 and EED proteins (supplemental Figure 6F). We quantified the alteration of EZH2 and EED proteins using intracellular FCM analysis and found that 16 hours of gabapentin treatment was sufficient to suppress EZH2 and EED expression (supplemental Figure 6G-H).
The BCAA metabolism activity is required for the maintenance of core PRC2 components, including EZH2 and EED in human acute leukemia. (A) Enrichment plots of genes positively regulated by EZH2 in HSCs (KAMMINGA_EZH2_TARGETS) from THP-1, Kasumi-9, and Jurkat cells cultured in control or BCAA-free DMEM/F12 media supplemented with 10% FBS (upper panels). Heat map for the expression of HSC-related EZH2-target genes in THP-1, Kasumi-9, and Jurkat cells (lower panels). (B) Quantitative real-time PCR analysis of EZH2 and EED messenger RNA (mRNA) in primary CD34+ AML cells (n = 7) and CD34+ ALL cells (n = 6) cultured in control or BCAA-free media supplemented with 10% FBS for 48 hours. Relative expression of control samples is adjusted to 1.0. (C) Quantitative real-time PCR analysis of EZH2 and EED mRNA in primary CD34+ AML cells (n = 7) and CD34+ ALL cells (n = 6) treated with or without 20 mM of gabapentin for 24 hours. (D) A representative result of intracellular FCM analysis for quantification of EZH2 and EED levels in primary ALL cells treated with 1.0 IU/ml of L-asparaginase or 20mM of gabapentin for 24h. (E) Summary of the intracellular FCM analysis of EZH2 and EED levels in primary AML cells (n = 6) and ALL cells (n = 5) treated with L-asparaginase or gabapentin. The MFI of the control sample is set to 1.0. (F) A representative result of intracellular FCM analysis to quantify EZH2 and EED levels in AML cells cultured in Thr/Phe/Lys-free or BCAA-free media supplemented with 10% FBS for 48h. (G) Summary of the intracellular FCM analysis of EZH2 and EED levels in primary AML cells (n = 8) and ALL cells (n = 7) cultured in the control, the Thr/Phe/Lys-free, and the BCAA-free media supplemented with 10% FBS for 24 to 48 hours. The MFI of the control sample is set to 1.0. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, panels B, C, E, and G, mean ± SEM. EED, embryonic ectoderm development.
The BCAA metabolism activity is required for the maintenance of core PRC2 components, including EZH2 and EED in human acute leukemia. (A) Enrichment plots of genes positively regulated by EZH2 in HSCs (KAMMINGA_EZH2_TARGETS) from THP-1, Kasumi-9, and Jurkat cells cultured in control or BCAA-free DMEM/F12 media supplemented with 10% FBS (upper panels). Heat map for the expression of HSC-related EZH2-target genes in THP-1, Kasumi-9, and Jurkat cells (lower panels). (B) Quantitative real-time PCR analysis of EZH2 and EED messenger RNA (mRNA) in primary CD34+ AML cells (n = 7) and CD34+ ALL cells (n = 6) cultured in control or BCAA-free media supplemented with 10% FBS for 48 hours. Relative expression of control samples is adjusted to 1.0. (C) Quantitative real-time PCR analysis of EZH2 and EED mRNA in primary CD34+ AML cells (n = 7) and CD34+ ALL cells (n = 6) treated with or without 20 mM of gabapentin for 24 hours. (D) A representative result of intracellular FCM analysis for quantification of EZH2 and EED levels in primary ALL cells treated with 1.0 IU/ml of L-asparaginase or 20mM of gabapentin for 24h. (E) Summary of the intracellular FCM analysis of EZH2 and EED levels in primary AML cells (n = 6) and ALL cells (n = 5) treated with L-asparaginase or gabapentin. The MFI of the control sample is set to 1.0. (F) A representative result of intracellular FCM analysis to quantify EZH2 and EED levels in AML cells cultured in Thr/Phe/Lys-free or BCAA-free media supplemented with 10% FBS for 48h. (G) Summary of the intracellular FCM analysis of EZH2 and EED levels in primary AML cells (n = 8) and ALL cells (n = 7) cultured in the control, the Thr/Phe/Lys-free, and the BCAA-free media supplemented with 10% FBS for 24 to 48 hours. The MFI of the control sample is set to 1.0. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, panels B, C, E, and G, mean ± SEM. EED, embryonic ectoderm development.
The addition of a doubled concentration of BCAA to the culture media largely canceled the suppression of PRC2 components induced by gabapentin (supplemental Figure 6G). By contrast, the addition of L-asparaginase (Figure 6D-E; supplemental Figure 6I) or the restriction of other major EAAs, including Thr, Lys, and Phe (Figure 6F-G; supplemental Figure 6J), did not suppress EZH2 or EED. In addition, EZH2 and EED transcripts were significantly suppressed in human AML and ALL cells harvested from first NSG recipients fed the BCAA-free diet (Figures 7A-B). Notably, suppression of EZH2 and EED was observed irrespective of cell cycle status (data not shown) or protein synthesis activity analyzed by O-propargyl-puromycin labeling40 (supplemental Figure 6K). Collectively, these results suggest that BCAA metabolism is required to maintain the expression of EZH2 and EED in human acute leukemia.
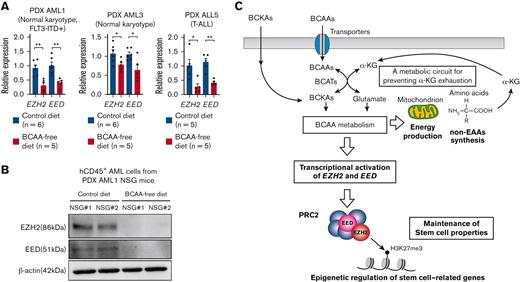
The BCAA metabolism maintains transcription of EZH2 and EED in human primary acute leukemia in vivo. (A) Quantitative real-time PCR analysis of EZH2 and EED transcription in primary AML and ALL cells in NSG mice fed the control and BCAA-free diet. Results of 3 independent xenotransplantation experiments using cells from 3 patients (PDX AML1, PDX AML3, and PDX ALL5) are shown. (B) Representative results of EED and EZH2 protein levels in hCD45+ cells purified from the NSG mice fed the control diet (n = 2) or BCAA-free diet (n = 2). (C) The schematic summary of the role of BCAA metabolism in human leukemias. Leukemia cells express LAT transporters to uptake BCAA. The BCAA catabolism generates critical substrates for the OXPHOS pathway to provide energy and for the non-EAA pathway to synthesize proteins. α-KG is a resultant product of both pathways. Although α-KG is used at the stage of BCAA to BCKA catabolism, both pathways produce α-KG to prevent decrease in the cellular α-KG level or even to increase its level in leukemia cells. The BCAA metabolism is required for the PRC2 function to maintain stemness in leukemia cells, at least through stimulating EZH2 and EED transcription. ∗P < .05, ∗∗P < .01, panel A, mean ± SEM.
The BCAA metabolism maintains transcription of EZH2 and EED in human primary acute leukemia in vivo. (A) Quantitative real-time PCR analysis of EZH2 and EED transcription in primary AML and ALL cells in NSG mice fed the control and BCAA-free diet. Results of 3 independent xenotransplantation experiments using cells from 3 patients (PDX AML1, PDX AML3, and PDX ALL5) are shown. (B) Representative results of EED and EZH2 protein levels in hCD45+ cells purified from the NSG mice fed the control diet (n = 2) or BCAA-free diet (n = 2). (C) The schematic summary of the role of BCAA metabolism in human leukemias. Leukemia cells express LAT transporters to uptake BCAA. The BCAA catabolism generates critical substrates for the OXPHOS pathway to provide energy and for the non-EAA pathway to synthesize proteins. α-KG is a resultant product of both pathways. Although α-KG is used at the stage of BCAA to BCKA catabolism, both pathways produce α-KG to prevent decrease in the cellular α-KG level or even to increase its level in leukemia cells. The BCAA metabolism is required for the PRC2 function to maintain stemness in leukemia cells, at least through stimulating EZH2 and EED transcription. ∗P < .05, ∗∗P < .01, panel A, mean ± SEM.
Discussion
In this study, we showed that BCAA metabolism is critical for maintaining the stemness of human primary acute leukemia, irrespective of their lineage type. Most AML and ALL cells had a high level of transporters, cellular BCAA, and BCAT1, whereas serum BCAA levels were decreased, suggesting active uptake of BCAAs. Isotope-tracing experiments revealed that the catabolism of BCAA to BCKA provides critical substrates for non-EAA synthesis and the TCA cycle in primary human leukemia. The BCAA restriction significantly impaired engraftment, expansion, and self-renewal of primary human acute leukemia cells in xenotransplantation experiments. Because a higher level of BCAT1 is associated with poor survival in both patients with AML and patients with ALL, these xenotransplantation data suggest that BCAA dietary restriction may prolong survival in human acute leukemias in combination with other antileukemic therapies. We also showed that BCAA metabolism is critical for PRC2 activity by promoting transcription of EZH2 and EED to maintain the stemness of acute leukemia (Figure 7C).
Recent studies have reported important role of BCAT1 in myeloid malignancies. Hattori et al showed that murine CML cells use BCAT1 to synthesize BCAA from BCKA to maintain cellular BCAA at a high level without the increment of BCAA uptake.12 Gu et al showed that EZH2 inactivation and oncogenic NRAS activation cooperate to induce leukemic transformation by activating BCAT1, which reversely synthesizes BCAA from BCKA in murine models.13 Both studies argued the significance of reversed BCAA synthesis by BCAT1 to enhance BCAA-dependent mTOR activity. We found that mTOR activity was suppressed by inhibiting BCAA catabolism (data not shown) and that reamination of BCKA can occur in primary human leukemia. However, it has been shown that mTOR is essential for leukemia propagation but not for self-renewal of murine leukemic stem cells,41 and it is insufficient to explain the BCAA catabolism-dependent reinforcement of stemness in leukemia cells observed in our experiments. Therefore, we sought to identify a BCAA metabolism–dependent mechanism that potentiates self-renewal in human acute leukemia and found that BCAA metabolism is required for PRC2 function in human acute leukemia.
Raffel et al showed that a high level of BCAT1 protein actively catabolizes BCAA to BCKA, while consuming α-KG, and a resultant low level of cellular α-KG disturbs TET2 function in DNA demethylation, leading to hypermethylation status in target genes.14 Our metabolome analysis of primary human leukemia samples, however, showed that the cellular α-KG did not decrease, whereas inhibition of BCAT1 or deprivation of BCAA reduced cellular α-KG, suggesting that the BCAA catabolism is essential to maintain α-KG. Our isotope-tracing experiments showed that BCAA catabolism involves both non-EAA synthesis and TCA cycle activity and that the metabolic processes necessarily produce α-KG. This process can compensate for the α-KG used in the catabolism of BCAA to BCKA and further accumulate α-KG in leukemia cells.
α-KG is one of the central metabolites essential for cellular function and regulates enzymes involved in hypoxic adaptation and epigenetic modifications.42 Recent studies have shown that a high level of intracellular α-KG plays a critical role in maintaining the stemness of ES and induced pluripotent stem cells,5,43,44 suggesting the possibility that α-KG can regulate stemness of acute leukemia. We here showed that the BCAA metabolism plays a critical role in the transcription of EZH2 and EED in human acute leukemia. The catalytic center of PRC2 resides in the SET domain of the EZH2 subunit, and EZH2 requires EED to maintain catalytic activity and stability.45 It is possible that the simultaneous suppression of EZH2 and EED by BCAA catabolism inhibition may synergistically impair the function of PRC2. Thus, the molecules related to the BCAA metabolism pathway should be critical targets for leukemia treatment. Further studies are required to identify molecules that transcriptionally activate PRC2 components downstream of BCAA catabolism.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research (S) (K.A., #16747244), a Grant-in-Aid for Scientific Research (B) (T.M., #16674756 and Y. Kikushige, #19109659), and a Grant-in-Aid for challenging Exploratory Research (Y. Kikushige, #20269344) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. This study was also supported in part by a Grant-in-Aid from the Japan Agency for Medical Research and Development (Y. Kikushige, 16768249 and K.A., 16770576). This study was also supported in part by a research grant of Princess Takamatsu Cancer Research Fund, SGH foundation, and Shin-nihon Foundation of Advanced Medical Treatment Research and Kaketsuken (Y. Kikushige).
Authorship
Contribution: Y. Kikushige, T.M., T. Soga, and K.A. designed the research; Y. Kikushige, Y. Kochi, Y.S., M.O., H.I., K.H., T. Sugio, T. Sakoda, Y. Kunisaki, and K.M. performed research and collected data; Y. Kikushige, Y.S., K.K., and K.A. analyzed and interpreted the data; and Y. Kikushige and K.A. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Koichi Akashi, Department of Medicine and Biosystemic Sciences, Kyushu University, 3-1-1 Maidashi, Higashi-Ku, Fukuoka 812-8582, Japan; e-mail: akashi.koichi.357@m.kyushu-u.ac.jp.
References
Author notes
The microarray data are available in the Gene Expression Omnibus (GEO) database under the accession numbers GSE 127181 for primary CD34+ AML cells, GSE 128146 for CD34+ ALL cells, GSE131813 for THP-1 cells, GSE131814 for Kasumi-9 cells, and GSE131815 for Jurkat cells, respectively. ChIP-Seq data are also available in GEO under the accession number GSE 132512. Additional details of the methods are provided in the supplemental data.
The full-text version of this article contains a data supplement.

![Acute leukemia cells catabolize BCAA to maintain mitochondrial OXPHOS and non-EAA synthesis, sustaining cellular α-KG levels. (A) The schema for the BCAA metabolism pathway. (B) Comparison of BCAT1 expression levels in normal CD34+ HSPCs (n = 6), CD34+ AML cells (n = 9), and CD34+ ALL cells (n = 11). The MFI ratio of the target and isotype control for each sample is shown. (C) Cellular contents of α-KG in CD34+ HSPCs- (n = 14), AML cells (n = 45), and ALL cells (n = 20) in our metabolome analysis. (D) The alteration of cellular α-KG levels in primary AML cells (n = 5) and ALL cells (n = 9) by the addition of gabapentin (20 mM) and L-asparaginase (1.0 IU/mL) for 24 hours are shown. The α-KG levels under control conditions are set to 1.0. (E) The alteration of cellular α-KG levels in primary AML cells (n = 7) and ALL cells (n = 8) after 24-hour culture in control and BCAA- or Thr/Phe/Lys-free DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS) are shown. The α-KG levels in control conditions are set to 1.0. (F) The schema and results for the isotope-tracing experiments using primary AML (n = 2) and ALL (n = 2) samples cultured in HPLM containing 160 uM of [13C6,15N1] Leu are shown. Percentages of [13C6,15N1] Leu–derived citrate (M+1 and M+2) and non-EAA (M+1) including Glu, Asp, Ala, and Ser among the total amount of corresponding metabolites quantified at the indicated time points are shown. Results from at least 3 independent experiments are shown. (G) The schema and results for isotope-tracing experiments using primary AML (n = 2) and ALL (n = 1) samples cultured in HPLM containing 30 uM of [13C6] α-KIC are shown. Results from at least 3 independent experiments are shown. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, panels B, C, D, E, F, and G, mean ± SEM. α-KIC, α-ketoisocaproate; EAA, essential amino acids; HPLM, human plasma-like medium; OXPHOS, oxidative phosphorylation; TCA, trichloroacetic acid.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/14/10.1182_bloodadvances.2022008242/2/m_blooda_adv-2022-008242-gr2.jpeg?Expires=1763524563&Signature=TIS4pOqEqI-n9Vp7jv9HLqmo-HrjWohWdXHG0yNOMBVCcQWq1UR~CazDQSFkbhS4l66N2OcZHYc7a6u-S2WkcccIHAlSzqaubu9lWQzl6RTkS~6dm9XXjWzLBVPoM~DU8jUF4bv~DuqQPBK3tT3LL0X1tWIaDeYpXECllM-dFcj2h8fYAEfPEPHhRfq8KclUuT6sYtLZzN-97xd16SjryILmiy0YZVkEG7CypMwfJSR~w4iig3r4SBzl3oIClwhTVTaHd31XbH~rx0s4HGjSBPX-fm6w2ACkARDLWReoqc9SqPRstdZjP9prrFVXHnuQ0KfptYLMzl27UERXios1Jg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)