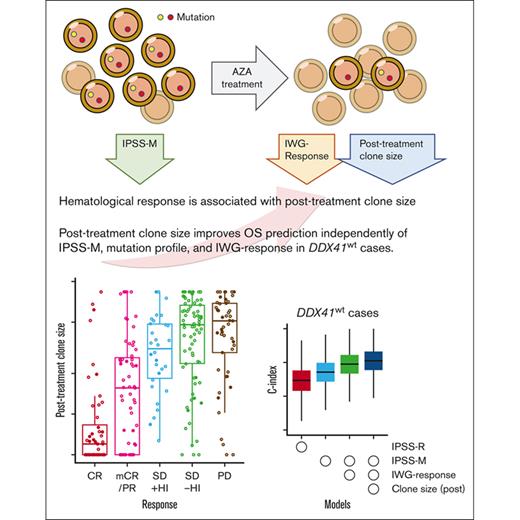
Key Points
Major clone sizes in posttreatment samples are strongly correlated with clinical response.
Inclusion of posttreatment clone size into the prognostic model allows better prognostication for MDS cases treated with azacitidine.
Abstract
Azacitidine is a mainstay of therapy for myelodysplastic syndrome (MDS)–related diseases. The purpose of our study is to elucidate the effect of gene mutations on hematological response and overall survival (OS), particularly focusing on their posttreatment clone size. We enrolled a total of 449 patients with MDS or related myeloid neoplasms. They were analyzed for gene mutations in pretreatment (n = 449) and posttreatment (n = 289) bone marrow samples using targeted-capture sequencing to assess the impact of gene mutations and their posttreatment clone size on treatment outcomes. In Cox proportional hazard modeling, multihit TP53 mutation (hazard ratio [HR], 2.03; 95% confidence interval [CI], 1.42-2.91; P < .001), EZH2 mutation (HR, 1.71; 95% CI, 1.14-2.54; P = .009), and DDX41 mutation (HR, 0.33; 95% CI, 0.17-0.62; P < .001), together with age, high-risk karyotypes, low platelets, and high blast counts, independently predicted OS. Posttreatment clone size accounting for all drivers significantly correlated with International Working Group (IWG) response (P < .001, using trend test), except for that of DDX41-mutated clones, which did not predict IWG response. Combined, IWG response and posttreatment clone size further improved the prediction of the original model and even that of a recently proposed molecular prediction model, the molecular International Prognostic Scoring System (IPSS-M; c-index, 0.653 vs 0.688; P < .001, using likelihood ratio test). In conclusion, evaluation of posttreatment clone size, together with the pretreatment mutational profile as well as the IWG response play a role in better prognostication of azacitidine-treated patients with myelodysplasia.
Introduction
Azacitidine is a standard choice of therapy for patients with high-risk myelodysplastic syndromes (MDS) and other related myeloid neoplasms, including myelodysplastic/myeloproliferative neoplasms (MDSs/MPNs) and oligoblastic acute myeloid leukemia (AML). It can induce a clinical remission in ∼50% to 60% of treated patients1,2 and has been shown to prolong survival compared with standard regimens3 whereas others either respond poorly or do not respond at all, and a major clinical question is, “who best benefits from azacitidine treatment?” In fact, many studies have investigated biomarkers that can predict initial response and long-term survival after azacitidine therapy, particularly in terms of gene mutations.4-27 However, despite many efforts, no reliable biomarkers have been identified, mainly because of the small numbers of enrolled patients and variable study designs.22
Another problem that complicates the identification of such biomarkers is the lack of reliable measures for the evaluation of the initial response to therapy. To date, the International Working Group (IWG) criteria have been widely used for the assessment of therapeutic response in MDS and related disorders,28,29 in which blast count and hematological improvement are evaluated. Although highly correlated with tumor clearance in AML, the blast count may not correctly reflect tumor burden in MDS,30 apart from an interobserver variance in blast cell quantification.31 In addition, blood cell counts could be influenced by cytotoxic agents, particularly when they are assessed before the bone marrow (BM) fully recovers between treatment cycles.29 In this regard, next-generation sequencing (NGS) might provide a more robust platform to measure a molecular response or a reduction in tumor burden in terms of a variant allele frequency, or clone size, of somatic mutations detected before and after therapy. In fact, several studies evaluated a molecular response in patients who were treated with hypomethylating agents using NGS.15,32-36 However, the size of each study was too small to fully evaluate the impact of tumor burden on clinical outcomes, although several studies have reported a correlation between a reduced tumor burden and clinical response, particularly in TP53-mutated AML and MDS.14,32,33
To our knowledge, we investigated the effects of gene mutations on clinical outcomes in the largest cohort of azacitidine-treated patients with MDS, MDS/MPN, and oligoblastic AML (blast percentage ≤ 30% at enrollment) ever studied (n = 449), in which genetic alterations were comprehensively analyzed by targeted-capture sequencing of major drivers for myeloid malignancies. In addition, mutational profiles before and after azacitidine treatment were assessed for 289 cases to explore their impact on clinical response and survival (supplemental Figure 1).
Methods
Targeted sequencing
BM samples were collected before and ∼4 cycles after the initiation of azacitidine treatment, followed by genomic DNA extraction and targeted-capture sequencing for the detection of single-nucleotide variations, small insertions/deletions, and structural variations including FLT3-ITD and KMT2A-PTD as well as copy number abnormalities (CNAs) and other allelic imbalances, including copy-neutral loss-of-heterogeneity (CN-LOH) (Figure 1A; supplemental Figure 2; supplemental Tables 1-5). Methods of mutation calling and sequencing-based detection of CNAs/CN-LOH are detailed elsewhere.37 A multihit TP53 mutation was thought to be present when a patient had multiple mutations or a mutation was accompanied by 17p LOH (either because of CN-LOH or deletion; supplemental Figure 3).38
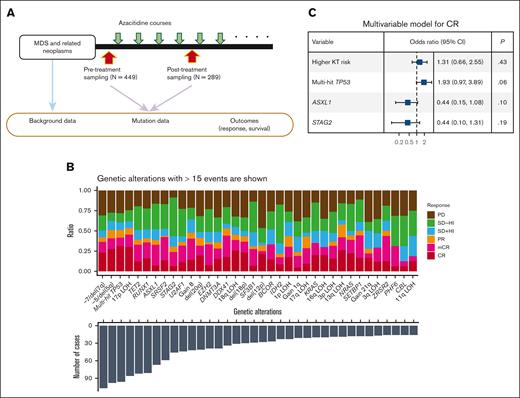
Genetic and clinical factors associated with response and OS. (A) A summary chart of the study design. (B) Bar plots showing the number of cases with the genetic alterations indicated on the x-axis (bottom) and with the proportion of the cases showing response indicated by color (top). (C) A forest plot showing the result of a multivariable logistic regression analysis for the factors associated with achieving CR with complete data for response analysis (n = 396). Explicative variables included in the multivariable model are high-risk karyotypes (poor and very poor groups based on IPSS-R–based karyotype risk classification), multihit TP53 mutations, and mutations in ASXL1 and STAG2. The x-axis is log10 scaled. CI, confidential interval.
Genetic and clinical factors associated with response and OS. (A) A summary chart of the study design. (B) Bar plots showing the number of cases with the genetic alterations indicated on the x-axis (bottom) and with the proportion of the cases showing response indicated by color (top). (C) A forest plot showing the result of a multivariable logistic regression analysis for the factors associated with achieving CR with complete data for response analysis (n = 396). Explicative variables included in the multivariable model are high-risk karyotypes (poor and very poor groups based on IPSS-R–based karyotype risk classification), multihit TP53 mutations, and mutations in ASXL1 and STAG2. The x-axis is log10 scaled. CI, confidential interval.
Single-cell sequencing library preparation and genotyping
Patient samples were washed and sorted to isolate viable blood cells (4′,6-diamidino-2-phenylindole and CD45+) using FACSAria III Cell Sorter (Becton & Dickinson). Single cells were encapsulated using a Tapestri microfluidics cartridge and were subjected to targeted sequencing for amplicons included in Tapestri Single-Cell DNA Myeloid Panel and were subjected to sequencing on an Illumina NovaSeq 6000 (Illumina).
Whole-genome sequencing
Fifty nanograms of DNA were subjected to library preparation using KAPA Hyper Prep Kit followed by sequencing using DNBSEQ-G400 (MGI Tech) with a target depth of 100× in 150 bp paired-end mode. Mutation calling was performed using the Genomon2 pipeline (version 2.6), as previously described.39
Statistical methods
To investigate genetic factors that were significantly associated with clinical responses, the association was first tested in univariate analysis using Fisher exact test, followed by multivariate analysis based on the logistic regression model, in which significant (P < .05) factors in univariate analysis with an incidence >5% in the cohort were included as explanatory variables, followed by parameter selection using Akaike information criteria. The predictability of different overall survival (OS) models was compared based on c-statistics. Univariate and multivariable analysis of OS was performed based on Cox proportional hazard modeling. OS models were constructed using those covariates that were significant in univariate analysis. The weight of each covariate was determined based on the coefficients of Cox proportional hazard model (log2 [hazard ratio]). Detailed methods are available in the supplemental Data.
Overlap of the data sets with the previous report
There exist sample overlaps with 2 previous reports (PMID 26959885 and PMID 32747829). The former reported the effect of gene mutations on the therapeutic effect of azacitidine in terms of short-term response and overall survival after azacitidine therapy. This study included 163 cases from the Karolinska Institute, of which 89 were included. However, in that study, the size of the entire cohort and the number of mutations tested were limited, and the posttherapeutic samples were not analyzed. Here, enrolling a larger number of samples and genotyping a larger set of driver genes, we investigated the effect of gene mutations more extensively and the result was confirmed using a validation cohort. In the latter study, we investigated the effect of the TP53 allelic state on clinical outcomes using extensive genotyping, where substantial numbers of cases overlapped (210 and 163 overlapped cases from 288 Japanese and 163 Karolinska Institute cases, respectively), but we did not investigate in a comprehensive manner the effect of other mutations or that of posttherapeutic clone size, both of which are the major topics of the current study.
Results
Patients
The entire cohort comprised 3 distinct cohorts of patients with MDS and related myeloid neoplasms who were treated with azacitidine: a prospective cohort of Japanese patients (n = 176),40 a retrospective cohort of Swedish patients (n = 163), and a retrospective cohort of Japanese patients (n = 110). The initial clinical response to azacitidine was evaluable in 396 patients. The treatment schedule, OS, and other demographic data are shown in supplemental Figure 2 and Table 1. Paired pre and posttreatment samples were analyzed for 289 cases, which were used to explore the effect of clone changes on outcomes and to construct an OS-predicting model. This study was approved by the institutional ethical committees at Kyoto University (G-608), Karolinska Institute (Dnr 2017/1090−31/4) and participating institutes and hospitals. All patients provided fully informed consent.
Characteristics of the study cohort
| Parameters . | Values . |
|---|---|
| Number of patients | 449 |
| Age at enrollment (median [range])--y | 72 (16-91) |
| Sex (%) | |
| Male | 304 (67.7) |
| Female | 145 (32.3) |
| WHO−classification at enrollment (%) | |
| MDS | 384 (85.5) |
| Isolated del(5q) | 5 (1.1) |
| MDS-SLD | 2 (0.4) |
| MDS-MLD | 37 (8.2) |
| MDS-RS | 4 (0.9) |
| MDS−U | 4 (0.9) |
| MDS-EB1 | 149 (33.2) |
| MDS-EB2 | 183 (40.8) |
| AML | 36 (8.0) |
| MDS/MPN | 29 (6.5) |
| Atypical CML, BCR-ABL1-negative | 1 (0.2) |
| CMML | 23 (5.1) |
| MDS/MPN−U | 5 (1.1) |
| IPSS-R number (%)∗ | |
| Very low | 4 (0.9) |
| Low | 19 (4.4) |
| Intermediate | 95 (22.1) |
| High | 126 (29.4) |
| Very high | 185 (43.1) |
| IPSS-M number (%)∗ | |
| Very low | 2 (0.5) |
| Low | 20 (4.7) |
| Moderate low | 26 (6.1) |
| Moderate high | 40 (9.4) |
| High | 109 (25.5) |
| Very high | 230 (53.9) |
| Karyotype risks (IPSS-R−based) number (%) | |
| Very good | 8 (1.8) |
| Good | 184 (41.1) |
| Intermediate | 78 (17.4) |
| Poor | 54 (12.1) |
| Very poor | 124 (27.7) |
| Peripheral blood | |
| WBC (median [range]) × 109/L | 2.7 (0.3−107.9) |
| ANC (median [range]) × 109/L | 1.0 (0−38.2) |
| HB (median [range]) g/dL | 9.1 (4.1−15) |
| PLT (median [range]) × 109/L | 71.0 (5−1237) |
| Bone marrow | |
| Blast (median [range])--% | 9.8 (0-30) |
| Post azacitidine samples available (%) | 289 (64.4) |
| RBC transfusion dependent (%) | 212 (47.2) |
| PLT transfusion dependent (%) | 48 (10.7) |
| Response (best response) | |
| CR (%) | 72 (18.2) |
| mCR (%) | 75 (18.9) |
| PR (%) | 21 (5.3) |
| SD (%) | 142 (35.9) |
| PD (%) | 86 (21.7) |
| Cases underwent transplantation (%) | 74 (16.5) |
| Parameters . | Values . |
|---|---|
| Number of patients | 449 |
| Age at enrollment (median [range])--y | 72 (16-91) |
| Sex (%) | |
| Male | 304 (67.7) |
| Female | 145 (32.3) |
| WHO−classification at enrollment (%) | |
| MDS | 384 (85.5) |
| Isolated del(5q) | 5 (1.1) |
| MDS-SLD | 2 (0.4) |
| MDS-MLD | 37 (8.2) |
| MDS-RS | 4 (0.9) |
| MDS−U | 4 (0.9) |
| MDS-EB1 | 149 (33.2) |
| MDS-EB2 | 183 (40.8) |
| AML | 36 (8.0) |
| MDS/MPN | 29 (6.5) |
| Atypical CML, BCR-ABL1-negative | 1 (0.2) |
| CMML | 23 (5.1) |
| MDS/MPN−U | 5 (1.1) |
| IPSS-R number (%)∗ | |
| Very low | 4 (0.9) |
| Low | 19 (4.4) |
| Intermediate | 95 (22.1) |
| High | 126 (29.4) |
| Very high | 185 (43.1) |
| IPSS-M number (%)∗ | |
| Very low | 2 (0.5) |
| Low | 20 (4.7) |
| Moderate low | 26 (6.1) |
| Moderate high | 40 (9.4) |
| High | 109 (25.5) |
| Very high | 230 (53.9) |
| Karyotype risks (IPSS-R−based) number (%) | |
| Very good | 8 (1.8) |
| Good | 184 (41.1) |
| Intermediate | 78 (17.4) |
| Poor | 54 (12.1) |
| Very poor | 124 (27.7) |
| Peripheral blood | |
| WBC (median [range]) × 109/L | 2.7 (0.3−107.9) |
| ANC (median [range]) × 109/L | 1.0 (0−38.2) |
| HB (median [range]) g/dL | 9.1 (4.1−15) |
| PLT (median [range]) × 109/L | 71.0 (5−1237) |
| Bone marrow | |
| Blast (median [range])--% | 9.8 (0-30) |
| Post azacitidine samples available (%) | 289 (64.4) |
| RBC transfusion dependent (%) | 212 (47.2) |
| PLT transfusion dependent (%) | 48 (10.7) |
| Response (best response) | |
| CR (%) | 72 (18.2) |
| mCR (%) | 75 (18.9) |
| PR (%) | 21 (5.3) |
| SD (%) | 142 (35.9) |
| PD (%) | 86 (21.7) |
| Cases underwent transplantation (%) | 74 (16.5) |
ANC, absolute neutrophil count; CML, chronic myeloid leukemia; CMML, chronic myelomonocytic leukemia; HB, hemoglobine; MDS/MPN-U, Myelodysplastic/myeloproliferative neoplasms unclassifiable; MDS-EB1/2, MDS with excess blasts 1/2; MDS-MLD, MDS with multilineage dysplasia; MDS-RS, refractory anemia with ring sideroblasts; MDS-SLD, MDS with single lineage dysplasia; MDS-U, MDS unclassifiable; PD, progressive disease; PLT, platelet; PR, partial remission; SD, stable disease; WBC, white blood cell.
IPSS-R and IPSS-M were not calculated for CMML cases with WBC ≥ 12 × 109/L.
Genetic abnormalities
With a median depth of 573× (supplemental Figure 4), targeted sequencing in the entire cohort of 449 patients who were treated with azacitidine disclosed 3.01 and 2.97 single-nucleotide variations or insertions/deletions per case and 2.73 and 1.55 CNAs/CN-LOHs per case in 449 pre and 289 posttreatment samples, respectively (supplemental Tables 2-5). An excellent reproducibility and concordance across different assays were confirmed in 2 independent sequencing experiments in a subset of samples (n = 26; supplemental Figure 5). Reflecting an overrepresentation of high-risk patients for azacitidine therapy, the current cohort was significantly enriched for poor-risk karyotypes/mutations, such as TP53 and -7/del(7q) mutations, followed by −5/del(5q), 17p LOH, and TET2 and RUNX1 mutations (supplemental Figure 6).
Correlations between genetic abnormalities and treatment response
We first investigated genetic alterations that could influence the initial clinical response to azacitidine based on the IWG criteria28 (“IWG response”). In univariate analysis, multihit TP53 mutation, high-risk karyotype, 17p LOH, and −5/del(5q) were significantly associated with a higher complete remission (CR) rate, whereas ASXL1 and STAG2 mutation predicted a lower response (Figure 1B; supplemental Figure 7A,B). A lower response in ASXL1-mutated cases has also been reported in a previous study of azacitidine-treated patients with chronic myelomonocytic leukemia.36 In multivariable analysis, multihit TP53 mutation, high-risk karyotype, ASXL1, and STAG2 mutations remained in the model, although none were statistically significant (Figure 1C; supplemental Methods). We observed no significant difference in CR rate itself or other responses between monoallelic vs multihit TP53 mutation, although this needs further evaluation because of the small number of patients with monoallelic mutation (supplemental Figure 7C).
Changes in clonal structure during azacitidine therapy
Given that our sequencing panel encompassed most of the driver genes implicated in MDS, we were able to estimate the size of both founder clones and subclones before and after azacitidine therapy in most cases. Posttreatment samples were obtained from 289 cases (64%) after a median of 4 treatment cycles (supplemental Figure 2C; supplemental Table 6). Among them, we detected 930 and 858 mutations in 278 pre and 255 posttreatment samples, respectively, of which 718 were found in both samples. Overall, mutations were more likely to disappear than to be newly acquired among those who achieve CR, marrow CR (mCR), or partial response (supplemental Figure 8). We detected 140 mutations newly acquired during azacitidine therapy, whereas 212 mutations present in pretreatment samples were lost in posttreatment samples (Figure 2A). Newly acquired mutations most commonly affected RUNX1, followed by TET2, CBL, DDX41, PPM1D, and TP53.
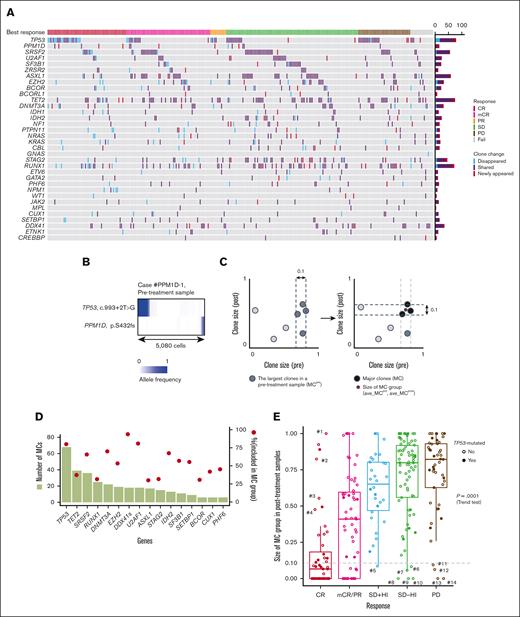
Clone changes during azacitidine treatment and genetic mechanisms of CR. (A) An oncoprint panel showing the change of gene mutation profiles between pre and postazacitidine treatment. Blue and red cells show gene mutations that disappeared and newly appeared after treatment, respectively. Purple cells show gene mutations that are shared between pre- and posttreatment samples. When 2 or more mutations are observed in a gene in a patient, the largest variant in pretreatment samples is assumed. Clinical response is shown at the top of the panel. (B) Single-cell analysis (n = 5080) with Tapestri platform of the case #PPM1D-1 (sample IDs correspond to those indicated in supplemental Table 7) that had mutations in TP53 and PPM1D. Shaded colors indicate the variant allele frequencies of the variants. (C) A schematic explanation of MC determined accounting for the clone size in pre (x-axis) and posttreatment (y-axis) samples from a patient. A set of mutations showing the largest and near largest size (difference in size is <0.10 compared with the largest mutation) in a pretreatment sample were defined as MCpre (left). Among the MCpre, a set of mutations showing the largest and near largest size in a posttreatment sample were assumed to be MC (right). The detailed description of definition appears in supplemental Methods. (D) Bar plots showing the numbers of MC (left) represented by the genes indicated on x-axis. Filled circles indicate the proportion of the variants (right) classified as MC out of all the mutations in the paired cohort. DDX41s indicates DDX41 somatic mutation. (E) Box plots showing the MC group sizes of posttreatment samples (ave_MCpost) in the paired cohort having the response indicated on x-axis. P = .0001, using Jonckheere-Terpstra tests. PR, partial response; SD, stable disease.
Clone changes during azacitidine treatment and genetic mechanisms of CR. (A) An oncoprint panel showing the change of gene mutation profiles between pre and postazacitidine treatment. Blue and red cells show gene mutations that disappeared and newly appeared after treatment, respectively. Purple cells show gene mutations that are shared between pre- and posttreatment samples. When 2 or more mutations are observed in a gene in a patient, the largest variant in pretreatment samples is assumed. Clinical response is shown at the top of the panel. (B) Single-cell analysis (n = 5080) with Tapestri platform of the case #PPM1D-1 (sample IDs correspond to those indicated in supplemental Table 7) that had mutations in TP53 and PPM1D. Shaded colors indicate the variant allele frequencies of the variants. (C) A schematic explanation of MC determined accounting for the clone size in pre (x-axis) and posttreatment (y-axis) samples from a patient. A set of mutations showing the largest and near largest size (difference in size is <0.10 compared with the largest mutation) in a pretreatment sample were defined as MCpre (left). Among the MCpre, a set of mutations showing the largest and near largest size in a posttreatment sample were assumed to be MC (right). The detailed description of definition appears in supplemental Methods. (D) Bar plots showing the numbers of MC (left) represented by the genes indicated on x-axis. Filled circles indicate the proportion of the variants (right) classified as MC out of all the mutations in the paired cohort. DDX41s indicates DDX41 somatic mutation. (E) Box plots showing the MC group sizes of posttreatment samples (ave_MCpost) in the paired cohort having the response indicated on x-axis. P = .0001, using Jonckheere-Terpstra tests. PR, partial response; SD, stable disease.
Of interest, almost half of PPM1D mutations were found in newly emerging clones, and most (6 of 9) were detected in patients with TP53-mutated clones (supplemental Figure 9), in which the single-cell sequencing analysis in a case suggest that TP53 and PPM1D mutations affected distinct cell populations (Figure 2B). In addition, many of these PPM1D mutations (n = 7) were shown to be already present in pretreatment samples, mostly in a small cell fractions (supplemental Table 7). Given that PPM1Dmutations are implicated in the downregulation of the p53 pathway,41 these results suggest the presence of a common selective pressure that favors both TP53- and PPM1D-mutated clones, of which the latter expanded after the effacement of TP53-mutant clones was induced by azacitidine.
Overall, patients with newly acquired mutations were less likely to achieve CR/mCR. However, their impact on outcome depended on their clone size and the affected genes; those with a larger clone size (variant allele frequency [VAF] > 0.30) were associated with a poor IWG response, whereas other mutations affecting PPM1D, TET2, and DDX41 tended to have a favorable IWG response. Finally, the cases with newly emerging mutations tended to have a shorter OS, although this was not significant (supplemental Figure 10).
Clone size after azacitidine therapy
Next, we evaluated the size of somatic mutations (n = 1026) detected in the paired cohort, in which both pre and posttreatment samples were analyzed (n = 289). We excluded FLT3-ITD and KMT2A-PTD because their clone sizes were difficult to correctly estimate using NGS platforms. Mutations were classified into those in the major clone (MC) and subclonal mutations (Figure 2C; supplemental Figure 11). MCs were most frequently explained by the presence of TP53 mutations, followed by TET2, SRSF2, RUNX1, and DNMT3A mutations. In contrast, DDX41, U2AF1, TP53, and DNMT3A mutations were more likely to represent the MC clone than subclones compared with ASXL1, RUNX1, and STAG2 (Figure 2D). Changes in clone size in pre- and posttreatment samples are summarized in supplemental Figure 12.
We correlated the response to azacitidine with the size of tumor component before and after azacitidine treatment, which was estimated based on the average size of MC mutations (ave_MCpre and ave_MCpost, respectively). We found that ave_MCpost showed a strong correlation with IWG response (Figure 2E), which was less remarkable for MDS/MPN cases (supplemental Figure 13), whereas only a modest correlation was noted between ave_MCpre and response (supplemental Figure 14A). Actually, in the 48 cases with CR, the median value of ave_MCpost was as small as 0.066, compared with 0.77 and 0.84 for stable disease and progressive disease (PD) cases, respectively. In particular, a substantial number of patients, including 22 CR and 13 mCR cases, achieved a complete disappearance of the MCs after treatment (molecular CR), in which the size of the MC could be reduced to as low as <1% VAF in 43 (15%) cases. This was most frequently associated with clones having multihit TP53 mutations (14/43; 30%) but also with SRSF2-, DDX41-, RUNX1-, NPM1-, and STAG2-mutated clones. Despite an excellent correlation between IWG response and posttreatment clone size in general, there were some exceptions. For example, 7 cases showed large clones with >0.40 ave_MCpost even after achieving CR, of which 4 had subclones that shrank after treatment (Figure 2E, from cases #1 to #4; supplemental Figure 14B). MC mutations that persisted in these cases after achieving CR were represented by TET2 (n = 3) and DNMT3A (n = 1) mutations, both of which are the most frequently mutated genes in clonal hematopoiesis,42-44 suggesting that these cases likely represent a reversion to clonal hematopoeisis after azacitidine treatment.
Effects of azacitidine therapy on clone size of DDX41 mutations
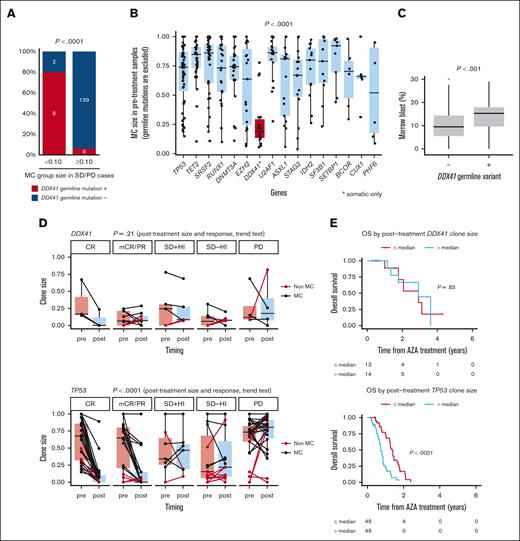
In accordance with a strong correlation between IWG response and ave_MCpost, patients who showed poor IWG response had a large ave_MCpost (Figure 2E). Among these, however, 10 showed a small ave_MCpost (< .10) (Figure 2E, cases #5-#14). Intriguingly, these cases were highly enriched for germ line DDX41 mutations, which accounted for 8 of the 10 cases (Figure 3A; supplemental Figure 14C). To explore possible mutations in other drivers that were not included in our target panel, we performed whole-genome sequencing in 4 of these 6 cases. Although a clonal population was suggested from the VAF distribution of somatic mutations, no known driver mutations were identified. Therefore, the poor IWG response in these cases remains unexplained (supplemental Figure 15; supplemental Table 8).
Somatic DDX41-mutated clone size does not correlate with blast ratio or response to treatment. (A) Percentage of the cases that have DDX41 germ line variants is shown among the cases showing SD/PD with MC group sizes <0.10 (n = 10) and ≥0.10 (n = 147). P < .0001, using Fisher exact test. (B) Box plots showing the size of MC groups in pretreatment samples that have mutations in the genes indicated on x-axis. MC groups with somatic DDX41 mutations are highlighted with red color. P value is derived from a two-sided t test for comparison of pretreatment MC sizes represented by DDX41 and the other genes. (C) Box plots showing marrow blast percentage for the cases with or without DDX41 germ line mutations. P value is derived from a two-sided t test. (D) Paired box plots showing the changes in clone size between pre- and posttreatment samples with mutations in DDX41 (top) and TP53 (bottom). The same mutations are connected by lines. Colors of lines represent whether they are MC (black) or not (red). P values are derived from Jonckheere-Terpstra tests under the hypothesis that there is no correlation between clinical response and posttreatment clone sizes. (E) Kaplan-Meier estimates of OS per clone sizes in posttreatment samples. Cases with somatic DDX41 mutations (top; n = 27) and TP53 mutations (bottom; n = 92) are shown. The posttreatment clone sizes were divided by the median value for each gene. The number of the cases at risk at each time is indicated in the table below. P values are derived from two-sided log-rank tests. HI, hematological improvement.
Somatic DDX41-mutated clone size does not correlate with blast ratio or response to treatment. (A) Percentage of the cases that have DDX41 germ line variants is shown among the cases showing SD/PD with MC group sizes <0.10 (n = 10) and ≥0.10 (n = 147). P < .0001, using Fisher exact test. (B) Box plots showing the size of MC groups in pretreatment samples that have mutations in the genes indicated on x-axis. MC groups with somatic DDX41 mutations are highlighted with red color. P value is derived from a two-sided t test for comparison of pretreatment MC sizes represented by DDX41 and the other genes. (C) Box plots showing marrow blast percentage for the cases with or without DDX41 germ line mutations. P value is derived from a two-sided t test. (D) Paired box plots showing the changes in clone size between pre- and posttreatment samples with mutations in DDX41 (top) and TP53 (bottom). The same mutations are connected by lines. Colors of lines represent whether they are MC (black) or not (red). P values are derived from Jonckheere-Terpstra tests under the hypothesis that there is no correlation between clinical response and posttreatment clone sizes. (E) Kaplan-Meier estimates of OS per clone sizes in posttreatment samples. Cases with somatic DDX41 mutations (top; n = 27) and TP53 mutations (bottom; n = 92) are shown. The posttreatment clone sizes were divided by the median value for each gene. The number of the cases at risk at each time is indicated in the table below. P values are derived from two-sided log-rank tests. HI, hematological improvement.
In most cases with DDX41 germ line variants, somatic DDX41 mutations represented the major clones (Figure 2D). Of interest, the size of these clones was significantly smaller than that of MCs in DDX41-unmutated cases, despite significantly higher blast counts in DDX41-mutated MDS45 (Figures 3B,C). Unlike TP53 mutations, the posttreatment clone size of somatic DDX41 mutations is poorly correlated with IWG response or OS (Figures 3D,E).
OS model on azacitidine therapy
We then investigated the effect of gene mutations on OS in azacitidine-treated patients, in which we first identified those variables that were significantly associated with OS in univariate analysis for 13 mutations/CNAs as well as 7 clinical variables, including all 449 patients in the current cohort, which were used for multivariable Cox proportional hazard modeling (supplemental Figure 16A,B). As expected from the observations that the revised International Prognostic Scoring System (IPSS-R) significantly predicted OS after azacitidine treatment (supplemental Figure 16C and also Ref #46), the established model contained many variables that overlapped with those of IPSS-R, including higher blast percentage, lower platelet count, and high-risk karyotypes (supplemental Figure 16B). In fact, these variables can be replaced by the IPSS-R score without significantly affecting the concordance index (c-index; supplemental Figure 16D). Largely, recapitulating previous reports, the mutation status of TP53,9,16,38,47-50EZH2, and DDX4145 was significantly associated with OS and contributes to model improvement. Note that multihit TP53 mutations predicted a significantly shorter progression free survival, although it was a significant predictor of a better initial response (supplemental Figure 16E).
As for the clinical effect of gene mutations in MDS, a novel prognostic model that integrates gene mutations and other variables used in IPSS-R has recently been proposed50 (molecular IPSS [IPSS-M]) and was shown to significantly predict OS after azacitidine in the current cohort (supplemental Figure 16F). Thus, we tested whether IPSS-M can better replace IPSS-R and gene mutations, except for DDX41 mutations, which are not included in IPSS-M. As shown in supplemental Figure 16G, the model was significantly improved by replacing IPSS-R and TP53 and EZH2 mutation states with IPSS-M, as shown by an increased c-index from 0.701 to 0.715.
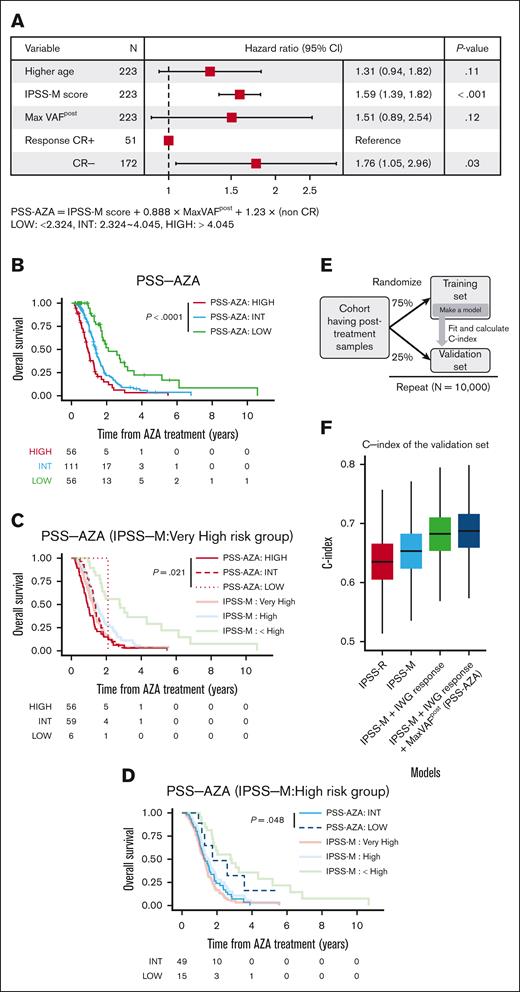
We then examined whether molecular response after treatment in terms of posttreatment clone size (maxVAFpost) and/or IWG response (CR or not) further improved this model, using the paired cohort in which both pre- and posttreatment samples are available. Because maxVAFpost of DDX41-mutated clones did not correlate with OS (Figure 3E), and the IPSS-M score does not include DDX4150 and also does not stratify OS of the patients with DDX41-mutatations,45 we excluded patients with DDX41-mutations from the subsequent analysis. We found that both initial IWG response and MaxVAFpost remained independent predictors of OS in the multivariable model (Figure 4A), based on which we constructed a scoring system (the prognostic scoring system for azacitidine treatment [PSS-AZA]), which was used to stratify azacitidine-treated patients into 3 age-adjusted risk categories: HIGH, INT, and LOW (Figure 4B). PSS-AZA recategorized IPSS-M–very high-risk or –high-risk groups into subgroups with distinct OS (Figures 4C,D). Of note, the 6 patients who were reclassified as being PSS-AZA LOW risk from IPSS-M–very high-risk had a considerably longer OS (24 months) than that of patients who were classified as IPSS-M–very high-risk (12 months; Figure 4C) We confirmed the improvement of the model using crossvalidation, in which we split the entire cohort into 75% training and 25% validation subsets, and for each split, a c-index was calculated for the validation set based on the model constructed for the training set (Figure 4E). The addition of IWG response and maxVAFpost data improved the prediction of IPSS-M, as explained by an increased c-index of 0.030 (IPSS-M vs IPSS-M + response) and 0.005 (IPSS-M + response vs PSS-AZA), respectively. Overall, the model was substantially improved by including all PSS-AZA variables, compared with the IPSS-M alone model, with an increment of c-index from 0.653 to 0.688 and improved goodness of fit (P < .001, using the likelihood ratio test; Figure 4F).
The role of posttreatment clone size on improvement of OS model. (A) A forest plot showing the result of Cox proportional hazards regression model for OS performed on the paired cohort with complete data for OS analysis (n = 223). Explicative variables are age, IPSS-M score, the largest VAF values adjusted for copy number alterations in posttreatment samples (MaxVAFpost), and clinical response per the IWG 2006 criteria (CR or not). The x-axis is log scaled. The new risk score of the novel OS model (prognostic scoring system after azacitidine treatment; PSS-AZA) is calculated according to the formula shown below the forest plot. The threshold for risk classification is also presented below the forest plot. (B) Kaplan-Meier estimates of OS per risk classes based on PSS-AZA are presented. The number of the cases at risk at each time is indicated in the tables below. P values are derived from two-sided log-rank tests. (C,D) Kaplan-Meier estimates of OS per risk classes based on PSS-AZA are presented for IPSS-M–very high-risk (C) and IPSS-M high-risk (D) group cases. Kaplan-Meier estimates of IPSS-M–based risk groups are overlaid with light-colored curves. (E) A schematic presentation of the analysis that examines the improvement of predictability. The paired cohort was randomly split into 75% training and 25% validation subsets 10 000 times and constructed a model for each training set to fit the model and calculated the concordance index (c-index) for the corresponding validation set. (F) Box plots indicating the distribution of c-index in the validation cohorts of 10 000 simulations.
The role of posttreatment clone size on improvement of OS model. (A) A forest plot showing the result of Cox proportional hazards regression model for OS performed on the paired cohort with complete data for OS analysis (n = 223). Explicative variables are age, IPSS-M score, the largest VAF values adjusted for copy number alterations in posttreatment samples (MaxVAFpost), and clinical response per the IWG 2006 criteria (CR or not). The x-axis is log scaled. The new risk score of the novel OS model (prognostic scoring system after azacitidine treatment; PSS-AZA) is calculated according to the formula shown below the forest plot. The threshold for risk classification is also presented below the forest plot. (B) Kaplan-Meier estimates of OS per risk classes based on PSS-AZA are presented. The number of the cases at risk at each time is indicated in the tables below. P values are derived from two-sided log-rank tests. (C,D) Kaplan-Meier estimates of OS per risk classes based on PSS-AZA are presented for IPSS-M–very high-risk (C) and IPSS-M high-risk (D) group cases. Kaplan-Meier estimates of IPSS-M–based risk groups are overlaid with light-colored curves. (E) A schematic presentation of the analysis that examines the improvement of predictability. The paired cohort was randomly split into 75% training and 25% validation subsets 10 000 times and constructed a model for each training set to fit the model and calculated the concordance index (c-index) for the corresponding validation set. (F) Box plots indicating the distribution of c-index in the validation cohorts of 10 000 simulations.
Outcome of allo-SCT
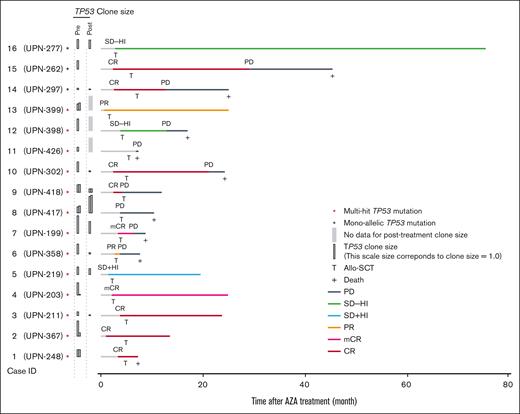
Among the paired cohort, 73 patients received allogeneic stem cell transplantation (allo-SCT) at a median of 5.2 months (range, 1.7-22 months) after the initiation of azacitidine therapy. The median posttransplant OS was 81.4 months (range, 36 months to not reached)(supplemental Figure 17A) after a median follow-up period of 49 months. Next, we focused on patients with TP53 mutations who are expected to have a dismal prognosis even with allo-SCT.37,51,52 Among 13 patients with multihit TP53 mutations who underwent transplantation, the median OS after the initiation of azacitidine therapy and allo-SCT were 24.3 and 19.2 months, respectively (supplemental Figures 17B,C). Six cases (Figure 5) were progression-free after a median follow-up of 23.7 months. Notably, all 4 cases in which the mutant TP53 clone size decreased to <0.10 maintained CR/mCR, with a median follow-up of 23.7 months. This suggests that a significant reduction in TP53-mutant clone size with azacitidine therapy could improve clinical outcomes when consolidated with allo-SCT.
The role of posttreatment clone size in predicting posttransplant outcomes. A swimmer plot showing the clinical course for the 16 cases who underwent transplant with TP53 mutations. Red and black asterisks indicate TP53 allelic state. Colors of horizontal bars indicate the period having the clinical state. Vertical bars show the clone size of TP53 variants in pre- and posttreatment samples.
The role of posttreatment clone size in predicting posttransplant outcomes. A swimmer plot showing the clinical course for the 16 cases who underwent transplant with TP53 mutations. Red and black asterisks indicate TP53 allelic state. Colors of horizontal bars indicate the period having the clinical state. Vertical bars show the clone size of TP53 variants in pre- and posttreatment samples.
Discussion
NGS-based mutational profiling has successfully been applied to longitudinal disease monitoring to depict clonal dynamics in representative patients with myeloid neoplasms who were treated with a variety of drugs, including azacitidine.33,35,38,53,54 However, the role of longitudinal NGS-based profiling in the evaluation of therapeutic response has not fully been investigated in a large cohort of patients uniformly evaluated with standardized response criteria. Here, enrolling a large number of patients who were treated with azacitidine and uniformly assessed using the IWG criteria, we have delineated the impact of NGS-based mutation profiling of both pre and posttreatment samples on the IWG response and long-term survival.
Here, through the analysis of several posttreatment samples, we demonstrated that the posttherapy clone size (ie, ave_MCpost) was highly correlated with IWG response. In particular, a substantial number of patients (15%) showed a complete or almost complete clearance (<1%) of major driver-mutated clones, or “molecular CR,” after azacitidine therapy, which was most frequent in TP53-mutated cases, but also obtained in many TP53–wild-type (WT) cases. Despite this high correlation, there existed some exceptions. Although CR in general accompanied a substantial reduction of MCs, it can be achieved even with persistent large MCs having multiple mutations, typically including those affecting TET2. This confirmed a previous observation that multiple residual and/or newly emerging mutations are compatible with apparently healthy hematopoiesis, as reported in CR in AML55 or clonal hematopoiesis in healthy individuals.42,44 By contrast, other cases showed PD even though a complete clearance of MCs was obtained, which was typically seen in cases with somatic DDX41 mutations. The clearance (<10% VAF) of somatic DDX41 mutations was found in 13 cases, of which 7 remained stable disease or PD. Although representing the MC in most cases and associated with elevated blast counts, somatic DDX41 mutants had significantly smaller clone sizes than expected for a major clone having other driver mutations, which together with their unique response to azacitidine, is among the unique features of DDX41-mutated myeloid neoplasms.
Another key finding in this study is the impact of gene mutations in both pre- and posttreatment samples on IWG response and OS. Prognostication of MDS has conventionally been performed relying on the IPSS or IPSS-R model that was established using a large cohort of untreated patients based on clinical and cytogenetic markers, which has been shown to be also applied in the prediction of OS after azacitidine therapy.46 Here, enrolling a large number of azacitidine-treated patients, we not only confirmed this but also demonstrated that the prediction was significantly improved by including gene mutation data, including those of TP53 (multihit), EZH2, and DDX41, through de novo construction of the prognostic model. The role of mutation profiling in MDS prognostication has recently been clearly demonstrated by the development of novel molecular prediction models.50,56,57 Among which IPSS-M has been shown to better predict leukemia progression and OS compared with the conventional IPSS-R by incorporating mutation status of 31 common driver genes in MDS together with the conventional clinical and cytogenetic markers used in IPSS-R in common.50 We showed in this study that IPSS-M also outperformed IPSS-R in the prediction of OS in azacitidine therapy, although IPSS-M does not include DDX41 mutations and also poorly predicted OS in DDX41-mutated cases.45 Therefore, IPSS-M should be applied only to patients with unmutated DDX41–. Importantly, however, the model including clinical factors and pretreatment gene mutations, such as IPSS-M, could be further improved by including posttreatment variables, that is, IWG response and, to a lesser extent, posttreatment clone size, highlighting the benefit of measuring posttreatment samples. It not only enables molecular assessment of the response but also contributes to better prediction of OS compared with IPSS-M.
Finally, it should be noted that DDX41-mutated cases intrinsically showed better response than DDX41-WT cases upon azacitidine treatment and are not stratified into subgroups with distinct OS even using IPSS-M or posttreatment clone size, suggesting that DDX41-mutated MDS should be separately evaluated in OS prediction. This is in line with the observation that posttreatment clone size of somatic DDX41 mutants and also with our recent study of large-scale analysis of DDX41-mutated myeloid neoplasms, which demonstrated a poor impact of the IPSS-M score on predicting the prognosis of patients with DDX41 mutatations.45
Limitations of this study include a single point assessment of posttreatment samples that potentially prevented a full description of clonal dynamics during treatment. In addition, we could not identify the optimal timing to evaluate posttreatment clones to monitor treatment response. Our framework that incorporates posttreatment clone size into the OS-predicting model can only be applied to cases that remained on treatment for about 4 courses; however, this is not considered a drawback as long-term outcomes cannot be achieved for early dropout cases. Lack of methylation58-60 or expression61 assessment is another drawback of this study that could have disclosed the pathogenesis that remained unexplained by genetic profiles alone, although these assays are not widely available in practical settings and clinical application is limited. Finally, this is among the largest studies ever reported, and the results are, at least partly, supported by an independent validation or crossvalidation cohort; the size of our cohort is still limited, compared with that used to establish IPSS or IPSS-R and should be confirmed in a larger set of samples.
Acknowledgments
The authors acknowledge the patients who participated in this study and their families. The authors thank the clinical research staff and caregivers at all participating sites. The authors express their appreciation to Natsuki Hosho, Kazuhide Ohnishi, Rikako Onoi, Toshiko Sato, and Takeshi Shirahari for their technical assistance.
The supercomputing resource was provided by the Human Genome Center, the Institute of Medical Science, the University of Tokyo.
This work was supported by the Japan Agency for Medical Research and Development (JP15cm0106056h0005, JP19cm0106501h0004, JP16ck0106073h0003, and JP19ck0106250h0003) (S. Ogawa), (JP19ck0106353h0003) (Y.N.), and (JP21ck0106470h0003) (H.M.); (JP16cm0106505h) (S.C.) and the Core Research for Evolutional Science and Technology (JP19gm1110011) (S. Ogawa); the Ministry of Education, Culture, Sports, Science and Technology of Japan; the High Performance Computing Infrastructure System Research Project (hp200138, hp210167, hp180198, and hp190158) (S. Ogawa and S.M.); the Japan Society for the Promotion of Science; Scientific Research on Innovative Areas (JP15H05909) (S. Ogawa. and S.M.), (JP15H05912) (S.M.), and KAKENHI (JP26221308 and JP19H05656) (S. Ogawa); (JP15H05707) (S.M.); (JP18H02836) (Y.N.); (JP15H05668) (K.Y.); (JP19H01053) (H.M.). S. Ogawa is a recipient of the JSPS Core-to-Core Program A: Advanced Research Networks. Y.M. received the grant for Clinical Cancer Research from the Ministry of Health, Labour and Welfare of Japan (H25-GanRinsho-Ippan-006). E.H.-L. and S. Ohtake were supported by a grant from the Knut and Alice Wallenberg Foundation, E.H.-L. was supported by grants from the Swedish Cancer Society, the Stockholm Cancer Society, the Stockholm County Council, and the Scientific Research Council of Sweden. The funders of the study did not have any impact on study design, data collection, interpretation of the results, or manuscript writing.
Authorship
Contribution: Y.N., M.T., Y.M., E.H.-L., and S. Ogawa contributed to the conception and design of the study; M.T., S.S., E.B., S. Ohtake, J.T., M.C., L.Z., M.K., Y. Shibata, N.N., Mizuki Watanabe., N.H., K.U., Mitsumasa Watanabe, K.I., H.H., M.T., T.K., K.O., T.I., A.T.-K., H. Tsurumi, S.K., S.C., T.N., S.M., E.P., and Y.M. collected the data, Y.N., M.T., J.T., Y. Shiozawa, Y. Shiraishi, H. Tanaka, K.Y., N.K., H.M., M.N., S.M., E.P., Y.M., E.H.-L., and S. Ogawa analyzed and interpreted the data; Y.N., M.T., M.C., E.H.-L., and S. Ogawa contributed in manuscript writing; and all the authors gave final approval of the manuscript and are accountable for all aspect of work.
Conflict-of-interest disclosure: Y.N. reports a consulting or advisory Role at Otsuka Pharmaceutical. K.U. received research funding from Otsuka Pharmaceutical and Nippon Shinyaku; and is on speakers’ bureau at Otsuka Pharmaceutical. K.I. received honoraria from Nippon Shinyaku. T.K. received grants from and is on speakers’ bureau at Nippon Shinyaku. K.O. received honoraria from Nippon Shinyaku. Y.M. received honoraria from Nippon Shinyaku.
Correspondence: Eva Hellström Lindberg, Karolinska Institutet, HERM, Neo, 141 83 Huddinge; e-mail: eva.hellstrom-lindberg@ki.se; and Seishi Ogawa, Yoshida Konoe-cho, Sakyo-ku, Kyoto City, Kyoto, Japan; e-mail: sogawa-tky@umin.ac.jp.
References
Author notes
∗Y.N. and M.T. contributed equally to the work.
†E.H.-L. and S. Ogawa are joint senior authors.
The sequence bam files are deposited to European Genome-Phenome Archive with accession number EGAS00001007030 and is availabe to access upon request to the corresponding authors, Eva Hellström Lindberg (eva.hellstrom-lindberg@ki.se) and Seishi Ogawa (sogawa-tky@umin.ac.jp).
The full-text version of this article contains a data supplement.