Key Points
Mice with a truncating mutation in GATA1 have lifelong anemia that is, in part, due to altered erythroid progenitor populations.
Gata1s mutant mouse erythrocytes have a reduced lifespan due to an unknown etiology.
Abstract
GATA1 mutations that result in loss of the N-terminal 83 amino acids are a feature of myeloid leukemia in children with Down syndrome, rare familial cases of dyserythropoietic anemia, and a subset of cases of Diamond-Blackfan anemia. The Gata1s mouse model, which expresses only the short GATA1 isoform that begins at methionine 84, has been shown to have a defect in hematopoiesis, especially impaired erythropoiesis with expanded megakaryopoiesis, during gestation. However, these mice reportedly did not show any postnatal phenotype. Here, we demonstrate that Gata1s mutant mice display macrocytic anemia and features of aberrant megakaryopoiesis throughout life, culminating in profound splenomegaly and bone marrow fibrosis. These data support the use of this animal model for studies of GATA1 deficiencies.
Introduction
GATA1 is a key regulator of maturation of several blood lineages including erythroid cells, megakaryocytes, and mast cells. Mouse models reveal that GATA1 regulates transcription of lineage-specific genes in combination with cofactors such as FOG1 and SCL.1-4 The first human GATA1 mutation was discovered in a family with 2 half-brothers that displayed anemia during gestation.5 These children were born with thrombocytopenia and anemia, owing to the inability of the GATA1 V205M mutant isoform to bind FOG1. Many other inherited GATA1 missense mutations in the N-terminal zinc finger have been identified, which impair the ability of GATA1 to bind either FOG1 or chromatin.6-9 In all cases, these mutations resulted in a spectrum of defects in erythroid cells and megakaryocytes. More recently, a novel missense mutation in GATA1, which is predicted to affect chromatin occupancy through an alteration in the intrinsically disordered region, was shown to be associated with congenital hemolytic anemia.10
A second class of GATA1 mutations, which lead to expression of a shortened isoform that lacks the N-terminal activation domain, were discovered in 2002.11 These mutations, which include insertions and deletions within exon 2 or mutations that disrupt inclusion of exon 2, are a unifying feature of transient abnormal myelopoiesis and myeloid leukemia in Down syndrome (ML-DS). Although the precise mechanism by which loss of the N-terminus leads to leukemia remains unclear, studies have shown that the GATA1s isoform has altered DNA-binding properties and leads to reduced H3K27 trimethylation of genes that are normally downregulated during erythroid maturation, including Runx1 and Gata2.12 Similar GATA1 mutations were identified in a subset of cases of Diamond-Blackfan anemia (DBA) that lack ribosomal gene mutations.13-15 Finally, rare cases of inherited GATA1 mutations that lead to exclusive expression of GATA1s have been reported.16 These affected individuals display macrocytic anemia.
A mouse model of GATA1 mutations that cause GATA1s expression, referred to here as Gata1s or G1s, was reported in 2005.17 These animals were shown to have impaired erythropoiesis and expanded megakaryopoiesis during gestation, with the abnormalities most prominently seen in late yolk sac through midfetal liver hematopoiesis. By contrast, hematopoiesis in the embryos was reported to be normalized by embryonic day 16.5 (E16.5), and the hematopoiesis in postnatal animals was also purportedly normal. Specifically, animals up to 6 weeks of age were reported to have normal platelet (PLT) count, normal white blood cell count (WBC), and a nonsignificant reduction in red blood cell (RBC) count. The splenic architecture and megakaryocyte content were also described as normal. Hematopoiesis beyond 6 weeks of age was not reported.
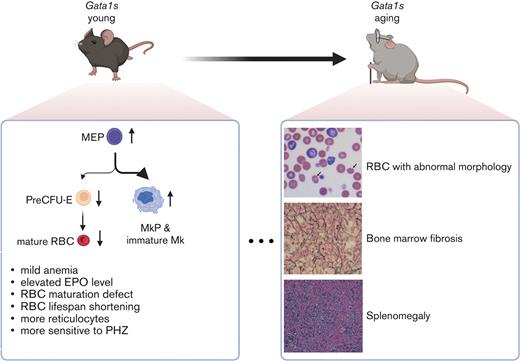
We performed an in-depth analysis of hematopoiesis in the Gata1s strain from 2 months to 20 months and observed persistent macrocytic anemia with expanded megakaryopoiesis at all ages. With time, the animals developed prominent splenomegaly and bone marrow (BM) fibrosis. Therefore, our data reveal that the Gata1s model is more applicable to studies of GATA1 mutations than previously thought.
Materials and methods
Animals
Gata1Δex2 mice (referred to as Gata1s or G1s), which have a deletion of exon 2 that results in unique expression of GATA1s was provided by Stuart Orkin (Children’s Hospital, Boston, MA). For genotyping, tail tips were collected at weaning, digested, and then the recovered DNA was amplified using primers sets wild-type (WT) (forward 5′-AACCCTTTCTGTCCTC-3′, reverse 5′-TCTATGGCAACCCTGA-3′) and G1s (forward 5′-GATGGAGTAGTAGAGG-3′, reverse 5′-TAGCATAAGGTGAGCC-3′). Ly5.1 mice were ordered from The Jackson Laboratory (stock number. 002014). Mice were housed in a barrier facility. Animal studies were approved by the St. Jude Children’s Research Hospital Institutional Animal Care and Use Committee.
BM transplantation and treatment
BM cells (BMCs) from Gata1 WT, Gata1s, or Ly5.1-recipient mice were isolated by flushing femurs and tibias followed by ammonium-chloride-potassium (ACK) RBC lysis (NH4Cl, 150 mM; KHCO3, 1 mM; EDTA, 0.1 mM; pH 7.2-7.4). One million live BMC were transplanted into lethally irradiated recipients via tail vein injection. Complete blood count (CBC) analysis and peripheral chimerism (peripheral blood mononuclear cells [PBMCs]) were performed at indicated time points. Phenylhydrazine (PHZ) (Sigma) was used to induce acute anemia in Gata1s mice. Mice received sterile PHZ solution intraperitoneally to achieve a desired dose on day 0 and day 1 with a dose of 80 mg/kg body weight. The recovery of erythropoiesis was monitored by CBC after injection.
Flow cytometry
Cells were suspended in sterile fluorescence-activated cell sorting buffer (phosphate-buffered saline [PBS] containing 0.5% bovine serum albumin and 2 mM EDTA) and stained with indicated surface markers for 30 minutes at 4°C. All flow cytometry data were analyzed using FlowJo. Chimerism in transplantations was checked by staining PBMCs with conjugated antibodies against CD45.1-APC (BD Biosciences, 561873) and CD45.2-PerCP-Cy5.5 (BD Biosciences, 552950). Erythroid cells were analyzed by staining of peripheral blood (PB) and unfractionated BMCs with conjugated antibodies against Ter119-APC (Biolegend, 116212), CD71-PE (BD Biosciences, 553267), and CD44-V450 (BD Biosciences, 560452). Megakaryocytes were analyzed by staining of ACK-lysed BMCs with conjugated antibodies against CD41-PE-Cy7 (Biolegend, 133916) and CD42-DyLight649 (Emfret Analytics, M040-3). Myeloerythroid progenitor cells were analyzed by staining of unfractionated BMCs with conjugated antibodies against lineage cocktail V450 (BD Biosciences, 51-9006957), c-Kit-APC-eFluro780 (eBioscience, 47-1171-82), Sca-1-FITC (eBioscience, 11-5981-81), CD150-PE (Biolegend, 115904), CD41-PE-Cy7 (Biolegend, 113916), CD105-APC (eBioscience, 17-1051-82), and CD16/32-PerCP-Cy5.5 (BD Biosciences, 560540). For myeloid progenitor staining, ACK-lysed BMCs were stained with conjugated antibodies against lineage cocktail V450 (BD Bioscences, 51-9006957), c-Kit-APC (eBioscience, 17-1171-82), Sca-1-PE-Cy7 (eBioscience, 25-5981-82), CD34-PE (BD, 551387), and CD16/32-PerCP-Cy5.5 (BD Biosciences, 560540). CD55 and CD59a on RBCs from PB were analyzed using PE-conjugated antibodies (Biolegend; anti-CD55, 131804 and anti-CD59, 143104). RBC lifespan was determined by biotin labeling (Thermo Fisher Scientific, 21336) of RBCs followed by serial flow cytometry (PE Streptavidin from BD Biosciences, 554061) as previously described.18 Measurement of reactive oxygen species in RBCs was performed following the manufacturer’s guidelines (Thermo Fisher Scientific, C10443). Briefly, 2 μL of PB was diluted in 2 mL Dulbecco’s PBS and CellROX Orange Reagent was added to a final concentration of 5 μM. After incubation at 37°C for 30 minutes, RBCs were washed 3 times with Dulbecco’s PBS and then analyzed by flow cytometry.
ELISA and colorimetric/fluorometric assays
The serum erythropoietin level was determined by enzyme-linked immunosorbent assays (ELISA) using a Legend Max Mouse EPO ELISA kit (Biolegend, 442707) according to the manufacturer’s guidelines. Plasma haptoglobin and hemopexin were determined by ELISA using 1:10 000 and 1:20 000 dilutions, respectively (Abcam, ab157714 and ab157716). The glucose-6-phosphate dehydrogenase (G6PD) activity assay kit (Cell Signaling Technology, 12581) was used to measure G6PD activity in Gata1s RBCs and whole blood. Briefly, 20 μL of whole blood or washed RBCs from 30 μL of PB were resuspended in 100 μL lysis buffer and 30 μL of supernatant was used in the assay. Plasma pyruvate kinase levels were measured using commercially available pyruvate kinase assay kits measuring fluorometric detection at 100-fold plasma dilution (Abcam, ab83432). Adenosine deaminase (ADA) levels were measured utilizing a commercially available colorimetric assay kit at recommended dilutions (Abcam, ab211093). Data were analyzed with GraphPad Prism9.
Sensitivity toward complement-mediated lysis (Ham test)
The Ham test was conducted as previously described.19 Pooled human complement serum was purchased from Innovative Research (ICSER10ML) and was diluted using normal saline (0.9% NaCl). Heat inactivation was performed by incubation at 56°C for 30 minutes to deactivate the complement system. Packed RBCs were obtained by centrifugation of PB with sodium citrate anticoagulant at 1000g for 10 minutes. Packed RBCs were washed with normal saline, and cells were incubated with dilutions of the serum at 37°C for 1 hour. Free hemoglobin (Hb) was detected by measuring absorbance at 412 nm, and results were normalized to a hypotonic lysis buffer.
Red cell deformability test
Measurement of deformability of RBCs from PB was performed on the laser-assisted rotational red cell analyzer (Lorrca) following manufacturer’s guidelines. This entailed mixing anticoagulated PB with manufacturer’s diluent (Elon ISO, Reference # QPR030901-B01, Viscosity: 28.38 mPa). For the deformability measurement, we diluted 12.5 μL PB in 2.5 mL Elon ISO and loaded 880 μL into the Lorcca instrument. We diluted 120 μL PB into 2.5 mL Elon ISO for Osmoscan.
Histology
Mouse tissues (sternum and spleen) were fixed in 10% neutral buffered formalin for at least 24 hours before processing for hematoxylin and eosin staining or reticulin staining.
Statistical analysis
All statistical analysis was performed with GraphPad Prism9. For quantitative assays, different genotypes were reported as mean ± standard deviation and compared using the unpaired Student t test or 2-way analysis of variance (ANOVA) test as indicated; P ≤ .05 was considered statistically significant.
Results
Adult Gata1s mice have macrocytic anemia
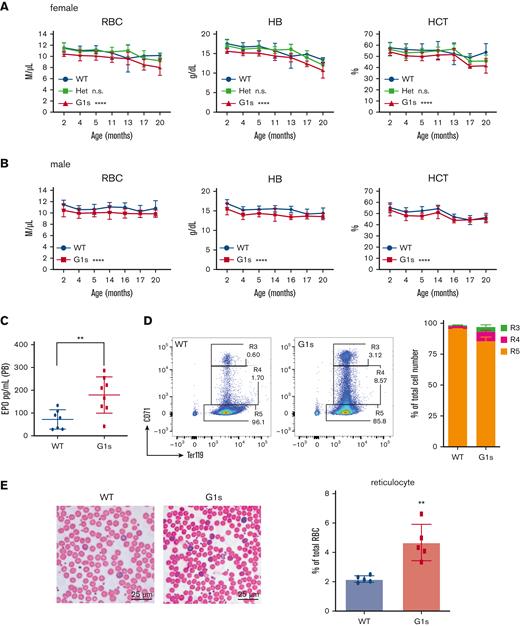
Studies from multiple groups have shown that Gata1s mice, which express exclusively the short isoform of GATA1, have anemia and expanded megakaryopoiesis in utero.12,17,20 However, the initial publication on these mice reported that there were no defects in adults, based on analysis at 3 through 6 weeks of age. Given that human GATA1 mutations, which cause exclusively GATA1s expression, are associated with congenital anemia including DBA,21 we performed a detailed assessment of hematopoiesis in Gata1s adult mice beyond 6 weeks. CBC analysis of 2-month-old mice revealed macrocytic anemia in both Gata1s homozygous females and hemizygous males, with significant reductions in RBC count and Hb, and increased mean corpuscular volume in hemizygous males (Figure 1; supplemental Figure 1A). By contrast, anemia was not observed in Gata1s heterozygous females indicating that 1 WT allele of Gata1 is sufficient for normal hematopoiesis, similar to that seen in female carriers of GATA1 mutations5 (Figure 1; supplemental Figure 1A). For unclear reasons, an elevated PLT count was restricted to hemizygous Gata1s males (supplemental Figure 1B). Of note, the changes in erythroid parameters persisted through 20 months of age (Figure 1A-B). Consistent with the anemia phenotype, we also observed an elevated erythropoietin level in the PB of Gata1s mice as determined by ELISA (Figure 1C). With respect to erythroid progenitors, flow cytometry analysis of 2 murine erythrocyte surface markers, transferrin receptor CD71 and Ter119, revealed an accumulation of CD71+ erythrocytes in Gata1s PB (Figure 1D). Moreover, a 2.14-fold increase of reticulocytes was also observed (Figure 1E). Howell-Jolly bodies were detected in aging Gata1s RBCs (supplemental Figure 1C), indicative of splenic dysfunction in the Gata1s-aged mice. Together, our observations indicate that the impairment of erythropoiesis in Gata1s mice persists into adulthood.
Defect of erythropoiesis in Gata1s adult mice. (A-B) PB indices for WT, Gata1s heterozygous (Het) females, Gata1s homozygous females (F), and Gata1s hemizygous males (M) between 2 and 20 months of age. NWT M ≥ 10, NG1s M ≥ 10, NWT F ≥ 4, NHet F ≥ 7, NG1s F ≥ 5. ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001 by 2-way analysis of variance (ANOVA). (C) Erythropoietin (EPO) levels in the PB of 2-month-old animals. Nwt = 7, NG1s = 8. ∗∗P ≤ .01 by the unpaired Student t test. (D) Representative flow cytometry plots and bar graph of mean ± standard deviation (SD for erythroid cells in the PB of Gata1s (G1s) mice as assessed by staining with antibodies against Ter119 and CD71. Nwt = 3, NG1s = 4. (E) Photos (left) and quantitation (right) of reticulocytes in the PB of WT and Gata1s littermates (2 months old). In panel E, scale bars represent 25 μm. The graph depicts the percentage of reticulocytes in 5 fields. N = 5. ∗∗P ≤ .01 by the unpaired Student t test. n.s., not significant.
Defect of erythropoiesis in Gata1s adult mice. (A-B) PB indices for WT, Gata1s heterozygous (Het) females, Gata1s homozygous females (F), and Gata1s hemizygous males (M) between 2 and 20 months of age. NWT M ≥ 10, NG1s M ≥ 10, NWT F ≥ 4, NHet F ≥ 7, NG1s F ≥ 5. ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001 by 2-way analysis of variance (ANOVA). (C) Erythropoietin (EPO) levels in the PB of 2-month-old animals. Nwt = 7, NG1s = 8. ∗∗P ≤ .01 by the unpaired Student t test. (D) Representative flow cytometry plots and bar graph of mean ± standard deviation (SD for erythroid cells in the PB of Gata1s (G1s) mice as assessed by staining with antibodies against Ter119 and CD71. Nwt = 3, NG1s = 4. (E) Photos (left) and quantitation (right) of reticulocytes in the PB of WT and Gata1s littermates (2 months old). In panel E, scale bars represent 25 μm. The graph depicts the percentage of reticulocytes in 5 fields. N = 5. ∗∗P ≤ .01 by the unpaired Student t test. n.s., not significant.
Abnormal megakaryocytes and erythroid precursors in adult Gata1s mice
To gain further insights into the nature of the hematopoietic defects in Gata1s mice, we performed flow cytometry analysis for myeloerythroid progenitors and erythroid precursors in the BM from 2-month-old mice. We used a previously described method that incorporates analysis of CD44 and Ter119 expression to divide erythropoiesis into 5 stages.22 Analysis of the Gata1s BM showed an accumulation of cells in stage IV (32.4% vs 27.6%, P < .001) and a decreased proportion of fully mature stage V cells (37.5% vs 43.6%, P < .05) (Figure 2A-B; supplemental Figure 2A). Wright-Giemsa staining of sorted population IV, which is composed of orthochromatic erythroblasts and reticulocytes, confirmed a twofold increase in the orthochromatic stage relative to reticulocytes in Gata1s mice (supplemental Figure 2B). Furthermore, by subdividing Lin−Sca-1−c-Kit+ cells with antibodies against CD16/32 and CD34,23 we detected elevated megakaryocyte-erythroid progenitors (MEPs) (49.0% vs 34.1%, P < .01) and fewer granulocyte-monocyte progenitors (GMPs) (14.0% vs 26.0%, P < .05) in Gata1s BM but no difference in common myeloid progenitors (CMPs) (Figure 2C-D). Given that MEPs give rise to RBCs and megakaryocytes, we further tracked the downstream differentiation of Gata1s cells through a combination of anti–CD105, CD41, CD16/32, and CD150 antibodies.24 In Gata1s mouse BM, we observed no difference in premegakaryocytic/erythroid progenitors (preMegEs) (KLS-CD41−CD16/32−CD105−CD150+), decreased pre-erythroid colony-forming unit (preCFU-E) (KLS-CD41−CD16/32−CD105+CD150+) (4.3% vs 9.9%, P < .001), or elevated megakaryocyte progenitors (MkPs) (KLS-CD105+CD41+) (8.1% vs 4.8%, P < .01) (Figure 2E-J). These data suggest a skewing of megakaryopoiesis during megakaryocytic-erythroid lineage specification downstream of the MEP. Immunostaining of Gata1s BMCs with antibodies against CD41 and CD42 showed a 2.1-fold expansion of immature megakaryocytes (CD41+CD42−) without a significant change in mature megakaryocytes (CD41+CD42+) (supplemental Figure 3A-B). Consistently more megakaryocytes could be observed in BM sections from male Gata1s mice and Gata1s homozygous females (supplemental Figure 3C). At this age, we did not observe BM fibrosis or disrupted splenic architecture. Taken together, the pre-erythroid CFU population is decreased in Gata1s mice while there is an expansion of immature megakaryocytes.
Defect in erythroid precursors in BM of Gata1s mice. (A-B) Representative flow cytometry plots (A) and bar graph (B) of mean ± SD for various stages of BM erythropoiesis in 2-month-old WT or Gata1s (G1s) male mice as assessed by staining with antibodies against Ter119 and CD44. (C-J) Analysis of the composition of BMCs by flow cytometry for hematopoietic progenitors in 2-month-old mice. Numbers indicate the percentage of cells within each gate as shown in the representative plots. (C-D) Representative flow cytometry plots and mean ± SD of MEP (Lin−c-kit+Sca-1−CD34−CD16/32−), granulocyte-monocyte progenitor (GMP) (Lin−c-kit+Sca-1−CD34+CD16/32+), and common myeloid progenitor (CMP) (Lin−c-kit+Sca-1−CD34+CD16/32−) cell populations from WT and G1s mice. (E-F) Lineage−Sca-1−c-Kit+ cells were further subdivided by expression of CD105 and CD41 into the megakaryocyte progenitors (MkPs, CD105+CD41+). (G-J) Lineage−Sca-1−c-Kit+CD34−CD16/32− cells were further subdivided by expression of CD105 and CD150 into pregranulocyte-macrophage progenitors (pre-GMs, CD105−CD150−), the premegakaryocytic/erythroid progenitors (preMegEs, CD105−CD150+), and the pre-CFU erythroid cells (preCFU-Es, CD105+CD150+). Graphs depict mean (± SD) percentage of flow cytometry data. Nwt = 3, NG1s = 4. ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001 by the unpaired Student t test.
Defect in erythroid precursors in BM of Gata1s mice. (A-B) Representative flow cytometry plots (A) and bar graph (B) of mean ± SD for various stages of BM erythropoiesis in 2-month-old WT or Gata1s (G1s) male mice as assessed by staining with antibodies against Ter119 and CD44. (C-J) Analysis of the composition of BMCs by flow cytometry for hematopoietic progenitors in 2-month-old mice. Numbers indicate the percentage of cells within each gate as shown in the representative plots. (C-D) Representative flow cytometry plots and mean ± SD of MEP (Lin−c-kit+Sca-1−CD34−CD16/32−), granulocyte-monocyte progenitor (GMP) (Lin−c-kit+Sca-1−CD34+CD16/32+), and common myeloid progenitor (CMP) (Lin−c-kit+Sca-1−CD34+CD16/32−) cell populations from WT and G1s mice. (E-F) Lineage−Sca-1−c-Kit+ cells were further subdivided by expression of CD105 and CD41 into the megakaryocyte progenitors (MkPs, CD105+CD41+). (G-J) Lineage−Sca-1−c-Kit+CD34−CD16/32− cells were further subdivided by expression of CD105 and CD150 into pregranulocyte-macrophage progenitors (pre-GMs, CD105−CD150−), the premegakaryocytic/erythroid progenitors (preMegEs, CD105−CD150+), and the pre-CFU erythroid cells (preCFU-Es, CD105+CD150+). Graphs depict mean (± SD) percentage of flow cytometry data. Nwt = 3, NG1s = 4. ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001 by the unpaired Student t test.
Adult Gata1s mice have an undefined defect in mature erythrocytes
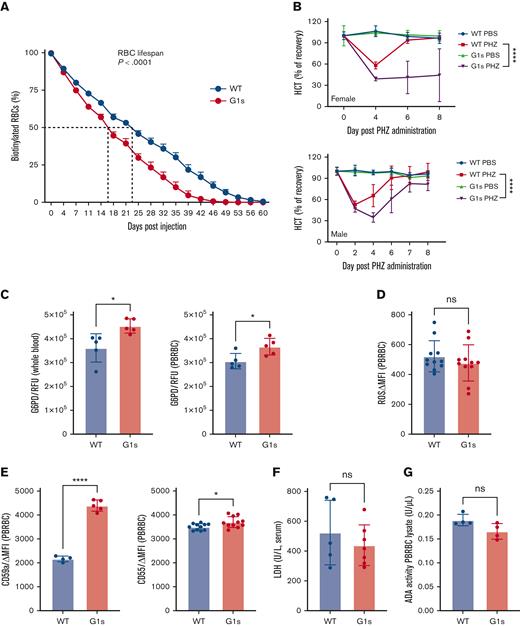
Given the anemia coupled with an elevated reticulocyte count, we delved deeper into the phenotype of mature erythrocytes in Gata1s mice. First, we compared RBC lifespan in Gata1s vs WT littermates. We performed an in vivo biotinylation assay and followed the levels of biotinylated erythrocytes by serial flow cytometry. Gata1s erythrocytes showed a significantly shorter lifespan, with a 16-day half-life compared with 23 days for the WT littermates (Figure 3A). Next, we induced acute hemolysis by intraperitoneal injection of PHZ on 2 consecutive days and then monitored recovery of erythropoiesis by serial CBC analysis. As expected, because Gata1s mice are anemic, the hematocrit (HCT) baselines of Gata1s mice and WT littermates were different (Figure 3B). Consistent with an underlying erythroid defect, we found that both Gata1s homozygous females and hemizygous males showed delayed recovery of erythropoiesis. Of note, Gata1s homozygous females were more sensitive to the PHZ-induced stress with a considerable, albeit variable, delay in recovery.
Adult Gata1s mice have a defect in mature erythrocytes. (A) Sulfo-NHS-biotin was injected IV on day 0, and the fraction of biotinylated RBCs was quantified serially by streptavidin labeling and flow cytometry, N = 4. (B) Mice were treated with PHZ solution by intraperitoneal injection on days 0 and 1. Serial HCT of WT and Gata1s (G1s) mice are shown at different time points after induced acute anemia. Normalized recovery was calculated through dividing the HCT mean value at each time point by the mean value at day 0 before treatment. Mean ± SD are shown, N ≥ 3, age = 2 months. ∗∗∗∗P ≤ .0001 by 2-way ANOVA. (C-G) Determination of PB parameters associated with hemolytic anemia, including G6PD activity (C), reactive oxygen species (ROS) levels (D), CD55 and CD59a expression (E), lactate dehydrogenase (LDH) activity (F), and ADA activity (G). ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗∗P ≤ .0001 by the unpaired Student t test. MFI, mean fluorescence intensity; RFU, relative fluorescence units; PBRBC, peripheral blood red blood cell.
Adult Gata1s mice have a defect in mature erythrocytes. (A) Sulfo-NHS-biotin was injected IV on day 0, and the fraction of biotinylated RBCs was quantified serially by streptavidin labeling and flow cytometry, N = 4. (B) Mice were treated with PHZ solution by intraperitoneal injection on days 0 and 1. Serial HCT of WT and Gata1s (G1s) mice are shown at different time points after induced acute anemia. Normalized recovery was calculated through dividing the HCT mean value at each time point by the mean value at day 0 before treatment. Mean ± SD are shown, N ≥ 3, age = 2 months. ∗∗∗∗P ≤ .0001 by 2-way ANOVA. (C-G) Determination of PB parameters associated with hemolytic anemia, including G6PD activity (C), reactive oxygen species (ROS) levels (D), CD55 and CD59a expression (E), lactate dehydrogenase (LDH) activity (F), and ADA activity (G). ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗∗P ≤ .0001 by the unpaired Student t test. MFI, mean fluorescence intensity; RFU, relative fluorescence units; PBRBC, peripheral blood red blood cell.
Next, we interrogated whether the Gata1s erythrocytes were undergoing hemolysis or had altered membrane properties through several conventional assays. First, we assayed G6PD levels in both whole blood and RBCs from Gata1s mice and observed an increase in enzymatic activity (Figure 3C), which likely resulted from the fact that reticulocytes and younger erythrocytes have higher G6PD activity than older erythrocytes.25 Second, we measured the levels of reactive oxygen species in the 2 genotypes and observed no significant differences (Figure 3D). Third, we investigated whether Gata1s erythrocytes were more susceptible to complement-mediated hemolysis, which occurs in patients with paroxysmal nocturnal hemoglobinuria, by virtue of mutations in the X-linked PIGA gene. Erythrocytes in paroxysmal nocturnal hemoglobinuria are deficient in glycosyl-phosphatidylinositol anchor proteins, such as CD55 (decay-accelerating factor) and CD59 (membrane attack complex inhibition factor), resulting in complement-mediated hemolysis.26 By flow cytometry we found that neither CD55 nor CD59a were reduced (Figure 3E). Ham test results confirmed that Gata1s erythrocytes were not more sensitive to complement-mediated lysis (supplemental Figure 4A-B). Fourth, we measured free Hb, another indicator of intravascular hemolysis that is normally cleared by haptoglobin and hemopexin.27 We did not observe alterations in plasma haptoglobin or hemopexin in Gata1s mice (supplemental Figure 5A-B). Fifth, we assayed for pyruvate kinase enzymatic activity and found it was not altered in the Gata1s mice (supplemental Figure 5C). Moreover, the plasma level of lactate dehydrogenase, which is also elevated in patients with hemolysis, was not altered in the Gata1 mice (Figure 3F). A recent publication reported that GATA1 C-terminal mutations (R307C/H) can cause hemolytic anemia with greatly increased levels of ADA.10 Our data did not reveal any changes in ADA levels (Figure 3G). Lastly, deformability assays uncovered no defect in Gata1s erythrocyte membranes unlike cells from a mouse model of sickle cell anemia (supplemental Figure 5D-E).
The Gata1s anemia phenotype is cell autonomous
Next, we investigated whether the anemic phenotype of Gata1s mice is cell autonomous. First, we transplanted 1 million total WT or Gata1s BMCs after RBC lysis into lethally irradiated Ly5.1 recipients and monitored peripheral chimerism and CBC monthly (Figure 4A). Chimerism was determined by checking the percentage of CD45.2 (donor cells) in PBMCs, which were obtained from PB after RBC lysis. The results revealed that both WT and Gata1s BMCs engrafted equally well in WT recipient mice (Figure 4B). Of note, the recipients of Gata1s BMCs phenocopied the anemia of parental Gata1s mice, with significantly lower RBC parameters, that is, RBCs, Hb, and HCT, than WT littermates up to 5 months posttransplantation (Figure 4C). Elevated reticulocytes were observed in recipient mice (supplemental Figure 6A-B) similar to the phenotype of primary Gata1s mice, indicating that this change is cell autonomous (Figure 1D-E). We also observed a defect in production of stage V erythrocytes accompanied by an increase in stage IV erythroblasts (supplemental Figure 6C), as seen in the primary Gata1s mice (Figure 2A-B). Next, given that the BM microenvironment plays a key role in supporting hematopoiesis, we assessed the engraftment and hematopoietic parameters of WT BMCs in Gata1s mice. One million Ly5.1 WT BMCs were transplanted into irradiated WT or Gata1s recipients and monitored for peripheral chimerism and CBC monthly for 5 months (Figure 4D). We observed no significant difference in PB chimerism, white blood cell, RBC, Hb, HCT, or PLT counts in Gata1s recipients compared with the WT control group (Figure 4E-F), an expected finding given that GATA1 expression is restricted to hematopoietic cells. Taken together, this study confirmed that the anemic phenotype of Gata1s mice is cell autonomous.
The Gata1s anemia phenotype is transplantable. (A) Schematic of the experimental design of transplantation of Ly5.2 Gata1s BM to irradiated Ly5.1 recipients. (B) PB chimerism in transplant recipients at different time points after transplantation. Mean ± SD are shown. (C) Graphs depict serial white blood cell (WBC) count, RBC count, Hb, and PLT count in transplant recipients over time. (D) Ly5.1 donor BMCs were transplanted into lethally irradiated WT and G1s Ly5.2 recipient mice. Peripheral chimerism (E) and WBC, RBC, Hb, HCT, and PLT (F) were monitored after transplantation. Mean ± SD are shown, N ≥ 5, age = 2 months. ∗P ≤ .05, ∗∗∗∗P ≤ .0001 by 2-way ANOVA.
The Gata1s anemia phenotype is transplantable. (A) Schematic of the experimental design of transplantation of Ly5.2 Gata1s BM to irradiated Ly5.1 recipients. (B) PB chimerism in transplant recipients at different time points after transplantation. Mean ± SD are shown. (C) Graphs depict serial white blood cell (WBC) count, RBC count, Hb, and PLT count in transplant recipients over time. (D) Ly5.1 donor BMCs were transplanted into lethally irradiated WT and G1s Ly5.2 recipient mice. Peripheral chimerism (E) and WBC, RBC, Hb, HCT, and PLT (F) were monitored after transplantation. Mean ± SD are shown, N ≥ 5, age = 2 months. ∗P ≤ .05, ∗∗∗∗P ≤ .0001 by 2-way ANOVA.
Gata1s mice display defects in BM and spleen with age
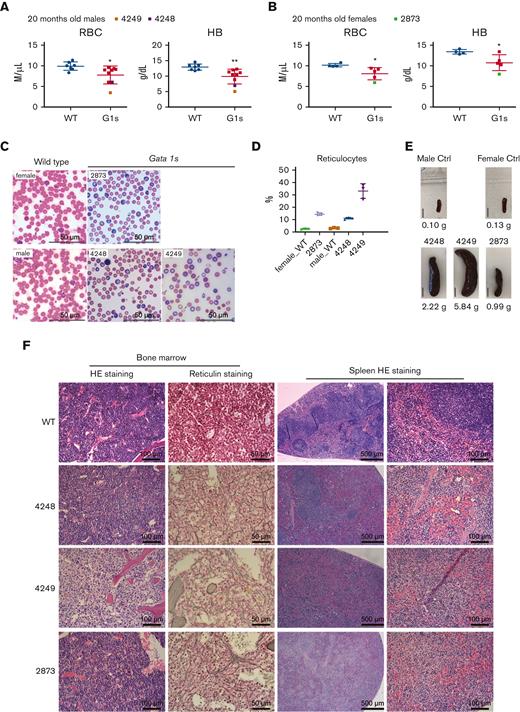
Finally, we examined hematopoiesis in 20-month-old Gata1s mice and saw that the anemic phenotype persisted as evidenced by lower RBC and Hb indices (Figure 5A-B). Among the aged mice, 3 animals displayed phenotypes consistent with severe anemia (2/9 males and 1/5 females), with an elevation in reticulocytes as well as polychromasia and anisopoikilocytosis (Figure 5C-D). We also observed enlarged spleens with abnormal architecture in the Gata1s mice with severe anemia (Figure 5E-F). Along with the severe anemia, BM fibrosis was detected in these 3 Gata1s mice (Figure 5F). Morphological assessment of 16 additional mice aged between 20 and 29 months, revealed that all of them displayed BM fibrosis, whereas only 1 of 14 WT mice presented with BM fibrosis.
Hematopoietic phenotype of aged Gata1s mice. PB analysis of RBC count and Hb level from WT and Gata1s (G1s) 20-month-old male (A) and female (B) Gata1s mice. The values of animals #4248, #4249, and #2873 are highlighted in different colors. (C) Photos of PB smears showing the increased reticulocytes in aging Gata1s (G1s) mice highlighted in panels A and B. Arrows indicate RBCs with abnormal morphology. Scale bars represent 50 μm. (D) Percentage of reticulocytes in the PB in panel C was determined by enumerating total RBCs and reticulocytes in 3 different fields. (E) Photos of enlarged spleens from animal #4248, #4249, and #2873 and 2 WT controls. Bars represent 1 cm. (F) Photomicrographs of hematoxylin and eosin (HE)– or reticulin-stained BM and spleen sections from mice in panel E. Scale bars represent 100 μm (left) and 50 μm (right) in BM sections, and represent 500 μm (left) and 100 μm (right) in spleen sections. Mean ± SD are shown in graphs, age = 20 months. ∗P ≤ .05, ∗∗P ≤ .01 by unpaired Student t test.
Hematopoietic phenotype of aged Gata1s mice. PB analysis of RBC count and Hb level from WT and Gata1s (G1s) 20-month-old male (A) and female (B) Gata1s mice. The values of animals #4248, #4249, and #2873 are highlighted in different colors. (C) Photos of PB smears showing the increased reticulocytes in aging Gata1s (G1s) mice highlighted in panels A and B. Arrows indicate RBCs with abnormal morphology. Scale bars represent 50 μm. (D) Percentage of reticulocytes in the PB in panel C was determined by enumerating total RBCs and reticulocytes in 3 different fields. (E) Photos of enlarged spleens from animal #4248, #4249, and #2873 and 2 WT controls. Bars represent 1 cm. (F) Photomicrographs of hematoxylin and eosin (HE)– or reticulin-stained BM and spleen sections from mice in panel E. Scale bars represent 100 μm (left) and 50 μm (right) in BM sections, and represent 500 μm (left) and 100 μm (right) in spleen sections. Mean ± SD are shown in graphs, age = 20 months. ∗P ≤ .05, ∗∗P ≤ .01 by unpaired Student t test.
Discussion
GATA1 mutations that lead to exclusive production of the truncated isoform GATA1s were first identified in children with megakaryocytic leukemia who have ML-DS.11 Similar mutations were subsequently found to be associated with transient abnormal myelopoiesis in DS.28 In 2006, Costa and colleagues16 discovered germ line mutations in GATA1, which lead to exclusive GATA1s expression, in a large family over 2 generations. These cases were characterized by macrocytic anemia and trilineage dysplasia.16 More recently, GATA1s-producing mutations have been found in a small number of patients with DBA who lack ribosomal protein (RP) gene mutations.14-16,29-31
Soon after the discovery of GATA1 mutations in ML-DS, Orkin and colleagues generated mouse models in which full-length GATA1 is replaced by GATA1s.11,17 Two of their models, Gata1ΔN (deleted for amino acids 3-63) and Gata1Δe2 (deleted for amino acids 1-83) have indistinguishable phenotypes. A detailed characterization of the Gata1ΔN model revealed the following: (1) a prominent defect in erythropoiesis before E14.5; (2) CD41+ megakaryocytic fetal liver cells were elevated in number and exhibited a hyperproliferative phenotype in ex vivo cultures; (3) hyperproliferative colony forming unit-megakaryocyte were only transiently detectable between E9.5 and E14.5 but not found in liver at later gestational stages or in the BM after birth; and (4) 3- to 6-week-old mice displayed typical PB indices and PLT parameters within the normal range. The difference between the Gata1s murine phenotype and persistent erythroid defects observed in humans with GATA1s-causing mutations was attributed to species-specific differences.
In a recent study, we confirmed that Gata1s mice have prominent defects in fetal liver erythropoiesis accompanied by an expansion of the megakaryocytic lineage, similar to a previous report.12 In contrast to the report that adult Gata1s mice are normal, as determined by assessment of PB counts and splenic architecture as well as CFU activity in 3- to 6-week-old animals,17 here, we show that Gata1s mice exhibit numerous hematopoietic defects, including postnatal macrocytic anemia. The abnormalities in adult hematopoiesis in Gata1s mice also include impaired stress erythropoiesis, aberrant erythroid cell maturation, decreased erythroid progenitor cells, and an accumulation of immature megakaryocytes. The lifespan of mature erythrocytes in the Gata1s adult mice was found to be reduced compared with the WT. This decrease in the erythrocyte half-life could not be explained by a membrane defect or intravascular hemolysis. Nevertheless, histological analysis supports a model in which there is splenic dysfunction by the presence of Howell-Jolly bodies in mature erythrocytes.
In a recent study, Gialesaki et al32 examined the stage-specific effects of GATA1s in human hematopoiesis by comparing the effect of loss of full-length GATA1 in fetal, neonatal, and adult hematopoietic stem and progenitor cells. They observed perturbed erythro-megakaryocytic differentiation and an accumulation of immature erythroid progenitors in cultures of fetal hematopoietic stem and progenitor cells engineered to have the GATA1s mutation; this effect was not seen in similar experiments performed with neonatal or adult progenitors. Although this study further confirms that the most striking effect of GATA1s mutations is seen in gestation, the study was performed ex vivo and patients with GATA1s mutations in the absence of trisomy 21 have persistent defects in erythropoiesis, which are partially recapitulated in the Gata1s mouse model, as shown here.
DBA is a rare inherited BM-failure disorder characterized by a significant decrease in erythroid precursors in BM without signs of dysplasia or prominent defects in other cell lineages at young ages. CBC and reticulocyte evaluation reveal moderate to severe anemia that is usually macrocytic, and this is frequently accompanied by reticulocytopenia. Although neutrophil and PLT counts are usually normal, transient thrombocytosis, mild thrombocytopenia, and leukopenia have been noted at diagnosis or during DBA evolution.33 Patients with DBA show persistent defects in the erythroid lineage and thus the Gata1s strain is a reasonable model for studying the disease. Although GATA1 mutations in DBA are rare, it is possible that defective erythropoiesis in the more common group of RP-coding gene mutant DBA cases is caused by a GATA1 deficiency. Studies from Sankaran et al suggest that the length and structure of the GATA1 5′ untranslated region makes GATA1 translation more sensitive to impaired ribosomal biogenesis than other messenger RNAs,30,34 leading to selective downregulation of GATA1 protein levels and defective hematopoiesis. By introducing exogenous GATA1 complementary DNA into RPL11- or RPL5-deficient CD34+ cells from patients with DBA, the authors observed improvement of erythropoiesis accompanied by increased expression of genes critical for terminal erythroid maturation.30 Therefore, efficient production of full-length GATA1 is critical for RBC maturation, and perturbation of this process can be found in anemia.
With respect to the mechanism by which loss of the N-terminus affects erythropoiesis, we recently demonstrated that there were few differences in chromatin occupancy when comparing GATA1 and GATA1s.12 However, we observed substantial reductions in the levels of H3K27Me3 at key sites in genes that are repressed by GATA1s, including Gata2 and Runx1. The persistent expression of these genes likely causes the defects in erythropoiesis because haploinsufficiency of Gata2 ameliorated the defective fetal development of RBCs.12 Ongoing studies are aimed at determining how loss of the N-terminus alters the ability of GATA1 to recruit factors that govern the epigenetic regulation of GATA1 target genes.
Finally, given that GATA1s mutations are a defining feature of ML-DS, it is notable that Gata1s mice do not develop leukemia in their lifetime. We surmise that this is owing to the absence of cooperating events, including trisomy 21 and mutations in other genes, such as those that comprise the cohesin complex. Two recent studies have shed light on the progression of ML-DS in humans. First, Hasle et al and Cantor35 described 3 cases of ML-DS that were caused by acquisition of trisomy 21 in hematopoietic cells from individuals with germ line GATA1s-causing mutations.35 Second, Wagenblast et al36 reported that the combination of a GATA1s mutation and the STAG2 mutation is sufficient to promote a megakaryocytic leukemia by human fetal liver progenitors with or without trisomy 21. By contrast, cohesin mutations could not confer leukemia in Gata1 mutant murine fetal liver cells, again pointing to a potential species-specific difference or a definitive requirement for trisomy 21.37 Although enlarged spleens and BM fibrosis can be detected in Gata1s mice with age, we did not observe any progression to leukemia. Thus, our study confirms that GATA1s itself is not sufficient to drive the transformation to megakaryocytic leukemia, even though it causes enhanced megakaryopoiesis at the expense of erythropoiesis.
Acknowledgments
The authors thank Mitchell Weiss for providing peripheral blood from the mouse model of sickle cell disease.
This work was supported by the National Institutes of Health, National Cancer Institute (CA253096), the Samuel Waxman Cancer Research Foundation, and St. Jude/American Lebanese Syrian Associated Charities (ALSAC).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: T.L., K.Z., and J.Y. conceived and performed the experiments, analyzed the data, and contributed to writing the manuscript; and S.G. and J.D.C. conceived the experiments, analyzed the data, and contributed to writing the manuscript.
Conflict-of-interest disclosure: J.D.C. receives consulting fees from Cellarity and is a scientify advisory board member of Alethiomics. The remaining authors declare no competing financial interests.
Correspondence: John D. Crispino, St. Jude Children’s Research Hospital, 262 Danny Thomas Pl, MS341, Memphis, TN 38105; e-mail: john.crispino@stjude.org.
References
Author notes
Data are available on request from the corresponding author, John D. Crispino (john.crispino@stjude.org).
The full-text version of this article contains a data supplement.