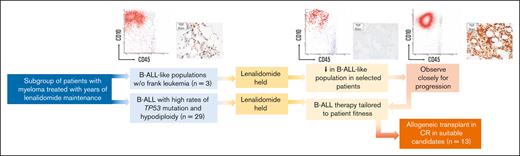
Key Points
Lenalidomide-associated B-ALL has high rates of TP53 mutation and hypodiploidy.
Combination chemotherapy, antigen-targeted therapies, and allogeneic transplantation are effective in achieving durable responses.
Abstract
Lenalidomide is an effective component of induction and maintenance therapy for multiple myeloma, though with a risk of secondary malignancies, including acute lymphoblastic leukemia (ALL). In contrast to therapy-related myeloid neoplasia, lenalidomide-associated lymphoblastic neoplasia remains poorly characterized. We conducted a dual institution retrospective study of 32 ALL cases that arose after lenalidomide maintenance (all B-lineage, 31/32 BCR::ABL-negative). B-cell ALL (B-ALL) was diagnosed at median 54 months (range, 5-119) after first exposure to lenalidomide and after median 42 months of cumulative lenalidomide exposure (range, 2-114). High incidence of TP53 mutations (9/19 evaluable cases) and low hypodiploidy (8/26 patients) were identified. Despite median age of 65 years and poor-risk B-ALL features observed in the cohort, rates of complete response (CR) or CR with incomplete hematologic recovery were high (25/28 patients receiving treatment). Median event-free survival was 35.4 months among treated patients (not reached among those undergoing allogeneic hematopoietic cell transplantation [HCT]). Sixteen patients remain alive without evidence of B-ALL after HCT or extended maintenance therapy. We also describe regression of B-ALL or immature B-cell populations with B-ALL immunophenotype after lenalidomide discontinuation in 5 patients, suggesting lenalidomide may drive leukemic progression even after initiation of lymphoblastic neoplasia and that lenalidomide withdrawal alone may be an appropriate first-line intervention in selected patients. Monitoring for early B-ALL–like proliferations may offer opportunities for lenalidomide withdrawal to prevent progression. Established combination chemotherapy regimens, newer surface antigen-targeted approaches, and allogeneic HCT are effective in many patients with lenalidomide-associated B-ALL and should be offered to medically fit patients.
Introduction
Recent therapeutic advances have improved the prognosis and life expectancy for patients with multiple myeloma (MM), making secondary malignancies an increasingly relevant clinical consideration. The immunomodulatory agent lenalidomide is a crucial component of many frontline remission induction regimens for MM and is frequently given as extended maintenance therapy. Lenalidomide directs substrate specificity of the CRBN-CRL4 E3 ubiquitin ligase, including to IKZF1 and IKZF3, essential transcription factors in MM.1,2 Observational series demonstrate increased incidence of secondary hematologic malignancies in lenalidomide-treated patients, including therapy-related myeloid neoplasia (t-MN; myelodysplastic syndrome [MDS] and acute myeloid leukemia [AML]).3,4 In addition, an increased incidence of acute lymphoblastic leukemia (ALL) has been reported in this patient population.5,6 In contrast to t-MN, therapy-related ALL is not robustly characterized, and small reports suggest clonally unrelated B-cell ALL (B-ALL) arising after lenalidomide therapy for MM may be a distinct clinical entity.5,7 Herein, we characterize clinical and pathologic characteristics of ALL and abnormal immature B-cell populations with B-ALL immunophenotype arising during lenalidomide maintenance for MM in patients treated at 2 large cancer centers and response to subsequent interventions for ALL. In addition to favorable response rates to standard ALL therapies, we observed regression of ALL in several patients after lenalidomide discontinuation alone, in the absence of immediate ALL therapy. These findings highlight the need to understand the leukemogenic effects of prolonged lenalidomide maintenance and the potential of lenalidomide to act as a driver even after the initiation of lymphoblastic neoplasia.
Methods
Study design
We conducted a retrospective cohort study. Eligible patients included adults (age ≥18 years) with either a pathologically confirmed diagnosis of ALL or an abnormal immature B-cell population with the B-ALL immunophenotype by multiparameter flow cytometry (MFC) (B-ALL–like population [BALLLP]), with a previous diagnosis of MM treated with lenalidomide at Memorial Sloan Kettering Cancer Center (MSK) and The Ohio State University Comprehensive Cancer Center (OSU) between May 2014 and February 2022.
The primary objective was to define the clinical and pathologic characteristics of patients with ALL that arose in patients treated for MM using lenalidomide. Secondary objectives included describing ALL treatment and outcomes (clinical responses and event-free survival [EFS]/overall survival [OS]) in this population. The relevant retrospective research protocol and biospecimen research protocol were approved by the institutional review board and privacy board at the participating institutions. All research was conducted according to the Declaration of Helsinki.
Genomic profiling
Paired tumor (bone marrow [BM] or blood) and normal samples from MSK patients were analyzed using MSK IMPACT-Heme (13 patients) where possible;8 the FoundationOne Heme platform (Foundation Medicine, Cambridge, MA) for DNA and targeted RNA sequencing (RNA-seq) was used for profiling in 3 patients, including 1 who also had MSK IMPACT-Heme data. In 2 MSK patients who did not have one of these comprehensive profiling studies performed, targeted sequencing was performed using a next-generation sequencing (NGS) platform panel comprised genes frequently mutated in myeloid neoplasms (RainDance Technologies ThunderBolts Myeloid Panel). BM from OSU patients with available samples (n = 2) were evaluated by the MSK HemePACT (research version of IMPACT-Heme) targeted deep sequencing assay.9-11 cBioPortal was used to visualize and analyze data from OSU patients.12 In total, 19 patients underwent targeted or comprehensive NGS. Targeted RNA-seq was performed in 13 MSK patients (FusionPlex, Archer, Boulder, CO). Clonal immunoglobulin heavy chain (IgH) rearrangement associated with MM and B-ALL populations was characterized when feasible using the Lymphotrack assay (Invivoscribe, Inc, San Diego, CA).
HCT
In patients undergoing allogeneic hematopoietic cell transplantation (HCT), donor-recipient HLA matching was established using high-resolution DNA sequence–specific oligonucleotide typing for HLA-A, -B, -C, -DQB1, and -DRB1 loci. CD34-selected peripheral blood stem cell products, when used, were formulated using the CliniMACS CD34 Reagent System (Miltenyi Biotec, Bergisch-Gladbach, Germany). Conditioning intensity was defined as either myeloablative conditioning or reduced-intensity/nonmyeloablative conditioning (RIC) per standard criteria.13
Patient demographics and clinical characteristics
| Characteristic . | All patients (N = 32) . |
|---|---|
| Sex, M/F (%) | 14/18 (44/56) |
| Age at MM diagnosis, median (range), y | 59 (46-77) |
| Age at ALL/BALLP diagnosis, median (range), y | 65 (50-86) |
| Time from MM to ALL/BALLLP, median (range), y | 5.5 (0.6-11.7) |
| Length of lenalidomide exposure, median (range), mo | 41.8 (1.8-114.1) |
| MM characteristics, n (%) | |
| IgG heavy chain | 19 (59) |
| IgA heavy chain | 8 (25) |
| Unknown heavy chain | 3 (9) |
| Light chain only MM | 2 (6) |
| κ light chain | 19 (59) |
| λ light chain | 12 (38) |
| Unknown light chain | 1 (3) |
| MM presentation, n (%) | |
| Hypercalcemia | 2 (6) |
| Renal insufficiency | 5 (16) |
| Anemia | 9 (2) |
| Bony lesions | 25 (7) |
| CRAB criteria status unknown | 3 (9) |
| Leptomeningeal disease | 1 (3) |
| ALL/BALLLP lineage and presentation, n (%) | |
| T-cell | 0 |
| B-cell | 32 (100) |
| Overt B-ALL at diagnosis | 29 (91) |
| BALLLP at diagnosis | 3 (9) |
| BCR::ABL-positive | 1 (3) |
| BCR::ABL-negative | 31 (91) |
| CD10 expression | 29 (91) |
| CD20 expression | 18 (56) |
| Low hypodiploidy∗ | 8 (31)† |
| 11q23 rearrangement‡ | 2 (8)† |
| Characteristic . | All patients (N = 32) . |
|---|---|
| Sex, M/F (%) | 14/18 (44/56) |
| Age at MM diagnosis, median (range), y | 59 (46-77) |
| Age at ALL/BALLP diagnosis, median (range), y | 65 (50-86) |
| Time from MM to ALL/BALLLP, median (range), y | 5.5 (0.6-11.7) |
| Length of lenalidomide exposure, median (range), mo | 41.8 (1.8-114.1) |
| MM characteristics, n (%) | |
| IgG heavy chain | 19 (59) |
| IgA heavy chain | 8 (25) |
| Unknown heavy chain | 3 (9) |
| Light chain only MM | 2 (6) |
| κ light chain | 19 (59) |
| λ light chain | 12 (38) |
| Unknown light chain | 1 (3) |
| MM presentation, n (%) | |
| Hypercalcemia | 2 (6) |
| Renal insufficiency | 5 (16) |
| Anemia | 9 (2) |
| Bony lesions | 25 (7) |
| CRAB criteria status unknown | 3 (9) |
| Leptomeningeal disease | 1 (3) |
| ALL/BALLLP lineage and presentation, n (%) | |
| T-cell | 0 |
| B-cell | 32 (100) |
| Overt B-ALL at diagnosis | 29 (91) |
| BALLLP at diagnosis | 3 (9) |
| BCR::ABL-positive | 1 (3) |
| BCR::ABL-negative | 31 (91) |
| CD10 expression | 29 (91) |
| CD20 expression | 18 (56) |
| Low hypodiploidy∗ | 8 (31)† |
| 11q23 rearrangement‡ | 2 (8)† |
F, female; M, male.
Includes patients with low hypodiploid features by traditional karyotyping (n = 5) or by FISH (n = 3).
Because low disease burden and/or lack of comprehensive cytogenetic data for 6 patients in the series, total evaluable patients here are considered as 26 rather than 32.
Refer to Discussion in the manuscript; possible but not definite in 1 of 2 patients.
Disease characteristics of patients developing B-ALL/BALLLP after lenalidomide treatment for MM
| ID# . | Sex . | Length of Len exposure (mo) . | Time from MM to ALL/BALLLP dx, y . | Age at ALL/BALLLP dx, y . | B-ALL induction . | B-ALL after induction (pre-HCT if HCT) . | Best response (pre-HCT if HCT) . | HCT donor (if HCT) . | B-ALL relapse? . | F/U from B-ALL diagnosis (mo) . | Last clinical status . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 01 | F | 53 | 5.4 | 66 | MSK L-2014 | MSK L-20 | MRD+ CR | MUD | N | 104.3 | Alive in CR after HCT |
| 02 | M | 57 | 5.6 | 66 | MSK L-20 | None | MRD− CR | Matched related donor | N | 98.0 | Alive in CR after HCT |
| 03 | M | 23 | 3.0 | 50 | MSK L-20 | MSK L-20 | CR | Matched related donor | N | 7.1 | Died in CR after HCT |
| 04 | F | 40 | 3.8 | 81 | MSK L-20 | POMP | NE | N/A | N | 27.1 | Developed therapy-related MDS and died subsequently; ALL was in CR |
| 05 | M | 69 | 7.7 | 64 | MSK 12-26615 | POMP | MRD− CR | MUD | N | 65.3 | Alive in CR after HCT |
| 06 | M | 68 | 6.1 | 63 | Hyper-CVAD | POMP, Hyper-CVAD | MRD− CR | MRD | Y | 35.8 | Relapsed after HCT and died with relapsed ALL |
| 07 | F | 61 | 7.6 | 68 | Augmented Hyper-CVAD | POMP, Blina | MRD− CR | N/A | N | 73.2 | Alive; ALL in CR though receiving therapy for MM |
| 08 | F | 69 | 5.9 | 52 | V + P | NE | NE | NE | N | 4.1 | Alive in CR as of last follow-up |
| 09∗ | M | 50 | 4.7 | 64 | Hyper-CVAD | Hyper-CVAD, Blina | MRD− CR | Haploidentical | N | 66.1 | Alive in CR after HCT |
| 10∗ | F | 6 | 0.6 | 75 | None; CR w/ holding Len | MRD− CR | N/A | N | 56.1 | Alive w/o B-ALL | |
| 11 | M | 39 | 5.0 | 60 | Hyper-CVAD | Hyper-CVAD | MRD+ CR | MMUD | N | 47.1 | Alive in CR after HCT |
| 12 | F | 54 | 5.3 | 53 | MSK 12-266 | MSK 12-266 | MRD− CR | N/A | N | 35.6 | Died with B-ALL in CR after subsequent HCT for therapy-related AML |
| 13 | F | 42 | 6.5 | 65 | Hyper-CVAD | Hyper-CVAD, Blina | MRD− CR | MUD | N | 12.3 | Died in CR after HCT |
| 14 | M | 69 | 7.2 | 64 | Hyper-CVAD + R | Hyper-CVAD + R | MRD− CR | MUD | N | 49.2 | Alive w/ in CR after HCT |
| 15‡ | F | 5 | 0.6 | 64 | None; CR w/ holding Len | MRD− CR | Autologous (for MM) | N | 43.9 | Alive w/o B-ALL after autologous HCT for MM | |
| 16 | M | 120+† | 15.7+ | 72 | Hyper-CVAD + R | Blina | MRD− CR | N/A | N | 36.1 | Alive in CR |
| 17 | F | 34 | 5.5 | 66 | Hyper-CVAD | Blina, m-HCVD + I, liposomal V | No CR | N/A | Never in CR | 14.5 | Died with refractory ALL |
| 18 | F | 102 | 9.8 | 68 | m-HCVD + I | m-HCVD + I | MRD− CR | MUD | N | 23.6 | Alive in CR after HCT |
| 19 | M | 17 | 5.8 | 83 | None | N/A | N/A | N/A | N | 1.2 | Died with ALL; not treated |
| 20 | F | 53 | 10.3 | 86 | V + P | V | MRD+ CR | N/A | Y | 5.4 | Died after salvage (I) for relapsed ALL |
| 21 | F | 114 | 10.1 | 64 | m-HCVD + I | m-HCVD + I | MRD− CR | MUD | N | 9.9 | Alive in CR after HCT |
| 22 | M | 95 | 10.0 | 61 | MSK 12-266 | MSK 12-266 | MRD− CR | N/A | N | 7.9 | Alive in CR |
| 23 | M | 35 | 3.5 | 68 | Hyper-CVAD | Methotrexate | CR | N/A | Y | 14.8 | Died after relapse of ALL |
| 24 | F | 45 | 4.0 | 63 | Hyper-CVAD – V | V + P | MRD− CR | N/A | Y | 7.7 | Died after relapse of ALL |
| 25 | F | 39 | 4.3 | 65 | Hyper-CVAD | Hyper-CVAD | MRD− CR | MUD | N | 66.4 | Alive in CR after HCT |
| 26 | M | 37 | 5.2 | 68 | V + P | Blina | CR‡ | N/A | Y | 33.1 | Died with relapsed ALL |
| 27 | F | 32 | 3.3 | 67 | Hyper-CVAD | Hyper-CVAD | MRD-CR | N/A | Y | 30.6 | Died after relapse of ALL |
| 28 | M | 32 | 4.1 | 58 | Hyper-CVAD + R | POMP | MRD-CR | N/A | N | 35.4 | Alive in CR after HCT |
| 29 | F | 49 | 6.0 | 68 | Hyper-CVAD | Hyper-CVAD | MRD-CR | MUD | Y | 48.2 | Died with relapsed ALL |
| 30 | F | 23 | 8.0 | 65 | CALGB 1010216 | NE | NE | N/A | N | 0.5 | Died during induction therapy |
| 31 | M | 2 | 11.7 | 86 | None | N/A | N/A | N/A | N | 0.4 | Died with ALL; not treated |
| 32 | F | 7 | 2.6 | 54 | Dexamethasone + dasatinib | NE | NE | N/A | N | 1.0 | Died during induction therapy |
| ID# . | Sex . | Length of Len exposure (mo) . | Time from MM to ALL/BALLLP dx, y . | Age at ALL/BALLLP dx, y . | B-ALL induction . | B-ALL after induction (pre-HCT if HCT) . | Best response (pre-HCT if HCT) . | HCT donor (if HCT) . | B-ALL relapse? . | F/U from B-ALL diagnosis (mo) . | Last clinical status . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 01 | F | 53 | 5.4 | 66 | MSK L-2014 | MSK L-20 | MRD+ CR | MUD | N | 104.3 | Alive in CR after HCT |
| 02 | M | 57 | 5.6 | 66 | MSK L-20 | None | MRD− CR | Matched related donor | N | 98.0 | Alive in CR after HCT |
| 03 | M | 23 | 3.0 | 50 | MSK L-20 | MSK L-20 | CR | Matched related donor | N | 7.1 | Died in CR after HCT |
| 04 | F | 40 | 3.8 | 81 | MSK L-20 | POMP | NE | N/A | N | 27.1 | Developed therapy-related MDS and died subsequently; ALL was in CR |
| 05 | M | 69 | 7.7 | 64 | MSK 12-26615 | POMP | MRD− CR | MUD | N | 65.3 | Alive in CR after HCT |
| 06 | M | 68 | 6.1 | 63 | Hyper-CVAD | POMP, Hyper-CVAD | MRD− CR | MRD | Y | 35.8 | Relapsed after HCT and died with relapsed ALL |
| 07 | F | 61 | 7.6 | 68 | Augmented Hyper-CVAD | POMP, Blina | MRD− CR | N/A | N | 73.2 | Alive; ALL in CR though receiving therapy for MM |
| 08 | F | 69 | 5.9 | 52 | V + P | NE | NE | NE | N | 4.1 | Alive in CR as of last follow-up |
| 09∗ | M | 50 | 4.7 | 64 | Hyper-CVAD | Hyper-CVAD, Blina | MRD− CR | Haploidentical | N | 66.1 | Alive in CR after HCT |
| 10∗ | F | 6 | 0.6 | 75 | None; CR w/ holding Len | MRD− CR | N/A | N | 56.1 | Alive w/o B-ALL | |
| 11 | M | 39 | 5.0 | 60 | Hyper-CVAD | Hyper-CVAD | MRD+ CR | MMUD | N | 47.1 | Alive in CR after HCT |
| 12 | F | 54 | 5.3 | 53 | MSK 12-266 | MSK 12-266 | MRD− CR | N/A | N | 35.6 | Died with B-ALL in CR after subsequent HCT for therapy-related AML |
| 13 | F | 42 | 6.5 | 65 | Hyper-CVAD | Hyper-CVAD, Blina | MRD− CR | MUD | N | 12.3 | Died in CR after HCT |
| 14 | M | 69 | 7.2 | 64 | Hyper-CVAD + R | Hyper-CVAD + R | MRD− CR | MUD | N | 49.2 | Alive w/ in CR after HCT |
| 15‡ | F | 5 | 0.6 | 64 | None; CR w/ holding Len | MRD− CR | Autologous (for MM) | N | 43.9 | Alive w/o B-ALL after autologous HCT for MM | |
| 16 | M | 120+† | 15.7+ | 72 | Hyper-CVAD + R | Blina | MRD− CR | N/A | N | 36.1 | Alive in CR |
| 17 | F | 34 | 5.5 | 66 | Hyper-CVAD | Blina, m-HCVD + I, liposomal V | No CR | N/A | Never in CR | 14.5 | Died with refractory ALL |
| 18 | F | 102 | 9.8 | 68 | m-HCVD + I | m-HCVD + I | MRD− CR | MUD | N | 23.6 | Alive in CR after HCT |
| 19 | M | 17 | 5.8 | 83 | None | N/A | N/A | N/A | N | 1.2 | Died with ALL; not treated |
| 20 | F | 53 | 10.3 | 86 | V + P | V | MRD+ CR | N/A | Y | 5.4 | Died after salvage (I) for relapsed ALL |
| 21 | F | 114 | 10.1 | 64 | m-HCVD + I | m-HCVD + I | MRD− CR | MUD | N | 9.9 | Alive in CR after HCT |
| 22 | M | 95 | 10.0 | 61 | MSK 12-266 | MSK 12-266 | MRD− CR | N/A | N | 7.9 | Alive in CR |
| 23 | M | 35 | 3.5 | 68 | Hyper-CVAD | Methotrexate | CR | N/A | Y | 14.8 | Died after relapse of ALL |
| 24 | F | 45 | 4.0 | 63 | Hyper-CVAD – V | V + P | MRD− CR | N/A | Y | 7.7 | Died after relapse of ALL |
| 25 | F | 39 | 4.3 | 65 | Hyper-CVAD | Hyper-CVAD | MRD− CR | MUD | N | 66.4 | Alive in CR after HCT |
| 26 | M | 37 | 5.2 | 68 | V + P | Blina | CR‡ | N/A | Y | 33.1 | Died with relapsed ALL |
| 27 | F | 32 | 3.3 | 67 | Hyper-CVAD | Hyper-CVAD | MRD-CR | N/A | Y | 30.6 | Died after relapse of ALL |
| 28 | M | 32 | 4.1 | 58 | Hyper-CVAD + R | POMP | MRD-CR | N/A | N | 35.4 | Alive in CR after HCT |
| 29 | F | 49 | 6.0 | 68 | Hyper-CVAD | Hyper-CVAD | MRD-CR | MUD | Y | 48.2 | Died with relapsed ALL |
| 30 | F | 23 | 8.0 | 65 | CALGB 1010216 | NE | NE | N/A | N | 0.5 | Died during induction therapy |
| 31 | M | 2 | 11.7 | 86 | None | N/A | N/A | N/A | N | 0.4 | Died with ALL; not treated |
| 32 | F | 7 | 2.6 | 54 | Dexamethasone + dasatinib | NE | NE | N/A | N | 1.0 | Died during induction therapy |
Blina, blinatumomab; CALGB 10102, off-protocol per published regimen, but without alemtuzumab; dx, diagnosis; F/U=follow-up; I, inotuzmab ozogamicin; Len, lenalidomide; MMUD, 1-locus mismatched unrelated donor; MUD, matched unrelated donor; N, no; N/A, not applicable; NE, not evaluable; P, corticosteroids; POMP, prednisone, vincristine, methotrexate, and mercaptopurine; R, rituximab; V, vincristine; Y, yes.
B-ALLLP by flow cytometry (not overt B-ALL) at diagnosis.
Exact date of lenalidomide start unclear but >120 months exposure.
CR achieved following blinatumomab.
Response definitions
Complete response (CR) was defined as achievement of <5% blasts with approximately normal cellularity and trilineage hematopoiesis by BM aspirate (BMA) and biopsy, resolution of central nervous system or other extramedullary disease, and recovery of peripheral blood counts (absolute neutrophil count ≥1000/μL without growth factor support and platelets ≥105/μL without transfusion); CR with incomplete hematologic recovery (CRi) was defined as meeting criteria for CR with the exception of achieving the above thresholds for absolute neutrophil count and/or platelet recovery. In patients with non–central nervous system extramedullary disease, CR for those sites was defined by Lugano criteria.17 Measurable (minimal) residual disease (MRD) was assessed by MFC (fluorescence-activated cell sorter [FACS]) by each institution’s laboratory; the MSK methodology for MRD determination by FACS has been described previously.15 OSU methodology for MRD assessment for B-ALL by FACS has been described in the context of their participation in a multicenter study with modification to use a 10-color panel and the lowest level of detection of 0.01% in adequately cellular specimens with the acquisition of 500,000 events and ≥50 positive events identified.18
Statistical analyses
Continuous variables were summarized as median and range. OS and EFS were estimated using the Kaplan-Meier method and compared between the groups using log-rank tests. OS was defined as time from the date of ALL diagnosis to death from any cause, with surviving patients censored at last follow-up. EFS was defined as the time from initiation of B-ALL therapy until the date of morphologic relapse or MRD relapse from a prior state of MRD negativity, confirmed refractory disease, or death from any cause; patients not known to have any of these events were censored on the date of the last follow-up. Kaplan-Meier curves were constructed, and log-rank tests were performed using GraphPad Prism version 8.2.1 for Windows (GraphPad Software, San Diego, CA).
Results
Patient population and disease characteristics
We identified 32 patients (male, n = 14; female, n = 18), with a median age of 59 years (range, 46-77) at the time of the MM diagnosis and 65 years (range, 50-86) at the time of the ALL/BALLLP diagnosis at MSK (patients #1-22, n = 22) or OSU (patients #23-32, n = 10) (Table 1). MM diagnoses were made between February 2004 and June 2018; B-ALL diagnoses were made between May 2008 and February 2022. All patients received ≥1 line of systemic therapy for MM. Twenty-two patients underwent autologous HCT after melphalan 200 mg/m2 (for all cases for which the conditioning regimen was confirmed), and 1 patient subsequently underwent a second autologous HCT for MM. All received lenalidomide maintenance after induction therapy and/or autologous HCT, and some received lenalidomide during prior lines of therapy, with the median total duration of lenalidomide exposure being 42 months (range, 2-114 months; interquartile range, 32-59). Lenalidomide and proteasome inhibitor therapy (most commonly bortezomib) were incorporated into induction or salvage therapy (preautologous HCT, where applicable) in 23 of 31 and 24 of 31 patients with known therapeutic history, respectively. Eight patients additionally underwent radiation therapy as local treatment for MM before the diagnosis of ALL. Ten patients had been treated on 1 of 6 prospective investigator-initiated or cooperative group clinical trials incorporating lenalidomide into therapy (with 322 accruals across these 6 studies).
ALL/BALLLP was identified at a median 65.6 months (range, 7.3-141.0) after MM diagnosis, and at a median 53.8 months (range, 5.5-119.0) after first exposure to lenalidomide. At the time of diagnosis, 29 of 32 patients had overt ALL (>20% blasts in blood/BM or extramedullary disease), and 3 had BALLLP (progressed to overt disease in 1 patient). Lenalidomide was discontinued a median of 13.5 days before ALL/BALLLP diagnosis, most commonly because of cytopenias heralding an ALL/BALLLP diagnosis, though several patients had completed their planned course of lenalidomide (range, 2430 days before 42 days after ALL/BALLLP diagnosis). All cases were B-lineage, and 31 of 32 cases were Philadelphia chromosome (Ph)/BCR::ABL fusion-negative. Cytogenetic features at diagnosis included a low hypodiploid karyotype (≤43 chromosomes with or without a near-triploid karyotype) in 5 patients, with 3 additional cases demonstrating hypodiploid karyotype features by fluorescence in situ hybridization (FISH) signal patterns or single-nucleotide polymorphism array. FISH evaluation and array for patient #1 suggested a KMT2A translocation with deletion of the reciprocal (nonfunctional) genomic fusion. FISH evaluation in patient #13 reflected possible KMT2A translocation at a level just above limits of detection.
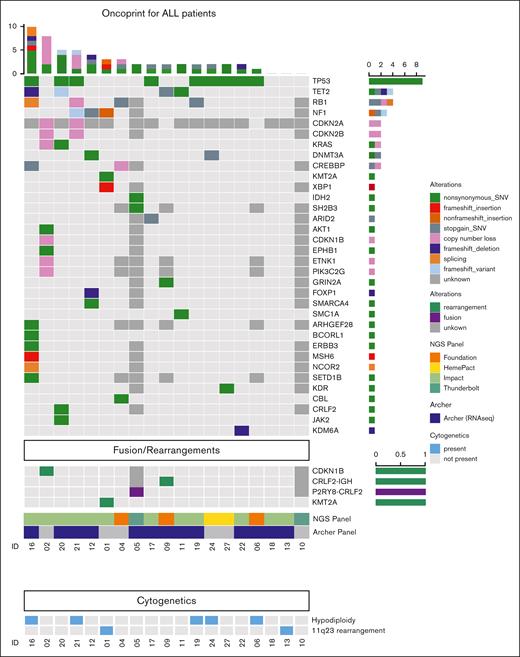
Two of the 14 patients evaluated with targeted RNA-seq exhibited Ph-like ALL (P2RY8::CRLF2 fusion; IGH::CRLF2 fusion). Somatic alterations in patients who had undergone NGS profiling included TP53 mutation (9/19), TET2 mutation (5/19), RB1 mutation (5/17), CDKN2A/B mutation or loss (2/17), CREBBP mutation (2/17), KRAS mutation (1/19), and IKZF2 loss (1/17); none (0/17) had IKZF1/3 mutation or loss (Figure 1). None of the patients had known germ line TP53 mutations; MSK IMPACT-Heme excluded germ line mutations in those sequenced. Clonal IgH rearrangements associated with both MM and B-ALL were separately characterized in 1 patient and were found to be distinct, supporting that the malignancies were clonally unrelated.
Cytogenetic and molecular features of lenalidomide-associated ALL. Oncoprint depicting somatic alterations and rearrangements/fusions identified by one of several NGS panels and targeted RNA-seq panels (eg, FoundationOne Heme and Archer FusionPlex) identified in evaluable patients developing B-ALL after lenalidomide therapy, including single-nucleotide variations (SNVs), insertions, copy number loss, deletions, and splicing variants. Dark grey boxes indicate the target gene/fusion was not evaluated in that patient; ∗, FoundationOne Heme panel includes RNA-seq.
Cytogenetic and molecular features of lenalidomide-associated ALL. Oncoprint depicting somatic alterations and rearrangements/fusions identified by one of several NGS panels and targeted RNA-seq panels (eg, FoundationOne Heme and Archer FusionPlex) identified in evaluable patients developing B-ALL after lenalidomide therapy, including single-nucleotide variations (SNVs), insertions, copy number loss, deletions, and splicing variants. Dark grey boxes indicate the target gene/fusion was not evaluated in that patient; ∗, FoundationOne Heme panel includes RNA-seq.
Spontaneous regression
Five MSK patients notably exhibited regression of B-ALL/BALLLP after lenalidomide withdrawal alone, before the initiation of antileukemic therapy, with 2 never requiring B-ALL–specific therapy.
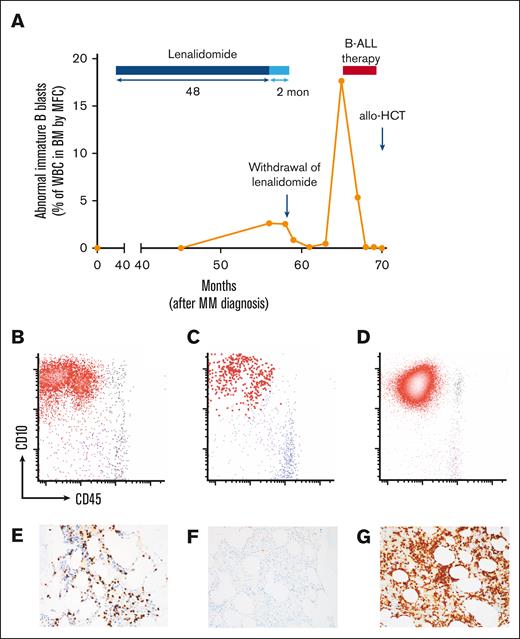
Patient #9 underwent a routine BMA and biopsy 48 months into maintenance lenalidomide. This revealed no evidence of plasma cell neoplasm, but 8% blasts by aspirate smear and abnormal B lymphoblasts representing 2.6% of total white blood cells (WBCs) by flow cytometry (Figure 2A). Concern was raised for the evolving B-ALL. BMA was repeated 59 days later and revealed 10% to 15% blasts by CD34 and TdT immunostain and a persistent abnormal B-lymphoblast population comprising 2.5% of total WBCs (Figure 2A,B,E). Lenalidomide was held and repeated BMA 31 days thereafter revealed decreased B-ALL burden by flow cytometry (abnormal B lymphoblasts representing 0.84% of WBCs), with further decrease evident 62 days thereafter (0.11% of WBCs by BMA flow cytometry) with observation alone (Figure 2A,C,F). At 284 days from initial detection of BALLLP, morphologic B-ALL became evident (57% blasts by BMA), and B-ALL therapy was started (Figure 2A,D,G).
Clinical course of lenalidomide-associated ALL in one patient. (A) Clinical course of patient #9, who was found to have an immature B-cell population with B-ALL immunophenotype at 48 months into lenalidomide maintenance for MM, with regression of B-ALL after lenalidomide withdrawal and subsequent progression to overt B-ALL at 284 days from the initial finding of abnormal B lymphoblasts. He received B-ALL therapy consisting of hyper-CVAD followed by blinatumomab and achieved CR with MRD negativity, followed by haploidentical allogeneic HCT and remains in continuous, ongoing MRD− CR. Flow cytometry plots from BMAs and TdT immunostains on core biopsies depict evolution in B-lymphoblast population from initial finding 48 months into lenalidomide maintenance (B,E) to regression after lenalidomide withdrawal (C,F) to overt progression to B-ALL (D,G). All blast percentages are from BMAs.
Clinical course of lenalidomide-associated ALL in one patient. (A) Clinical course of patient #9, who was found to have an immature B-cell population with B-ALL immunophenotype at 48 months into lenalidomide maintenance for MM, with regression of B-ALL after lenalidomide withdrawal and subsequent progression to overt B-ALL at 284 days from the initial finding of abnormal B lymphoblasts. He received B-ALL therapy consisting of hyper-CVAD followed by blinatumomab and achieved CR with MRD negativity, followed by haploidentical allogeneic HCT and remains in continuous, ongoing MRD− CR. Flow cytometry plots from BMAs and TdT immunostains on core biopsies depict evolution in B-lymphoblast population from initial finding 48 months into lenalidomide maintenance (B,E) to regression after lenalidomide withdrawal (C,F) to overt progression to B-ALL (D,G). All blast percentages are from BMAs.
Patient #10 underwent a routine BMA at 6 months into therapy with carfilzomib, lenalidomide, and dexamethasone, which revealed no morphologic or immunophenotypic evidence of myeloma but demonstrated abnormal BALLLP representing 0.11% of the WBCs (Figure 3) and an atypical myeloid blast population representing 0.33% of the WBCs, with the myeloid blast population not clearly thought to represent neoplasia. Lenalidomide was held, but no corticosteroids or other antileukemic therapy were administered. Subsequent BMA at 1.5 months thereafter demonstrated no abnormal B-lymphoblast or myeloid blast population; she has never demonstrated evidence of acute leukemia in the subsequent >5 years.
Flow cytometric features of B-ALL-like cell populations arising during lenalidomide therapy. BM flow cytometry dot plots depicting abnormal immature B-cell populations arising during lenalidomide therapy, which ultimately resolved with holding lenalidomide. Plots reflect normal case (blue dots) with abnormal immature B cells (A) (red, emphasized) in patient #15 showing abnormally increased CD20 and CD34 with abnormally decreased CD38 and abnormal immature B cells (B) (red dots) in patient #10 with similar abnormal phenotype.
Flow cytometric features of B-ALL-like cell populations arising during lenalidomide therapy. BM flow cytometry dot plots depicting abnormal immature B-cell populations arising during lenalidomide therapy, which ultimately resolved with holding lenalidomide. Plots reflect normal case (blue dots) with abnormal immature B cells (A) (red, emphasized) in patient #15 showing abnormally increased CD20 and CD34 with abnormally decreased CD38 and abnormal immature B cells (B) (red dots) in patient #10 with similar abnormal phenotype.
Patient #11 similarly underwent a routine staging BMA 37.5 months into maintenance lenalidomide after autologous HCT, with BMA revealing 37% blasts by aspirate and FACS confirming B-ALL. Lenalidomide was held. Repeat BMA 1 week later revealed 10% blasts by aspirate, and B-ALL therapy was commenced.
Patient #15 was scheduled to undergo autologous HCT for myeloma but was found to have BALLLP, representing 0.027% of WBCs and 0.067% of mononuclear cells on BMA (Figure 3), as well as minimal residual involvement by myeloma. Lenalidomide was discontinued, and no ALL-directed therapy was given. Repeat BMA 2 months thereafter showed resolution of the BALLLP by flow cytometry, with minimal residual myeloma, and she proceeded to undergo autologous HCT; she has never developed B-ALL in the subsequent >4 years.
Patient #21 presented to an outside medical center with WBC 84.9 × 103/μL and 86% circulating blasts after 114 months of lenalidomide exposure. Lenalidomide was held, and in the absence of corticosteroids, hydroxyurea, or other cytoreductive therapy, WBC declined over 3 days to 14.3 × 103/μL with 86% circulating blasts and over 8 days to 2.3 × 103/μL with 64% circulating blasts. She then began taking corticosteroids, followed by definitive B-ALL therapy.
Treatment
Patients were managed with several different approaches after the diagnosis of B-ALL/BALLLP, depending on age, comorbidities, time period of diagnosis, and preference of the treating physician (Table 2). Induction approaches included hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone; or augmented hyper-CVAD) ± rituximab (n = 14),19 minihyper-CVD + inotuzumab ozogamicin (n = 2),20 an MSK pediatric-inspired regimen (MSK 12-266; n = 2),15 an MSK 4-drug adult regimen (vincristine, prednisone, cyclophosphamide, and doxorubicin, known as MSK L-20; n = 4),14 CALGB 10102 regimen (n = 1),16 vincristine + prednisone (n = 3), or dasatinib + dexamethasone (n = 1 had Ph+ ALL). In 4 patients, no therapy was administered as either the disease was low volume and cleared with holding lenalidomide alone (n = 2) or because of patient frailty and preferences, in which management consisted of the best supportive care (n = 2). Postremission therapy is summarized in Table 2. Thirteen patients underwent allogeneic HCT in CR1 (Table 3). Hematopoietic cell sources included fully HLA-matched related (n = 3) or unrelated (n = 7) donors, partially HLA-matched unrelated donors (n = 2), or a haploidentical donor (n = 1). Graft-versus-host disease prophylaxis was calcineurin inhibitor–based for all HLA-matched donor recipients, whereas posttransplant cyclophosphamide with mycophenolate mofetil and either sirolimus (n = 1) or tacrolimus (n = 2) was used for HLA-mismatched related or unrelated donor recipients. One patient underwent planned autologous HCT for MM after B-ALL clone had cleared with holding lenalidomide alone.
Characteristics of allogeneic HCT for B-ALL following lenalidomide treatment for MM
| ID# . | Sex . | Age at ALL dx (y) . | Best response before HCT . | HCT donor (if HCT) . | CD34 selection? (if HCT) . | Conditioning intensity . | Conditioning regimen . |
|---|---|---|---|---|---|---|---|
| 01 | F | 66 | MRD+ CR | MUD | N | RIC | Flu/Bu |
| 02 | M | 66 | MRD− CR | MRD | N | RIC | Flu/Mel |
| 03 | M | 50 | CR | MRD | Y | MAC | Total body irradiation/cyclophosphamide/thiotepa |
| 05 | M | 64 | MRD− CR | MUD | N | RIC | Flu/Mel |
| 06 | M | 63 | MRD− CR | MRD | N | RIC | Flu/Mel |
| 09 | M | 64 | MRD− CR | Haploidentical | N | MAC | Flu/Mel/thiotepa |
| 11 | M | 60 | MRD+ CR | MMUD | N | MAC | Flu/Bu |
| 13 | F | 65 | MRD− CR | MUD | N | RIC | Flu/Mel |
| 14 | M | 64 | MRD− CR | MUD | N | RIC | Flu/Mel |
| 18 | F | 68 | MRD− CR | MUD | N | RIC | Flu/Mel |
| 21 | F | 64 | MRD− CR | MUD | N | RIC | Flu/Mel |
| 25 | F | 65 | MRD− CR | MUD | Y | RIC | Flu/Bu |
| 29 | F | 68 | MRD− CR | MUD | Y | RIC | Flu/Bu |
| ID# . | Sex . | Age at ALL dx (y) . | Best response before HCT . | HCT donor (if HCT) . | CD34 selection? (if HCT) . | Conditioning intensity . | Conditioning regimen . |
|---|---|---|---|---|---|---|---|
| 01 | F | 66 | MRD+ CR | MUD | N | RIC | Flu/Bu |
| 02 | M | 66 | MRD− CR | MRD | N | RIC | Flu/Mel |
| 03 | M | 50 | CR | MRD | Y | MAC | Total body irradiation/cyclophosphamide/thiotepa |
| 05 | M | 64 | MRD− CR | MUD | N | RIC | Flu/Mel |
| 06 | M | 63 | MRD− CR | MRD | N | RIC | Flu/Mel |
| 09 | M | 64 | MRD− CR | Haploidentical | N | MAC | Flu/Mel/thiotepa |
| 11 | M | 60 | MRD+ CR | MMUD | N | MAC | Flu/Bu |
| 13 | F | 65 | MRD− CR | MUD | N | RIC | Flu/Mel |
| 14 | M | 64 | MRD− CR | MUD | N | RIC | Flu/Mel |
| 18 | F | 68 | MRD− CR | MUD | N | RIC | Flu/Mel |
| 21 | F | 64 | MRD− CR | MUD | N | RIC | Flu/Mel |
| 25 | F | 65 | MRD− CR | MUD | Y | RIC | Flu/Bu |
| 29 | F | 68 | MRD− CR | MUD | Y | RIC | Flu/Bu |
Bu, busulfan; Flu, fludarabine; MAC, myeloablative conditioning; Mel, melphalan.
Outcomes
Of the 28 patients treated actively for B-ALL (beyond best supportive care or holding lenalidomide alone), 25 achieved a best response of CR/CRi, 2 died during induction therapy before response assessment, and another never achieved CR/CRi. Twenty-one of 24 patients undergoing restaging after their first course of induction therapy were in CR/CRi at that time. MRD was undetectable by flow cytometry (to sensitivity <0.01%) in 12 of 21 patients in CR/CRi on first restaging (MRD+, n = 8; MRD not evaluated, n = 1). Rates of MRD negativity increased with further therapy; of the 25 treated patients achieving CR/CRi, 23 underwent MRD evaluation during therapy; 20 of 23 patients achieved MRD negativity without or before allogeneic HCT, 2 of 23 achieved MRD negativity after allogeneic HCT, and 1 never achieved MRD negativity and relapsed. Disease status before allogeneic HCT was CR in all 13 patients undergoing HCT (MRD–, n = 10; MRD+, n = 2; MRD not evaluated, n = 1).
The median OS from the time of B-ALL/BALLLP diagnosis was 35.8 months among all patients. The median EFS (as determined among treated patients) was 35.4 months (Figure 4A-B). Sixteen patients remain alive without evidence of B-ALL; the median follow-up among patients surviving at the last follow-up was 48.1 months (range, 4.1-104.3). Eight of 28 evaluable patients experienced morphologic or MRD-level relapse of B-ALL (n = 6) after beginning treatment or experienced refractory disease (n = 2). Initial therapies administered for relapsed or refractory disease included blinatumomab (n = 5), inotuzumab (n = 1), and ADCT-402 (loncastuximab tesirine; n = 1). Three patients received further lines of therapy (cyclophosphamide and dexamethasone after having achieved CR to blinatumomab for primary refractory disease, n = 1; inotuzumab ozogamicin for intracardiac and BM relapse after having achieved MRD response to blinatumomab after transplant, n = 1; mini-hyper-CVD + inotuzumab ozogamicin and subsequent liposomal vincristine for primary refractory disease, n = 1). All patients who exhibited relapsed or refractory B-ALL subsequently died. Only 3 patients received further therapy for MM during follow-up, 2 of whom had not received therapy for B-ALL beyond holding lenalidomide (supplemental Table 1).
Survival outcomes in patients with lenalidomide-associated ALL. (A) EFS among patients in cohort actively treated for B-ALL. (B) OS in the entire cohort. EFS (C) and OS (D) from time of transplant in subgroup of patients undergoing allogenic HCT.
Survival outcomes in patients with lenalidomide-associated ALL. (A) EFS among patients in cohort actively treated for B-ALL. (B) OS in the entire cohort. EFS (C) and OS (D) from time of transplant in subgroup of patients undergoing allogenic HCT.
Sixteen patients died during follow-up. Causes of death included progressive B-ALL (n = 8); undocumented cause of death after B-ALL relapse (n = 3); nonrelapse mortality after HCT, including regimen-related toxicity after allogeneic HCT for therapy-related AML with B-ALL in CR (n = 1) and Graft-versus-host disease (n = 2); complications of therapy-related MDS after B-ALL therapy (n = 1); sepsis during induction therapy for B-ALL (n = 1). Characteristics of AML/MDS after B-ALL therapy are in supplemental Table 2.
Among the 13 patients undergoing allogeneic HCT for B-ALL, median EFS (from start of B-ALL therapy) and OS (from diagnosis), as well as median EFS and OS from time of HCT, have not been reached at median 43.2 months follow-up after allogeneic HCT (Figure 4C-D). Two patients experienced relapse of B-ALL after allogeneic HCT and 2 died in CR post-HCT for B-ALL as above, with 1 additional death after allogeneic HCT for AML (not B-ALL).
Discussion
This report represents, to our knowledge, the largest series of patients with ALL diagnosed after lenalidomide therapy for MM. These results suggest several features possibly unique to this type of secondary ALL, including near-exclusive presentation as BCR::ABL-negative B-ALL despite the substantial proportion of BCR::ABL-positive ALL cases in this age group,21,22 as well as a strikingly high incidence of TP53 mutations (identified in nearly half of the evaluable patients) and hypodiploid karyotype. Low hypodiploid karyotype in ALL is itself known to be associated with high rates of TP53 mutations.23TP53 mutation and/or low hypodiploidy at ALL diagnosis are typically associated with unfavorable long-term outcomes with standard therapy, though independent prognostic significance of these factors is less clear in patients who clear MRD promptly.23,24 Despite the older age of the cohort at the time of B-ALL diagnosis, poor-risk features in many cases, and conventional induction regimens (as opposed to novel antigen-targeted approaches) used in most patients, most attained CR, and EFS/OS compares favorably to historical series in older adults with ALL (as we and others have reported25) even when including patients herein who were unfit for ALL therapy and managed with supportive care alone. Patients who underwent allogeneic HCT in CR1 had encouraging outcomes, even when considering the selection bias inherent in comparing this group with the whole cohort. Long-term remission after HCT was observed despite the use of RIC rather than myeloablative conditioning in most patients in this series (with RIC favored based on age/comorbidities). Whether further use of novel therapies with frontline therapy may result in outcomes comparable to allogeneic HCT in CR1 remains uncertain. It remains our practice to refer patients with lenalidomide-associated ALL for evaluation and consideration of allogeneic HCT in CR1.
t-MN after cytotoxic chemotherapy for MM has been well described for over 40 years.26-28 However, whether novel immunomodulatory agents such as lenalidomide are associated with a significant risk of secondary hematologic malignancies in the absence of high-dose melphalan has been questioned.29 One meta-analysis estimated a 5-year cumulative incidence of hematologic malignancy of 3.1%, with median time to occurrence of second primary hematologic malignancy of 29 months among those receiving lenalidomide, and with receipt of the combination of lenalidomide and oral melphalan particularly associated with increased risk of second primary hematologic malignancy; AML/MDS represented most cases.3 Subsequent reports, however, have described patients with B-ALL diagnosed 2.5 to 7 years into therapy with maintenance lenalidomide and offered insight into the possible unique disease biology, including a median latency of ∼5 years, high rates of TP53 deletion/mutation in the B-ALL clone, and distinct IgH clonotypes and mutational profiles between MM and B-ALL clones.5,7 Barnell et al also identified the B-ALL–specific TP53 mutations in non-MM hematopoietic cells in 3 BM samples (preceding B-ALL diagnosis), suggesting the possibility that B-ALL arose as an outgrowth of unrelated clonal hematopoiesis expanding in the presence of lenalidomide.7 Of interest, Sperling et al have demonstrated that lenalidomide provides a selective advantage to TP53-mutant hematopoietic cells that may contribute to risk of secondary myeloid neoplasia.30 In a lenalidomide-sensitive knockin murine cell line engineered to carry mutations recurrently mutated in clonal hematopoiesis using a CRISPR-Cas9 system, only TP53 loss led to lenalidomide resistance as well as an advantage in proliferative capacity. In lenalidomide-sensitive mice crossed with TP53–/– knockout mice that underwent transplantation with both TP53 mutant and wild-type hematopoietic progenitor cells, lenalidomide led to preferential outgrowth of TP53 mutant cells in all blood lineages.30 A case of lenalidomide-dependent MDS with the TP53 mutation has also been reported.31 Whether lenalidomide-driven expansion of preleukemic hematopoietic progenitor cells underlies secondary lymphoblastic neoplasia remains unclear.
A small group of patients exhibited apparent regression of either overt or incipient B-ALL clones with the discontinuation of lenalidomide alone, in the absence of any B-ALL–directed therapy, including corticosteroids (Figures 2 and 3), further suggesting a causative link between lenalidomide exposure and subsequent B-ALL. One recent report similarly describes regression of B-ALL in a 59-year-old woman with MM after lenalidomide withdrawal, without further B-ALL therapy, for ≥12 months.32 These observations and other recent reports further support the potential role of lenalidomide in leukemogenesis and leukemic maintenance.30 Although the generalizability of these findings is limited by small numbers of patients and rapid access to high-sensitivity FACS for B-ALL, these observations suggest discontinuation of lenalidomide promptly upon recognition of an abnormal immature B-cell population with the B-ALL immunophenotype may temporarily arrest the progression of B-ALL in selected patients and allow a period of very close monitoring without immediate B-ALL therapy. In patient #9, the patient’s B-ALL was observed for >9 months with lenalidomide discontinuation alone before progression, and in patients #10 and #15, the B-ALLLP disappeared completely and has never recurred after >5 and >4 years of observation, respectively (though patient #15 later received autologous HCT for MM). Patient-derived xenograft models of such cases, if feasible, would be of particular future interest in defining the pathogenesis of lenalidomide-associated ALL, as would bulk and B-ALL/BALLLP–specific RNA-seq before and after lenalidomide withdrawal to investigate affected pathways and IKZF1/3 expression.
We and others have described cases of ALL arising as a second primary malignancy.5,33-36 In 1 multi-institutional series of 116 adult patients diagnosed with ALL after having received chemotherapy or radiation therapy, a higher incidence of the “MDS-like” karyotype was observed (compared with patients with de novo ALL or ALL arising after primary malignancy untreated with cytotoxic therapy), with a subset of cases bearing mutations commonly observed in myeloid neoplasia or in DNA repair genes. Two of 20 patients with therapy-related ALL and NGS data had TP53 mutations. Incidence of BCR::ABL fusion and B- vs T-lineage was also not significantly different with respect to de novo vs therapy-related ALL in that report (though power to detect difference limited).34 The distinct cytogenetic and molecular features in the present series suggesting lenalidomide-associated ALL may bear different and unique features compared with other forms of secondary ALL, such as KMT2A-rearranged ALL arising after topoisomerase II inhibitors.37 Performance of HCT in the larger series of patients with therapy-related ALL was associated with improved OS in multivariable analysis; we also observed favorable outcomes in those undergoing HCT in CR1, though this small series was not intended to directly compare HCT vs non-HCT approaches and multiple confounders must be considered.
This report has several limitations, related in part to heterogeneity in evaluation and management during the prolonged timespan during which patients were identified. Patients with BALLLP (not meeting World Health Organization criteria for B-ALL) were included in this report given that unusual disease trajectories (regression or prolonged stability) were observed with lenalidomide withdrawal alone and as we confirmed the abnormal B-ALL–like immunophenotype, though we acknowledge this introduces further heterogeneity into the cohort. Different targeted DNA and RNA-seq platforms were used at the time of diagnosis, and in some cases, archival samples were unable to be retrieved for characterization years later. The small size of BALLLP in patients #10 and #15 did not lend themselves to molecular assessments of clonality, despite unequivocal B-ALL–like immunophenotype. Although we feel nonneoplastic immunophenotypic shift during lenalidomide therapy is unlikely, we acknowledge uncertainty, and it remains unknown whether BALLLP would have regressed even if lenalidomide were continued. Differences in BMA site and sampling in patient #11 could have contributed to variation in pretreatment blast enumeration as well, independent of lenalidomide discontinuation. Hydration could have accounted for some reduction in circulating lymphoblasts in patient #21 (though circulating blasts declined nearly 50-fold after lenalidomide was discontinued, without corticosteroids or other therapy). In addition, although we attempted to sequence the malignant clonal IgH rearrangements associated with the MM and B-ALL populations, in many cases we were unable to do so as MM diagnoses had occurred years before B-ALL diagnosis, and in several cases, MM diagnoses had been made at an outside institution. Sensitivity of MFC for MRD assessment improved throughout the period during which cases were accrued and flow cytometry was not performed/reviewed centrally. B-ALL cases were also not managed uniformly because of the therapeutic advances made during the period represented (including the Food and Drug Administration approvals of blinatumomab and inotuzumab ozogamicin), age and comorbidities of the patients, preferences of the patient and treating physician, and lack of standardized criteria for recommending allogeneic HCT in CR1. Nevertheless, the report describes the typical time course during which lenalidomide-associated ALL seems to arise, its typical presentation as BCR::ABL-negative B-ALL, and high frequency of poor-risk features, including TP53 mutation. Finally, these observations should be interpreted within the context of the established progression-free survival benefit of lenalidomide maintenance for patients with MM after autologous HCT.38-41
In summary, ALL arises in a clinically significant subgroup of patients treated with lenalidomide for MM, including in patients who have not received high-dose chemotherapy with autologous stem cell rescue. Our observations regarding the behavior of immature B-cell populations (or frank B-ALL) after lenalidomide discontinuation support the agent’s role in leukemogenesis and warrant further investigation regarding the underlying mechanism.
Acknowledgments
This work was funded in part by the National Cancer Institute (NCI) Cancer Center Core, National Institutes of Health (NIH) (grant P30-CA008748). W.X. is supported by Alex’s Lemonade Stand Foundation and the RUNX1 Research Program, MSK’s Cycle for Survival’s Equinox Innovation Award in Rare Cancers, MSK Leukemia SPORE Career Enhancement Program, and a grant from the NCI, NIH (grant K08CA267058-01). M.B.G. is supported by American Society of Hematology, MSK Comedy vs Cancer, and a grant from the NCI, NIH (grant R42 CA254685-01).
Authorship
Contribution: M.B.G. conceived and designed the analysis, collected the data, performed the analysis, and wrote the manuscript; B.C.S. conceived and designed the analysis, collected the data, and wrote the manuscript; B.B., A.S.M., and V.K. collected the data and wrote the manuscript; D.D., J.L.G., and G.L. contributed and described key analysis tools and performed portions of the analysis; H.H. and H.L. collected the data and wrote the manuscript; Y.Z., W.X., and M.R. collected the data, contributed and described key analysis tools, performed portions of the analysis, and wrote the manuscript; and J.H.P. conceived and designed the analysis.
Conflict-of-interest disclosure: M.B.G. received research support from Actinium Pharmaceuticals and Amgen; is on the advisory board of and received research support from Sanofi; and is a consultant for Allogene and Novartis. W.X. received research support from Stemline Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Mark B. Geyer, 530 East 74th St, New York, NY 10021; e-mail: geyerm@mskcc.org.
References
Author notes
Data are available on request from the corresponding author, Mark B. Geyer (geyerm@mskcc.org).
The full-text version of this article contains a data supplement.