Key Points
Of the patients treated with BsAbs, half developed an infection, and a quarter developed grade III/IV infections.
BCMA-targeting BsAbs are associated with a higher risk of infection than non-BMCA–targeting BsAbs.
Abstract
The use of bispecific antibodies (BsAbs) in the treatment of relapsed/refractory multiple myeloma (MM) is showing early promising overall response rates in heavily pretreated patients. Infectious complications related to the use of BsAbs are not well described. We conducted a pooled analysis that included all single-agent BsAbs used in MM with no prior use of different BsAbs. A total of 1185 patients with MM were treated with a BsAb in the studied period (71.6% of the patients treated with an agent targeting B-cell maturation antigen (BCMA). Pooled median follow-up was short at 6.1 months (7.5 vs 5.2 months for BCMA vs non-BCMA BsAbs, respectively). Adverse events of interest included all grade neutropenia in 38.6%, all grade infections in 50% (n = 542/1083), all grade cytokine release syndrome in 59.6% (n = 706/1185), grade III/IV neutropenia in 34.8% (n = 372/1068), grade III/IV infections in 24.5% (n = 272/1110), grade III/IV pneumonia in 10% (n = 52.4/506), and grade III/IV coronavirus disease 2019 in 11.4% (n = 45.4/395) of the patients. Non-BCMA–targeted BsAbs were associated with lower grade III/IV neutropenia (25.3% vs 39.2%) and lower grade III/IV infections (11.9% vs 30%) when compared with BCMA-targeted BsAbs. Hypogammaglobulinemia was reported in 4 studies, with a prevalence of 75.3% (n = 256/340) of the patients, with IV immunoglobulin used in 48% (n = 123/256) of them. Death was reported in 110 patients, of which 28 (25.5%) were reported to be secondary to infections. Certain precautions should be used when using BsAbs to mitigate the risk and/or identify and treat infections promptly.
Introduction
Multiple myeloma (MM), a plasma cell neoplasm, is associated with an increased risk of infections. The risk of infections is variable and related to tumor burden, underlying comorbidities, and/or treatments used. It is estimated that patients with MM had 7- and 10-fold increased bacterial and viral infections, respectively.1 The infections result in increased morbidity and mortality. A significant improvement in treatment options, including the use of immunomodulatory drugs, proteasome inhibitors, anti-CD38 monoclonal Abs, and high-dose chemotherapy followed by autologous stem cell transplantation results in improved MM outcomes.2
Bispecific antibodies (BsAbs) are currently studied for the management of relapsed/refractory MM with promising early results. BsAbs bind a target both on the malignant plasma cells and immune effector cells, which results in immunologic synapse and subsequent malignant plasma cell apoptosis.3 BsAbs used in MM can target B-cell maturation antigen (BCMA) or non-BCMA antigens, including G-protein coupled receptor family C group 5 member D (GPRC5D) and Fc Receptor-Like 5 (FcRH5), present mainly on the tumor cells to decrease toxicity, while also binding to anti-CD3 on effector T cells, leading to the activation of the immune system.
Multiple ongoing trials have highlighted some of the BsAbs-associated adverse events (AEs), such as cytokine release syndrome (CRS), cytopenia, hypogammaglobulinemia, and infections. Infectious complications because of any possible differences between BCMA and non-BCMA–targeted BsAbs related to its use are not well described. Our study aims to quantify and describe reported infectious AEs related to the use of BsAbs in MM treatment.
Methods
We conducted a cross sectional analysis of all the BsAbs used in MM treatment, with reported data either in an abstract format or full publication. A search using the terms “bispecific,” “bispecific antibody,” “T-cell engager,” “T-cell redirecting therapy,” “multiple myeloma,” “myeloma,” and “plasma cell dyscrasia” was performed using Embase, Medline/PubMed, CINAHL, Scopus, Web of Science, and Google Scholar for English-language literature published until December 2022. We used medical subject heading terms because they are universally used in indexing life science journal articles and books. Data from the most recent publications were used. The study was deemed exempt from review by the institutional review board of the University of Arkansas for Medical Sciences because data were publicly available.
BsAbs were categorized based on their targets as BCMA vs non-BCMA. BsAbs targeting non-BCMAs included GPRC5D and FcRH5. We excluded studies that reported on the use of BsAbs as part of combination therapy and allowed for prior use of other BsAbs. Trispecific antibodies were excluded. The drugs included in our analysis were teclistamab, elranatamab, REGN-5458 (linvoseltamab), AMG420, AMG701 (Pavurutamab), CC-93269 (alnuctamab), TNB-383B (ABBV-383), talquetamab (weekly dosing), talquetamab (biweekly dosing), talquetamab IV, and cevostamab.4-13 We collected data about the frequencies of neutropenia (absolute neutrophil count < 1.5× 103/mL); lymphopenia (<1 × 103/mL); infections, including bacterial, fungal, and viral; CRS; hypogammaglobulinemia; and death related to infections. Categorical data were compared using χ2 test. The pooled analysis of infectious complications was performed using R statistical software version 4.0.5 (R project for statistical computing).14
Results
Eleven trials were included in our analysis. A total of 1185 patients with MM were treated with a BsAb as a single agent in the studied period (71.6% of patients were treated with an agent targeting BCMA). Across all the trials included, no notable differences in enrollment criteria were noted. Patients had a history of relapsed/refractory MM with previous use of immunomodulatory drugs, proteasome inhibitors, and anti-CD38 monoclonal Abs. In MajesTEC-1, the median age was 64 years, the median time between diagnosis and the first dose was 6 years, and the median lines of prior therapy were 5, which were similar to those in the MonumenTAL −1 study.
Pooled median follow-up was short at 6.1 months (range, 1.7-14.1 months). In BCMA BsAbs studies, pooled median follow-up was relatively longer at 7.5 months (range, 1.7-14.1 months), whereas in non-BCMA BsAbs studies, pooled median follow-up was 5.2 months (range, 4-11.7 months).
Reported AEs varied between studies and are summarized in Table 1. Treatment-related AEs were reported in 100% of the included studies. AEs of interest reported in all included studies included all grade neutropenia in 38.6% (n = 441/1143), all grade infections in 50% (n = 542/1083), all grade CRS in 59.6% (n = 706/1185), grade III/IV neutropenia in 34.8% (n = 372/1068), grade III/IV infections in 24.5% (n = 272/1110), grade III/IV pneumonia in 10% (n = 52/506), and grade III/IV coronavirus disease 2019 (COVID-19) in 11.4% (n = 45/395) of the patients (Figure 1).
Characteristics of included clinical trials for BsAbs
| Agent . | Teclistimab . | Elranatamab . | REGN-5458 (linvoseltamab) . | AMG420 . | AMG701 (pavurutamab) . | CC-93269 (alnuctamab) . | ABBV-383 (TNB-383B) . | Talquetamab 405 ng SC weekly . | Talquetamab 800 ng SC biweekly . | Talquetamab IV . | Cevostamab . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | BCMA | BCMA | BCMA | BCMA | BCMA | BCMA | BCMA | GPRC5D | GPRC5D | GPRC5D | cRH5 |
| N | 165 | 123 | 252 | 42 | 75 | 68 | 124 | 30 | 44 | 102 | 160 |
| Median follow-up, mo | 14.1 | 10.4 | 3.2 | 7.5 | 1.7 | 2.6 | 10.8 | 11.7 | 4.2 | 4 | 6.1 |
| Neutropenia N (%) | 117 (70.90) | 59 (48) | 64 (25) | N/A | 17 (23) | 25 (37) | 46 (37) | 20 (67) | 16 (36) | 48 (47) | 29 (18) |
| Neutropenia G III/IV N (%) | 106 (64.2) | 59 (48) | 58 (23) | N/A | N/A | 22 (32) | 42 (34) | 18 (60) | 14 (32) | 27 (26) | 26 (16.3) |
| Lymphopenia N (%) | 57 (34.5) | 32 (26) | 42 (17) | N/A | N/A | N/A | 19 (15) | 12 (40) | 17 (39) | 53 (52) | N/A |
| Leukopenia N (%) | 29 (17.6) | N/A | N/A | N/A | N/A | N/A | N/A | 12 (40) | 8 (18) | 38 (37) | N/A |
| Infections N (%) | 126 (76.4) | 82 (66.7) | 136 (54) | 14 (33) | 13 (17) | 23 (34) | 51 (41) | 14 (47) | 15 (34) | N/A | 68 (42.5) |
| Infections G III/IV N (%) | 74 (44.8) | 43 (35) | 73 (29) | 8 (21.4) | N/A | 6 (9) | 28 (22.5) | 2 (7) | 3 (7) | 5 (4.9) | 30 (18.8) |
| CRS N (%) | 119 (72.1) | 71 (57.7) | 111 (44) | 16 (38) | 46 (61) | 36 (53) | 71 (57) | 23 (77) | 35 (80) | 50 (49) | 128 (80) |
| CRS G III/IV N (%) | 1 (0.6%) | 0 | 3 (1) | 1 (0.02) | 5 (7) | 0 | 3 (2) | 1 (3) | 0 | 5 (5) | 2 (1.8) |
| CRS tx N (%) | 110 (66.7) | 30 | 44 +24 +10 | 12 | 5 | 20 | 17 (14) | 23 (77) | 35 (80) | 47 (46.1) | 89 (69.5) |
| CRS tx with tocilizumab/steroids N (%) | 60 (36.4)/14 (8.5) | 27 (22.7)/10 (8.4) | 44 (18)/24 (10) | 1 (2.3)/12 (28.5) | 5 (6)/5 (6) | 12 (17.6)/8 (11.8) | 17 (13.7)/ N/A | 19 (63.3)/1 (3.3) | 24(54.5)/0 | 38 (37.3)/11 (10.8) | 56 (43.8)/33 (25.8) |
| Hypogammaglobulinemia N (%) | 123 (74.5) | 76 (75.2)∗ | N/A | N/A | N/A | N/A | N/A | 26 (87) | 31 (71) | N/A | N/A |
| hypogammaglobulinemia tx with IVIG N (%) | 65 | 50 (40.7) | N/A | N/A | N/A | N/A | N/A | 4 (13) | 4 (9.7) | N/A | N/A |
| Agent . | Teclistimab . | Elranatamab . | REGN-5458 (linvoseltamab) . | AMG420 . | AMG701 (pavurutamab) . | CC-93269 (alnuctamab) . | ABBV-383 (TNB-383B) . | Talquetamab 405 ng SC weekly . | Talquetamab 800 ng SC biweekly . | Talquetamab IV . | Cevostamab . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | BCMA | BCMA | BCMA | BCMA | BCMA | BCMA | BCMA | GPRC5D | GPRC5D | GPRC5D | cRH5 |
| N | 165 | 123 | 252 | 42 | 75 | 68 | 124 | 30 | 44 | 102 | 160 |
| Median follow-up, mo | 14.1 | 10.4 | 3.2 | 7.5 | 1.7 | 2.6 | 10.8 | 11.7 | 4.2 | 4 | 6.1 |
| Neutropenia N (%) | 117 (70.90) | 59 (48) | 64 (25) | N/A | 17 (23) | 25 (37) | 46 (37) | 20 (67) | 16 (36) | 48 (47) | 29 (18) |
| Neutropenia G III/IV N (%) | 106 (64.2) | 59 (48) | 58 (23) | N/A | N/A | 22 (32) | 42 (34) | 18 (60) | 14 (32) | 27 (26) | 26 (16.3) |
| Lymphopenia N (%) | 57 (34.5) | 32 (26) | 42 (17) | N/A | N/A | N/A | 19 (15) | 12 (40) | 17 (39) | 53 (52) | N/A |
| Leukopenia N (%) | 29 (17.6) | N/A | N/A | N/A | N/A | N/A | N/A | 12 (40) | 8 (18) | 38 (37) | N/A |
| Infections N (%) | 126 (76.4) | 82 (66.7) | 136 (54) | 14 (33) | 13 (17) | 23 (34) | 51 (41) | 14 (47) | 15 (34) | N/A | 68 (42.5) |
| Infections G III/IV N (%) | 74 (44.8) | 43 (35) | 73 (29) | 8 (21.4) | N/A | 6 (9) | 28 (22.5) | 2 (7) | 3 (7) | 5 (4.9) | 30 (18.8) |
| CRS N (%) | 119 (72.1) | 71 (57.7) | 111 (44) | 16 (38) | 46 (61) | 36 (53) | 71 (57) | 23 (77) | 35 (80) | 50 (49) | 128 (80) |
| CRS G III/IV N (%) | 1 (0.6%) | 0 | 3 (1) | 1 (0.02) | 5 (7) | 0 | 3 (2) | 1 (3) | 0 | 5 (5) | 2 (1.8) |
| CRS tx N (%) | 110 (66.7) | 30 | 44 +24 +10 | 12 | 5 | 20 | 17 (14) | 23 (77) | 35 (80) | 47 (46.1) | 89 (69.5) |
| CRS tx with tocilizumab/steroids N (%) | 60 (36.4)/14 (8.5) | 27 (22.7)/10 (8.4) | 44 (18)/24 (10) | 1 (2.3)/12 (28.5) | 5 (6)/5 (6) | 12 (17.6)/8 (11.8) | 17 (13.7)/ N/A | 19 (63.3)/1 (3.3) | 24(54.5)/0 | 38 (37.3)/11 (10.8) | 56 (43.8)/33 (25.8) |
| Hypogammaglobulinemia N (%) | 123 (74.5) | 76 (75.2)∗ | N/A | N/A | N/A | N/A | N/A | 26 (87) | 31 (71) | N/A | N/A |
| hypogammaglobulinemia tx with IVIG N (%) | 65 | 50 (40.7) | N/A | N/A | N/A | N/A | N/A | 4 (13) | 4 (9.7) | N/A | N/A |
G, grade; N/A, not available; SC, subcutaneous; tx, treatment.
Data available for 101 patients.
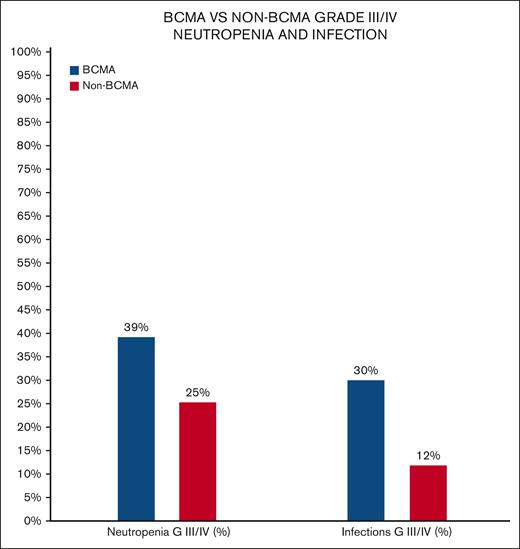
BCMA vs non-BCMA incidence of all grade and grade III/IV infections and neutropenia.
BCMA vs non-BCMA incidence of all grade and grade III/IV infections and neutropenia.
Treatment for CRS was used among 66% of the patients who developed CRS (n = 466/706): tocilizumab was used among 65% (n = 303/466) of them, whereas steroids were used for the remaining 25.3% (n = 118/466) of them.
Non-BCMA–targeting BsAbs were associated with lower grade III/IV neutropenia (25.3% vs 39.2%; P = .001) and lower grade III/IV infections (11.9% vs 30%; P = .01) when compared with BCMA-targeting BsAbs. Hypogammaglobulinemia was reported in 4 studies, with a prevalence of 75.3% (n = 256/340) with IV immunoglobulin (IVIG) used in 48% (n = 123/256) of the patients.
Significant reported infections included both typical and opportunistic infections. Typical infections reported included the following: pneumonia (including an episode of respiratory syncytial virus pneumonia), upper respiratory tract infection, adenovirus infection, central line-associated bloodstream infections, urinary tract infection, and Helicobacter pylori gastritis. Opportunistic infections, including cytomegalovirus (CMV) infection/reactivation, pneumocystis jiroveci pneumonia (PJP), candida esophagitis, ophthalmic herpes simplex virus, and progressive multifocal leukoencephalopathy were also reported. All grades of PJP infection was reported in 4.2% of the treated patients, whereas CMV infection and/or reactivation was reported in 8% of the treated patients.
Four studies included reporting about the microorganisms identified with infections caused with the use of specific drugs (teclistimab, elranatmab, AMG420, and talquetemab). PJP, progressive multifocal leukoencephalopathy, adenovirus infection, Streptococcus pneumoniae infection, and COVID-19 were reported with the use of teclistimab. Pseudomonas aeruginosa pneumonia, CMV infection, and COVID-19 were reported with the use of elranatamab. Adenovirus infections, aspergilliosis, and influenza were reported with the use of AMG 420. The most detailed reporting was in MonumenTAL-1 (talquetemab), which included information about the various organisms that caused pneumonia, urinary tract infections, and sepsis; drug dosing; and the day of onset and duration of infection. It included diseases caused by species of Enterococcus, Escherichia, Pseudomonas, and Pneuomococcus in addition to respiratory syncytial virus disease, influenza, COVID-19, and Helicobacter pylori gastritis. Specific opportunistic infections, such as candida esophagitis, disseminated varicella-zoster virus infection, adenovirus infection, and ocular herpes were also reported.
Death was reported in 110 patients, of which 28 (25.5%) were reported to be secondary to infections. Infections leading to death included COVID-19, Streptococcus pneumoniae infection, Pseudomonas aeruginosa pneumonia, influenza, aspergillosis, hepatitis, pneumonia (due to adenovirus infection), and sepsis (due to an unidentified infection).
Discussion
In our analysis, we quantified reported infectious complications associated with BsAbs. BsAbs function by bridging T cells through CD3 and specific antigens on MM cells, therefore, unleashing the apoptotic machinery of T cells against malignant myeloma cells. The use of BsAbs will continue to evolve.15 BCMA is expressed on both healthy and malignant plasma cells; however, the expression is markedly increased on malignant plasma cells. BCMA function is integral to the formation of IGs and thus immunity and the regulation of regulatory lymphocytes via intracellular signaling.16 The function of GPR5D is not well defined, whereas FcHR5 is responsible for B-cell proliferation.17
We found a higher risk of infection among patients treated with BCMA-targeting BsAbs than non-BMCA–targeting BsAbs (talquetmab and cevostumab). The risk of neutropenia was lower with the use of non-BCMA–BsAbs. BCMA antigen is present on mature B lymphocytes and is necessary for their function. The overiexpression of BCMA was found to activate nuclear factor κB and c-Jun N-terminal kinase, which plays a role in T-cell proliferation and cytokine release to increase the production of phagocytic cells and neutrophils.1
Our study showed that grade III/IV infections occurred in one-fourth (24.5%) of the patients treated with BsAbs. The risk of acquiring an infection in MM is multifactorial, and it is increasing with time; a Swedish study that included 9253 patients with MM from 1988 to 2004 showed increasing infection rates in the last decade, which can be because patients with MM are living longer with improved life expectancy and/or improved identification of infections but also raises the question about modern therapy contributing to the increased risk.1 In our analysis, we found a significant association between BsAbs and infectious complications.
The risk of acquiring infections in patients with MM is multifactorial, especially for those treated with BsAbs and is likely related to overall improved outcomes. First, almost all patients who received BsAbs have been treated with other forms of therapy leading to an already immunosuppressed state with a depletion of lymphocytes and IGs.15 Second, BsAb-targeted therapy is associated with significant neutropenia, lymphopenia, and hypogammaglobulinemia, directly increasing the risk of acquiring bacterial, fungal, and viral infections via the stimulation of regulatory T cells. Third, the results of the pooled analysis showed significant development of CRS, which is treated with immunosuppressive agents including glucocorticoids, tocilizumab, and tumor necrosis factor α blockers such as etanercept, indirectly causing further immunosuppression.3 The infectious complications seem to predominantly affect the upper and lower respiratory tract, in addition to opportunistic infections such as CMV and adenovirus infections, PJP, and aspergilliosis.
There is a lack of guidelines about the use of antimicrobial prophylaxis and/or IVIG among patients who receive BsAbs. Implementation of antimicrobial prophylaxis and IVIG infusions may help in decreasing the risk of infectious complications and should be added to future studies.
Our analysis revealed 4.2% of all grades of PJP and CMV infections and/or 8% reactivation in the treated patients. These findings can help guide the consideration of antibiotic prophylaxis to decrease the risk of PJP and periodic monitoring of CMV to help in the early diagnosis and management.
COVID-19 infections were frequent and resulted in death for some patients. In the MajesTEC-1 study, 68 deaths were reported of which 12 were due to COVID-19 infection.4 The MagnetisMM-3 trial reported various infections associated with elranatamab, including all grades of COVID-19 infections in 14.9% of the patients (n = 14) (grade III/IV: 8.5% n = 8).5 ABBV-383 (TNB-383B) was associated with 7 cases (6%) of COVID-19, whereas talquetamab was associated with 5 cases of COVID-19.10,12,18 Frequent COVID-19 infections are likely related to the timing of enrollment of patients in various BsAbs clinical trials; however, given the endemic nature of COVID-19 and the increased risk of infections in patients receiving BsAbs, efforts should be made to ensure that patients’ vaccination status is up to date along with early identification and/or treatment, as clinically appropriate.
Hypogammaglobulinemia was reported in 4 studies (75.3%, n = 256/340), and it was treated with IVIG in 48% of the patients (123/256). In MajesTEC-1, the use of teclistamab resulted in hypogammaglobulinemia (defined as <500 mg/dL serum IgG levels) in 74.5% of the patients (n = 123/165).4 ABBV-383 was associated with hypogammaglobulinemia in 14% of the patients (n = 17/124).10 The development of hypogammaglobulinemia with the use of talquetamab in MonumenTAL-1 varied based on the dosing frequency, with 87% (n = 26/30) of the patients with 405 ng subcutaneous once-a-week-dosing, and 70% (n = 31/44) of the patients with 800 ng subcutaneous once every 2 week dosing reporting hypogammaglobulinemia.12
Our analysis showed inconsistent reporting of infections across all included studies. Efforts should be made to capture infectious complications and report them in a better manner to help guide the treating physicians. Additionally, the use of prophylaxis for opportunistic infections was unclear across the studies. It is crucial to have an organized system for tracking and reporting infections and identifying patients who are at high risk and require prophylaxis and treatment. Another strategy would be to include infections in the research protocol's AEs of special interest category, which also includes CRS and requires stringent data collection to include timing, management, and reporting of all patients’ data.
Baseline and periodic testing for specific infections, including CMV, may be needed in clinical practice along with the use of IVIG and implementation of a mitigation system to help in decreasing infections and/or treating them earlier. Future BsAb studies should consider the incorporation of various prophylactic measures, including IVIG and/or prophylactic antimicrobials, to prevent serious infections.
Our analysis is limited because of the nature of it being retrospective and the heterogeneity of reporting on infections, prophylactic treatment, and the duration of follow-up. We also included BsAbs with different targets (GPRC5D, FcRH5) as non-BCMA–BsAbs. However, this is the first pooled analysis, to our knowledge, that aims to quantify the infectious complications associated with the use of BsAbs in patients with MM that may help in improving the supportive care plans focused on preventing and/or treating infections with expected use in clinical practice in the future.
In conclusion, BsAbs are associated with infectious complications that may be related to underlying MM, previous therapies, and/or their mechanism of action. Half of the patients treated with BsAbs developed an infection and a quarter of the patients developed grade III/IV infections. A quarter of deaths were secondary to infections. Certain precautions should be used when using BsAbs to mitigate the risk and/or identify and treat infections promptly. Limited duration of treatment with less frequent dosing may result in better outcomes and lower risks of complications.19,20 Guidelines should consider the implementation of prophylactic measures and/or baseline laboratory testing while bispecific antibodies are being studied in clinical trials and are being considered to be approved for clinical use.
Authorship
Contribution: S.A.H. conceived the research idea; F.M., L.M., and S.A.H. collected data, performed statistical analyses, and wrote the initial draft of the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Samer Al Hadidi, Myeloma Center, Winthrop P. Rockefeller Cancer Institute, University of Arkansas for Medical Sciences, Little Rock, AR 72205; e-mail: salhadidi@uams.edu.
References
Author notes
Portions of this work was presented at the 65th annual meeting of the American Society of Hematology from 10 to 13 December 2022 in New Orleans, LA.
Data are available on request from the corresponding author, Samer Al Hadidi (salhadidi@uams.edu).