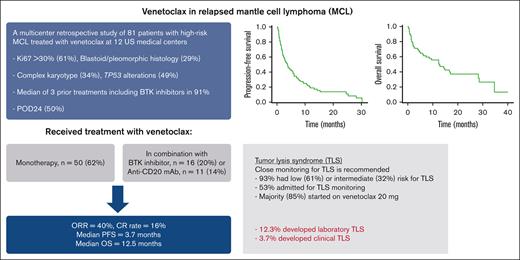
Key Points
In high-risk patients with relapsed MCL, most of whom received prior BTKi, venetoclax resulted in ORR of 40% and median PFS of 3.7 months.
Patients with MCL who initiate venetoclax should be closely monitored for TLS.
Abstract
To report the activity of venetoclax in patients with relapsed mantle cell lymphoma (MCL), we identified 81 patients treated with venetoclax monotherapy (n = 50, 62%) or in combination with a Bruton tyrosine kinase inhibitor (BTKi) (n = 16, 20%), an anti-CD20 monoclonal antibody (n = 11, 14%), or other active agents at 12 US academic medical centers. Patients had high-risk disease features including Ki67 >30% (61%), blastoid/pleomorphic histology (29%), complex karyotype (34%), and TP53 alterations (49%), and received a median of 3 prior treatments including BTKis in 91%. Venetoclax alone or in combination resulted in an overall response rate (ORR) of 40% and median progression-free (PFS) and overall survival (OS) of 3.7 and 12.5 months, respectively. The receipt of ≤3 prior treatments was associated with higher odds of response to venetoclax in a univariable analysis. In a multivariable analysis, having a high-risk Mantle Cell Lymphoma International Prognostic Index score before receiving venetoclax and disease relapse or progression within 24 months of diagnosis were associated with inferior OS whereas the use of venetoclax in combination was associated with superior OS. Although most patients (61%) had low risk for tumor lysis syndrome (TLS), 12.3% of patients developed TLS despite the implementation of several mitigation strategies. In conclusion, venetoclax resulted in good ORR but short PFS in patients with MCL who are at high risk, and may have a better role in earlier lines of treatment and/or in conation with other active agents. TLS remains an important risk in patients with MCL who initiate treatment with venetoclax.
Introduction
Mantle cell lymphoma (MCL) is an uncommon B-cell non-Hodgkin lymphoma characterized by an aggressive clinical course in most patients. Frontline treatment typically consists of chemoimmunotherapy with consideration for consolidative autologous stem cell transplantation in eligible patients and/or rituximab maintenance.1 Although most patients respond to frontline chemoimmunotherapy, relapses are inevitable. The Bruton tyrosine kinase inhibitors (BTKis) are a preferred second-line treatment option given their efficacy, duration of response, and favorable safety profile.1 However, the median progression-free survival (PFS) with BTKis is <2 to 3 years and outcomes after progression on a BTKi have historically been poor.2-10 Other approved novel agents (bortezomib, temsirolimus, and lenalidomide) are less effective in relapsed MCL. More recently, brexucabtagene autoleucel, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, is a highly effective treatment and is now approved for relapsed MCL.11 However, treatment with CAR T-cell therapy has important challenges including significant toxicities, which limit its use in older and unfit patients, and inadequate bridging therapy options for patients with rapidly progressing disease. In addition, manufacturing failures and post–CAR T-cell relapse are ongoing challenges for many patients. Overall, more effective treatment options are needed for patients with relapsed MCL, particularly after progression on BTKis.
Venetoclax is a BH3-mimetic drug that has shown significant clinical activity in B-cell lymphoid malignancies including MCL. In a phase 1 trial of venetoclax monotherapy in patients with relapsed or refractory B-cell non-Hodgkin lymphoma, the overall response rate (ORR) was 75% and the median PFS was 11 months in the 28 patients with MCL.12,13 Treatment with venetoclax was well tolerated, with the most common grade 3 and 4 toxicities being anemia, neutropenia, and thrombocytopenia. Similar to what is seen in chronic lymphocytic leukemia, although less well described, tumor lysis syndrome (TLS) was also reported in MCL and a ramp-up dose schedule of venetoclax along with TLS prophylaxis is recommended.12,14 Notably, none of the patients with MCL enrolled on this phase 1 trial received prior treatment with a BTKi. The efficacy of venetoclax in patients previously treated with BTKis was reported in 2 small retrospective studies that each included between 20 and 24 patients in whom venetoclax resulted in an ORR of between 50% and 53% and a median PFS of between 3 and 8 months.15,16 Based on the phase 1 data, venetoclax is also frequently included in studies of novel combinations in relapsed/refractory and/or newly diagnosed MCL. However, data regarding its efficacy in relapsed MCL are limited. In this study, we evaluated the clinical activity of venetoclax in MCL and identified factors associated with outcomes in a larger cohort of patients.
Methods
Patients
We retrospectively reviewed records of patients aged ≥18 years with a diagnosis of relapsed/refractory MCL treated at 12 US medical centers between 1 January 2010 and 1 November 2019, and identified patients treated with venetoclax alone or in combination with other agents. Institutional review board approval was obtained at each participating center before data collection.
We collected clinical, laboratory, pathologic, and outcome data for each patient at the time of diagnosis and before the start with venetoclax. We calculated the Mantle Cell Lymphoma International Prognostic Index (MIPI) score for each patient with available data, as previously described.17 We defined complex karyotype as having ≥3 chromosomal abnormalities, excluding t(11;14), and TP53 alterations as having chromosome 17p deletion by fluorescence in situ hybridization or conventional metaphase karyotyping, TP53 oncogenic mutations by next-generation sequencing, and/or mutant p53 protein overexpression by immunohistochemistry. Treatment responses were determined by the local investigator and were not centrally reviewed. We defined laboratory and clinical TLS per the Cairo-Bishop criteria,18 and defined TLS risk categories per venetoclax prescribing information in chronic lymphocytic leukemia based on the patient’s status at the time of venetoclax initiation: low risk as an absolute lymphocyte count (ALC) of <25 000 cells per μL and all lymph nodes <5 cm; intermediate risk as and ALC of >25 000 cells per μL or any lymph node between 5 and 10 cm; and high risk as any lymph node >10 cm, or an ALC of >25 000 cells per μL and any lymph node between 5 and 10 cm.19
Statistical analysis
Patient characteristics at diagnosis and before venetoclax initiation were summarized by descriptive statistics with the median and range presented for continuous variables, and frequency count and percentage provided for categorical variables. Univariable logistic regression models were used to estimate the association between patient characteristics and ORR. PFS was calculated from the time of venetoclax initiation to either progression or death, and overall survival (OS) from the time of venetoclax initiation to death due to all causes; patients without events were censored at the time of last follow-up. PFS and OS were estimated through the Kaplan-Meier method and compared using the log-rank test. Multivariable analyses (MVAs) were performed using Cox proportional hazards models to identify predictors of PFS and OS. For MVAs, we excluded variables with <50% data capture and a backward variable selection approach was used with an α of 0.1 as removal criteria. To account for the small sample size, Firth penalized likelihood bias-reduction approach was used in MVAs. The significance level (α) was set at 0.05. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Patient and disease characteristics
In total, 81 patients were included. Table 1 shows patient characteristics at diagnosis and before starting venetoclax. At diagnosis, the median age was 64 years (range, 38-87 years), 78% were male, and 95% had stage 3/4 disease. The MIPI score was low, intermediate, and high in 16%, 34%, and 49% of patients, respectively (data available for n = 61). Sixty-one percent had Ki-67 >30% (data available for n = 62), 29% had blastoid or pleomorphic histology (data available for n = 75), 34% had complex karyotype (data available for n = 62), and 49% had TP53 alterations (data available for n = 43), suggesting that a significant proportion of patients had high-risk features. Before venetoclax initiation, the MIPI score was low, intermediate, and high in 22%, 25%, and 53% of patients, respectively (data available for n = 66). Seventy-six percent had Ki-67 >30% (data available for n = 38), 45% had blastoid or pleomorphic histology (data available for n = 65), 36% had complex karyotype (data available for n = 28), and 33% had TP53 alterations (data available for n = 30).
Patient characteristics at diagnosis and before venetoclax initiation
| Variable . | At diagnosis . | Before venetoclax . |
|---|---|---|
| Age, median (range), y | 63.9 (38.0-86.9) | 69.0 (38.3-87.8) |
| Gender, n (%) | ||
| Male | 64 (79.0) | — |
| Female | 17 (21.0) | |
| MIPI score, n (%) | ||
| Low risk | 10 (16.4) | 15 (22.1) |
| Intermediate risk | 21 (34.4) | 17 (25.0) |
| High risk | 30 (49.2) | 36 (52.9) |
| Missing data | 20 | 13 |
| Ki-67 | ||
| >30%, n (%) | 38 (61.2) | 29 (76.3) |
| Median (range), % | 40 (5-90) | 70 (5-95) |
| Missing data | 19 | 43 |
| Blastoid or pleomorphic, n (%) | ||
| Yes | 22 (29.3) | 29 (44.7) |
| No | 53 (70.7) | 36 (55.4) |
| Missing data | 6 | 16 |
| Complex karyotype, n (%) | ||
| Yes | 21 (33.9) | 10 (35.7) |
| No | 41 (66.1) | 18 (64.3) |
| Missing data | 19 | 53 |
| TP53alterations, n (%) | ||
| Yes | 21 (48.8) | 10 (33.3) |
| TP53 mutation | 9 (20.9) | 5 (16.7) |
| Del 17p by fluorescence in situ hybridization | 10 (23.3) | 5 (16.7) |
| Del 17p by conventional cytogenetics | 2 (4.7) | 0 |
| No | 22 (51.2) | 20 (66.7) |
| Missing data | 38 | 51 |
| Variable . | At diagnosis . | Before venetoclax . |
|---|---|---|
| Age, median (range), y | 63.9 (38.0-86.9) | 69.0 (38.3-87.8) |
| Gender, n (%) | ||
| Male | 64 (79.0) | — |
| Female | 17 (21.0) | |
| MIPI score, n (%) | ||
| Low risk | 10 (16.4) | 15 (22.1) |
| Intermediate risk | 21 (34.4) | 17 (25.0) |
| High risk | 30 (49.2) | 36 (52.9) |
| Missing data | 20 | 13 |
| Ki-67 | ||
| >30%, n (%) | 38 (61.2) | 29 (76.3) |
| Median (range), % | 40 (5-90) | 70 (5-95) |
| Missing data | 19 | 43 |
| Blastoid or pleomorphic, n (%) | ||
| Yes | 22 (29.3) | 29 (44.7) |
| No | 53 (70.7) | 36 (55.4) |
| Missing data | 6 | 16 |
| Complex karyotype, n (%) | ||
| Yes | 21 (33.9) | 10 (35.7) |
| No | 41 (66.1) | 18 (64.3) |
| Missing data | 19 | 53 |
| TP53alterations, n (%) | ||
| Yes | 21 (48.8) | 10 (33.3) |
| TP53 mutation | 9 (20.9) | 5 (16.7) |
| Del 17p by fluorescence in situ hybridization | 10 (23.3) | 5 (16.7) |
| Del 17p by conventional cytogenetics | 2 (4.7) | 0 |
| No | 22 (51.2) | 20 (66.7) |
| Missing data | 38 | 51 |
Prior treatments
First-line treatment (data available for n = 80) was intensive chemotherapy (defined as including high-dose cytarabine and/or undergoing autologous hematopoietic cell transplantation [HCT] in first remission) in 51% of patients, less-intensive chemotherapy in 43%, and nonchemotherapy in 6% (Table 2). Twenty-six patients (32%) underwent autologous HCT in first remission and 27 (36%) received rituximab maintenance after first-line treatment. The median duration of first remission was 1.6 years (range, 0.04-6.4). Thirty-nine patients (50%) had early disease relapse/progression after first-line treatment (POD24, defined as disease relapse or progression within 24 months of diagnosis; missing, n = 3).20 The median number of therapies before venetoclax was 3 (range, 1-8), which included anti-CD20 monoclonal antibody in 99% of patients, alkylator in 93%, BTKis in 91%, anthracycline in 58%, cytarabine in 56%, lenalidomide in 37%, bortezomib in 28%, platinum in 21%, phosphoinositide 3-kinase inhibitor in 9%, autologous HCT in 32%, allogeneic HCT in 4%, and CAR T-cell therapy in 3%. Fifty-five percent of patients had refractory disease (defined as stable disease [SD] or progressive disease [PD]) to the last therapy before venetoclax. The ORR to the BTKi before venetoclax (n = 70) was 66% including complete response (CR) in 20%. Treatment with BTKis lasted for a median of 6 months (range, 0.5-69) and was stopped because of PD and toxicity in 82% and 18% of patients, respectively. The ORR to the last treatment before venetoclax was 45% (data available for n = 75).
Treatment data before initiation of venetoclax treatment
| Variable . | N (%)∗ . |
|---|---|
| First-line treatment | |
| Intensive chemotherapy | 41 (51.3) |
| Less-intensive chemotherapy | 34 (42.5) |
| Nonchemotherapy | 5 (6.25) |
| Autologous HCT in first remission | |
| Yes | 26 (32.1) |
| No | 55 (67.9) |
| Rituximab maintenance | |
| Yes | 27 (35.5) |
| No | 49 (64.5) |
| Duration of first remission, median (range), y | 1.63 (0.04-6.4) |
| Relapse/progression within 24 mo of diagnosis | 39 (50.0) |
| Lines of treatment before venetoclax | |
| Median (range) | 3 (1-8) |
| ≥4 | 24 (29.6) |
| Treatments before venetoclax | |
| Anti-CD20 monoclonal antibody | 80 (98.8) |
| Alkylator | 75 (92.6) |
| BTKi | 74 (91.4) |
| Anthracycline | 47 (58.0) |
| Cytarabine | 45 (55.6) |
| Lenalidomide | 30 (37.0) |
| Bortezomib | 23 (28.4) |
| Platinum | 17 (21.0) |
| Phosphoinositide 3-kinase inhibitor | 7 (8.6) |
| Autologous HCT | 26 (32.1) |
| Allogeneic HCT | 3 (4.1) |
| CAR T-cell therapy | 2 (2.7) |
| Best response to BTKi | |
| CR | 14 (20.0) |
| PR | 32 (45.7) |
| SD | 4 (5.7) |
| PD | 20 (28.6) |
| ORR (CR + PR) | 46 (65.7) |
| Unknown | 4 |
| No previous BTKi | 7 |
| Reason for stopping BTKi | |
| PD | 59 (81.9) |
| Toxicity | 13 (18.2) |
| Duration of treatment with BTKi, median (range), mo | 6.4 (0.5-69) |
| Best response to last treatment before venetoclax | |
| CR, PR | 34 (45.3) |
| SD, PD | 41 (54.7) |
| Variable . | N (%)∗ . |
|---|---|
| First-line treatment | |
| Intensive chemotherapy | 41 (51.3) |
| Less-intensive chemotherapy | 34 (42.5) |
| Nonchemotherapy | 5 (6.25) |
| Autologous HCT in first remission | |
| Yes | 26 (32.1) |
| No | 55 (67.9) |
| Rituximab maintenance | |
| Yes | 27 (35.5) |
| No | 49 (64.5) |
| Duration of first remission, median (range), y | 1.63 (0.04-6.4) |
| Relapse/progression within 24 mo of diagnosis | 39 (50.0) |
| Lines of treatment before venetoclax | |
| Median (range) | 3 (1-8) |
| ≥4 | 24 (29.6) |
| Treatments before venetoclax | |
| Anti-CD20 monoclonal antibody | 80 (98.8) |
| Alkylator | 75 (92.6) |
| BTKi | 74 (91.4) |
| Anthracycline | 47 (58.0) |
| Cytarabine | 45 (55.6) |
| Lenalidomide | 30 (37.0) |
| Bortezomib | 23 (28.4) |
| Platinum | 17 (21.0) |
| Phosphoinositide 3-kinase inhibitor | 7 (8.6) |
| Autologous HCT | 26 (32.1) |
| Allogeneic HCT | 3 (4.1) |
| CAR T-cell therapy | 2 (2.7) |
| Best response to BTKi | |
| CR | 14 (20.0) |
| PR | 32 (45.7) |
| SD | 4 (5.7) |
| PD | 20 (28.6) |
| ORR (CR + PR) | 46 (65.7) |
| Unknown | 4 |
| No previous BTKi | 7 |
| Reason for stopping BTKi | |
| PD | 59 (81.9) |
| Toxicity | 13 (18.2) |
| Duration of treatment with BTKi, median (range), mo | 6.4 (0.5-69) |
| Best response to last treatment before venetoclax | |
| CR, PR | 34 (45.3) |
| SD, PD | 41 (54.7) |
Missing data: first-line treatment, n = 1; autologous HCT in first remission, n = 5; rituximab maintenance, n = 5; duration of first remission, n = 5; relapse/progression within 2 years of diagnosis, n = 3; best response to BTKi, n = 11; reason for stopping BTKi, n = 9; duration of treatment with BTKi, n = 10; and best response to last therapy before venetoclax, n = 6.
Treatment with venetoclax
The median time from diagnosis to venetoclax initiation was 3.9 years (range, 0.2-17.8). Seven patients (9%) received treatment with venetoclax on a clinical trial. Fifty patients (62%) received venetoclax as monotherapy and 31 patients (38%) in combination with other agents: a BTKi (n = 16, 20%), an anti-CD20 monoclonal antibody (n = 11, 14%), or others (n = 4, 5%). The highest dose of venetoclax received was between 20 and 100 mg in 12% (n = 9), 200 mg in 11% (n = 8), 400 mg in 61% (n = 46), and 800 mg in 17% of patients (n = 13) (data available for n = 76). Venetoclax dose interruption and/or reduction occurred in 24 patients (30%). Treatment with venetoclax lasted for a median of 2.8 months (range, 0.1-30) and was stopped because of PD in 69% of patients, toxicity in 9%, before allogeneic HCT in 3%, or other reasons in 19%.
For the 67 patients (83%) with data available for response assessment, the best response to venetoclax was CR in 16% (n = 11), partial response (PR) in 24% (n = 16), SD in 10% (n = 7), and PD in 49% (n = 33) of patients, with an ORR of 40% (n = 27). Of the 10 patients with known TP53 alterations before starting venetoclax treatment, 7 had data available for response assessment, of whom 2 responded (ORR = 28.6%; 1 CR, 1 PR, and 5 PD). In a univariable logistic regression analysis (Table 3), receipt of >3 lines of treatment before venetoclax treatment was the only factor significantly associated with response to venetoclax (odds ratio, 0.26; 95% confidence interval [CI], 0.08-0.90; P = .033). The ORR was 66.7% in the 7 patients who received venetoclax as a second-line treatment (4 CR, 2 PD, and 1 with missing data). Best response (CR/PR vs SD/PD) to prior BTKi therapy or last prior therapy and whether venetoclax was given as monotherapy vs in combination were not associated with response to venetoclax. Notably, of the 16 patients treated with venetoclax in combination with a BTKi, 10 (63%) received prior treatment with a BTKi for a median of 7 months (range, 1-51) with an ORR to the BTKi of 40% (4 PR, 1 SD, and 5 PD). The ORR to the combination of venetoclax and a BTKi in patients previously treated with a BTKi was 33% (3 PR, 1 SD, 5 PD, and 1 with missing data).
Univariable logistic regression analysis of factors associated with ORR
| Variable . | Level . | n . | Odds ratio (95% CI) . | P value . |
|---|---|---|---|---|
| MIPI score at diagnosis | High risk | 24 | 0.70 (0.23-2.17) | .536 |
| Low/intermediate risk | 26 | — | ||
| TP53 alterations at diagnosis | Yes | 16 | 0.56 (0.12-2.54) | .448 |
| No | 16 | — | — | |
| MIPI score before venetoclax | High risk | 26 | 0.94 (0.32-2.75) | .906 |
| Low/intermediate risk | 30 | |||
| TP53 alterations before venetoclax | Yes | 7 | 0.57 (0.09-3.83) | .564 |
| No | 17 | — | — | |
| Blastoid/pleomorphic histology before venetoclax | Yes | 24 | 1.11 (0.38-3.26) | .854 |
| No | 30 | — | — | |
| Complex karyotype before venetoclax | Yes | 6 | 1.25 (0.20-7.96) | .813 |
| No | 18 | — | — | |
| No. of lines of treatment before venetoclax | >3 | 20 | 0.26 (0.08-0.90) | .033 |
| ≤3 | 47 | — | — | |
| POD24 | Yes | 32 | 1.30 (0.48-3.52) | .611 |
| No | 32 | — | ||
| Best response to last therapy before venetoclax | CR, PR | 28 | 2.27 (0.81-6.34) | .117 |
| SD, PD | 36 | — | — | |
| Duration of treatment with last therapy before venetoclax, mo | >4 | 30 | 2.27 (0.83-6.22) | .110 |
| ≤4 | 36 | — | — | |
| Best response to BTKi | CR, PR | 35 | 2.39 (0.76-7.49) | .136 |
| SD, PD | 23 | — | — | |
| Duration of treatment with BTKi, mo | >6 | 31 | 1.96 (0.66-5.80) | .227 |
| ≤6 | 27 | — | — | |
| Reason for stopping BTKi | PD | 51 | 0.73 (0.15-3.61) | .697 |
| Toxicity | 7 | — | — | |
| Venetoclax combined with other agent(s) | Combination | 29 | 1.79 (0.67-4.83) | .247 |
| Monotherapy | 38 | — | — | |
| Venetoclax highest dose received, mg | ≥400 | 53 | 1.65 (0.42-6.42) | .47 |
| <400 | 10 | — | — | |
| Venetoclax highest dose received, mg | 800 | 12 | 0.78 (0.2-3.03) | .72 |
| 400 | 41 | — | — |
| Variable . | Level . | n . | Odds ratio (95% CI) . | P value . |
|---|---|---|---|---|
| MIPI score at diagnosis | High risk | 24 | 0.70 (0.23-2.17) | .536 |
| Low/intermediate risk | 26 | — | ||
| TP53 alterations at diagnosis | Yes | 16 | 0.56 (0.12-2.54) | .448 |
| No | 16 | — | — | |
| MIPI score before venetoclax | High risk | 26 | 0.94 (0.32-2.75) | .906 |
| Low/intermediate risk | 30 | |||
| TP53 alterations before venetoclax | Yes | 7 | 0.57 (0.09-3.83) | .564 |
| No | 17 | — | — | |
| Blastoid/pleomorphic histology before venetoclax | Yes | 24 | 1.11 (0.38-3.26) | .854 |
| No | 30 | — | — | |
| Complex karyotype before venetoclax | Yes | 6 | 1.25 (0.20-7.96) | .813 |
| No | 18 | — | — | |
| No. of lines of treatment before venetoclax | >3 | 20 | 0.26 (0.08-0.90) | .033 |
| ≤3 | 47 | — | — | |
| POD24 | Yes | 32 | 1.30 (0.48-3.52) | .611 |
| No | 32 | — | ||
| Best response to last therapy before venetoclax | CR, PR | 28 | 2.27 (0.81-6.34) | .117 |
| SD, PD | 36 | — | — | |
| Duration of treatment with last therapy before venetoclax, mo | >4 | 30 | 2.27 (0.83-6.22) | .110 |
| ≤4 | 36 | — | — | |
| Best response to BTKi | CR, PR | 35 | 2.39 (0.76-7.49) | .136 |
| SD, PD | 23 | — | — | |
| Duration of treatment with BTKi, mo | >6 | 31 | 1.96 (0.66-5.80) | .227 |
| ≤6 | 27 | — | — | |
| Reason for stopping BTKi | PD | 51 | 0.73 (0.15-3.61) | .697 |
| Toxicity | 7 | — | — | |
| Venetoclax combined with other agent(s) | Combination | 29 | 1.79 (0.67-4.83) | .247 |
| Monotherapy | 38 | — | — | |
| Venetoclax highest dose received, mg | ≥400 | 53 | 1.65 (0.42-6.42) | .47 |
| <400 | 10 | — | — | |
| Venetoclax highest dose received, mg | 800 | 12 | 0.78 (0.2-3.03) | .72 |
| 400 | 41 | — | — |
Survival outcomes
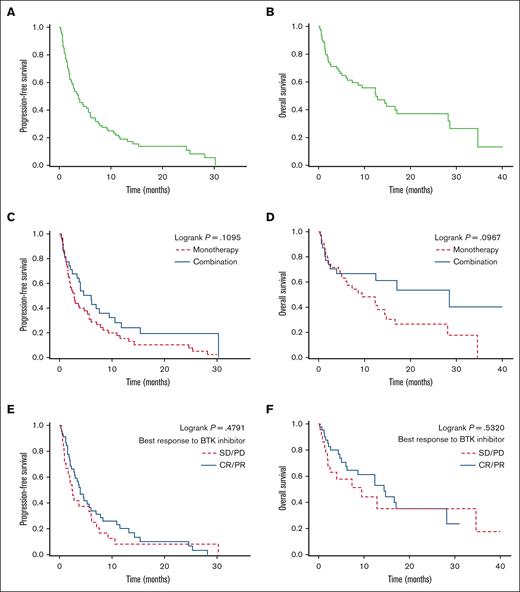
With a median follow-up of 16.4 months from the time of venetoclax treatment initiation, the median PFS and OS were 3.7 months (95% CI, 2.3-5.6) and 12.5 months (95% CI, 6.2-28.2), respectively, with 2-year PFS and OS of 13.8% (95% CI, 6.9-23.1) and 37.1% (95% CI, 24.0-50.2), respectively (Figure 1 A-B). PFS and OS were superior in patients who achieved CR vs PR with venetoclax: the 2-year PFS and OS were 57.7% (95% CI, 22.1-81.9) and 85.7% (95% CI, 33.4-97.9) vs 11.9% (95% CI, 0.9-38.4) and 35.9% (95% CI, 9.5-64.0), respectively (supplemental Figure). For the 7 patients who received venetoclax as second-line treatment, the median PFS and OS were 11.8 months (95% CI, 0.6 to not reached) and 28.6 months (95% CI, 0.6-28.6), respectively, and the 1-year PFS and OS were 42.9% (95% CI, 9.8-73.4) and 57.1% (95% CI, 17.2-83.7), respectively. For patients with known TP53 alterations before starting venetoclax treatment, the 1-year PFS and OS were 20.0% (95% CI, 3.1-47.5) and 31.7% (95% CI, 4.9-64.7), respectively. Compared with venetoclax monotherapy, combination therapy resulted in longer median PFS and OS but without reaching statistical significance (PFS = 6.0 vs 2.8 months, P = .11 and OS = 28.6 vs 9.5 months, P = .10, respectively) (Figure 1 C-D). The median PFS and OS did not significantly differ based on response (CR/PR vs SD/PD) to previous treatment with a BTKi (median PFS = 4.0 vs 2.5 months, P = .48 and median OS = 14.4 vs 9.5 months, P = .53, respectively) (Figure 1 E-F). Thirty-eight patients received postvenetoclax treatments with a median time of 0.24 months (range, 0-2.2) from stopping venetoclax to initiation of the next treatment. For the 33 patients with available data, the best response to the postvenetoclax treatment was CR, 24%; PR, 27%; SD, 9%; and PD, 39% with an ORR of 51%. Three patients achieved CR with venetoclax and underwent allogeneic HCT. Three patients received treatment with CAR T cells after venetoclax therapy and achieved CR (best response to venetoclax in these 3 patients was PD).
Survival outcomes with venetoclax. Median PFS was 3.7 months (A) and median OS was 12.5 months (B). (C-D) PFS and OS in patients treated with venetoclax monotherapy vs in those treated with combination therapy. (E-F) PFS and OS based on the best response to prior treatment with BTKis.
Survival outcomes with venetoclax. Median PFS was 3.7 months (A) and median OS was 12.5 months (B). (C-D) PFS and OS in patients treated with venetoclax monotherapy vs in those treated with combination therapy. (E-F) PFS and OS based on the best response to prior treatment with BTKis.
Prognostic factors
In univariable analyses (Tables 4 and 5), a high-risk MIPI score at diagnosis (hazard ratio [HR], 2.58; 95% CI, 1.43-4.65; P = .002) was associated with PFS, and a high-risk MIPI score at diagnosis (HR, 2.73; 95% CI, 1.26-5.88; P = .011), a high-risk MIPI score before venetoclax (HR, 2.12; 95% CI, 1.03-4.36; P = .041), POD24 (HR, 2.30; 95% CI, 1.22-4.45; P = .012), and duration of treatment with the last therapy before venetoclax of >4 months (HR, 0.48; 95% CI, 0.25-0.92; P = .026) were associated with OS.
Univariable and MVAs of factors associated with PFS
| Factor . | Level . | n . | Univariable analysis HR (95% CI) . | P value . | MVA HR (95% CI) . | P value . |
|---|---|---|---|---|---|---|
| MIPI score at diagnosis | High risk | 30 | 2.58 (1.43-4.65) | .002 | 2.56 (1.43-4.65) | .002 |
| Low/intermediate risk | 31 | — | — | |||
| TP53 alterations at diagnosis∗ | Yes | 21 | 1.39 (0.73-2.66) | .317 | ||
| No | 22 | — | ||||
| MIPI score before venetoclax | High risk | 35 | 1.24 (0.72-2.11) | .437 | ||
| Low/intermediate risk | 32 | — | — | |||
| Complex karyotype before venetoclax∗ | Yes | 10 | 1.17 (0.47-2.91) | .742 | ||
| No | 17 | — | ||||
| TP53 alterations before venetoclax∗ | Yes | 10 | 1.28 (0.55-2.84) | .556 | ||
| No | 20 | — | ||||
| Blastoid/pleomorphic before venetoclax | Yes | 29 | 1.10 (0.64-1.89) | .732 | ||
| No | 35 | — | ||||
| Lines of treatment before venetoclax | >3 | 24 | 1.15 (0.69-1.92) | .587 | ||
| ≤3 | 56 | — | ||||
| POD24 | Yes | 39 | 1.49 (0.91-2.44) | .116 | ||
| No | 38 | — | ||||
| Best response to last therapy before venetoclax | CR, PR | 33 | 0.62 (0.37-1.03) | .065 | ||
| SD, PD | 41 | — | ||||
| Best response to BTKi | CR, PR | 45 | 0.83 (0.49-1.40) | .483 | ||
| SD, PD | 24 | — | ||||
| Duration of treatment with BTKi, mo | >6 | 36 | 0.68 (0.41-1.12) | .128 | ||
| ≤6 | 34 | — | ||||
| Reason for stopping BTKi | PD | 59 | 1.21 (0.58-2.52) | .611 | ||
| Toxicity | 12 | — | ||||
| Duration of treatment with last therapy before venetoclax, mo | >4 | 35 | 0.68 (0.42-1.11) | .125 | ||
| ≤4 | 43 | — | ||||
| Venetoclax highest dose received, mg | ≥400 | 59 | 1.20 (0.64-2.25) | .571 | ||
| <400 | 17 | — | ||||
| Venetoclax combined with other agents | Yes | 31 | 0.67 (0.40-1.10) | .115 | ||
| No | 49 | — |
| Factor . | Level . | n . | Univariable analysis HR (95% CI) . | P value . | MVA HR (95% CI) . | P value . |
|---|---|---|---|---|---|---|
| MIPI score at diagnosis | High risk | 30 | 2.58 (1.43-4.65) | .002 | 2.56 (1.43-4.65) | .002 |
| Low/intermediate risk | 31 | — | — | |||
| TP53 alterations at diagnosis∗ | Yes | 21 | 1.39 (0.73-2.66) | .317 | ||
| No | 22 | — | ||||
| MIPI score before venetoclax | High risk | 35 | 1.24 (0.72-2.11) | .437 | ||
| Low/intermediate risk | 32 | — | — | |||
| Complex karyotype before venetoclax∗ | Yes | 10 | 1.17 (0.47-2.91) | .742 | ||
| No | 17 | — | ||||
| TP53 alterations before venetoclax∗ | Yes | 10 | 1.28 (0.55-2.84) | .556 | ||
| No | 20 | — | ||||
| Blastoid/pleomorphic before venetoclax | Yes | 29 | 1.10 (0.64-1.89) | .732 | ||
| No | 35 | — | ||||
| Lines of treatment before venetoclax | >3 | 24 | 1.15 (0.69-1.92) | .587 | ||
| ≤3 | 56 | — | ||||
| POD24 | Yes | 39 | 1.49 (0.91-2.44) | .116 | ||
| No | 38 | — | ||||
| Best response to last therapy before venetoclax | CR, PR | 33 | 0.62 (0.37-1.03) | .065 | ||
| SD, PD | 41 | — | ||||
| Best response to BTKi | CR, PR | 45 | 0.83 (0.49-1.40) | .483 | ||
| SD, PD | 24 | — | ||||
| Duration of treatment with BTKi, mo | >6 | 36 | 0.68 (0.41-1.12) | .128 | ||
| ≤6 | 34 | — | ||||
| Reason for stopping BTKi | PD | 59 | 1.21 (0.58-2.52) | .611 | ||
| Toxicity | 12 | — | ||||
| Duration of treatment with last therapy before venetoclax, mo | >4 | 35 | 0.68 (0.42-1.11) | .125 | ||
| ≤4 | 43 | — | ||||
| Venetoclax highest dose received, mg | ≥400 | 59 | 1.20 (0.64-2.25) | .571 | ||
| <400 | 17 | — | ||||
| Venetoclax combined with other agents | Yes | 31 | 0.67 (0.40-1.10) | .115 | ||
| No | 49 | — |
Variables with <50% data capture were excluded from the MVA.
Univariable and MVAs of factors associated with OS
| Factor . | Level . | n . | Univariable analysis HR (95% CI) . | P value . | MVA HR (95% CI) . | P value . |
|---|---|---|---|---|---|---|
| MIPI score at diagnosis | High risk | 28 | 2.73 (1.26-5.88) | .011 | ||
| Low/intermediate risk | 29 | — | — | |||
| TP53 alterations at diagnosis∗ | Yes | 18 | 1.42 (0.63-3.14) | .398 | ||
| No | 22 | |||||
| MIPI score before venetoclax | High risk | 34 | 2.12 (1.03-4.36) | .041 | 2.36 (1.17-5.03) | .022 |
| Low/intermediate risk | 29 | — | — | |||
| Complex karyotype before venetoclax∗ | Yes | 9 | 2.04 (0.49-8.50) | .329 | ||
| No | 15 | — | ||||
| TP53 alterations before venetoclax∗ | Yes | 9 | 2.63 (0.85-8.12) | .097 | ||
| No | 19 | — | ||||
| Blastoid/pleomorphic before venetoclax | Yes | 27 | 1.77 (0.86-3.64) | .123 | ||
| No | 33 | — | ||||
| Number of lines of treatment before venetoclax | >3 | 23 | 1.19 (0.62-2.29) | .592 | ||
| ≤3 | 53 | — | ||||
| POD24 | Yes | 35 | 2.30 (1.22-4.45) | .012 | 3.81 (1.73-8.85) | .001 |
| No | 38 | — | ||||
| Best response to last therapy before venetoclax | CR, PR | 33 | 0.58 (0.29-1.16) | .125 | ||
| SD, PD | 37 | — | ||||
| Best response to BTKi | CR, PR | 43 | 0.80 (0.39-1.63) | .534 | ||
| SD, PD | 22 | — | ||||
| Duration of treatment with BTKi, mo | >6 | 35 | 0.81 (0.42-1.57) | .533 | ||
| ≤6 | 31 | — | ||||
| Reason for stopping BTKi | PD | 55 | 1.30 (0.50-3.40) | .590 | ||
| Toxicity | 12 | — | ||||
| Duration of treatment with last therapy before venetoclax, mo | >4 | 35 | 0.48 (0.25-0.92) | .026 | ||
| ≤4 | 39 | — | ||||
| Venetoclax highest dose received, mg | ≥400 | 55 | 0.97 (0.44-2.12) | .933 | ||
| <400 | 17 | — | ||||
| Venetoclax combined with other agent(s) | Yes | 31 | 0.58 (0.30-1.12) | .102 | 0.32 (0.14-0.70) | .007 |
| No | 45 | — |
| Factor . | Level . | n . | Univariable analysis HR (95% CI) . | P value . | MVA HR (95% CI) . | P value . |
|---|---|---|---|---|---|---|
| MIPI score at diagnosis | High risk | 28 | 2.73 (1.26-5.88) | .011 | ||
| Low/intermediate risk | 29 | — | — | |||
| TP53 alterations at diagnosis∗ | Yes | 18 | 1.42 (0.63-3.14) | .398 | ||
| No | 22 | |||||
| MIPI score before venetoclax | High risk | 34 | 2.12 (1.03-4.36) | .041 | 2.36 (1.17-5.03) | .022 |
| Low/intermediate risk | 29 | — | — | |||
| Complex karyotype before venetoclax∗ | Yes | 9 | 2.04 (0.49-8.50) | .329 | ||
| No | 15 | — | ||||
| TP53 alterations before venetoclax∗ | Yes | 9 | 2.63 (0.85-8.12) | .097 | ||
| No | 19 | — | ||||
| Blastoid/pleomorphic before venetoclax | Yes | 27 | 1.77 (0.86-3.64) | .123 | ||
| No | 33 | — | ||||
| Number of lines of treatment before venetoclax | >3 | 23 | 1.19 (0.62-2.29) | .592 | ||
| ≤3 | 53 | — | ||||
| POD24 | Yes | 35 | 2.30 (1.22-4.45) | .012 | 3.81 (1.73-8.85) | .001 |
| No | 38 | — | ||||
| Best response to last therapy before venetoclax | CR, PR | 33 | 0.58 (0.29-1.16) | .125 | ||
| SD, PD | 37 | — | ||||
| Best response to BTKi | CR, PR | 43 | 0.80 (0.39-1.63) | .534 | ||
| SD, PD | 22 | — | ||||
| Duration of treatment with BTKi, mo | >6 | 35 | 0.81 (0.42-1.57) | .533 | ||
| ≤6 | 31 | — | ||||
| Reason for stopping BTKi | PD | 55 | 1.30 (0.50-3.40) | .590 | ||
| Toxicity | 12 | — | ||||
| Duration of treatment with last therapy before venetoclax, mo | >4 | 35 | 0.48 (0.25-0.92) | .026 | ||
| ≤4 | 39 | — | ||||
| Venetoclax highest dose received, mg | ≥400 | 55 | 0.97 (0.44-2.12) | .933 | ||
| <400 | 17 | — | ||||
| Venetoclax combined with other agent(s) | Yes | 31 | 0.58 (0.30-1.12) | .102 | 0.32 (0.14-0.70) | .007 |
| No | 45 | — |
Variables with <50% data capture were excluded from the MVA.
In a MVA (Table 4), high-risk MIPI scores at diagnosis (HR, 2.56; 95% CI, 1.43-4.65; P = .002) was associated with inferior PFS. In a MVA for OS (Table 5), high-risk MIPI scores before venetoclax (HR, 2.36; 95% CI, 1.17-5.03; P = .022) and POD24 (HR, 3.81; 95% CI, 1.73-8.85; P = .001) were associated with inferior OS whereas receiving venetoclax combination (vs monotherapy) (HR, 0.32; 95% CI, 0.14-0.70; P = .007) was associated with superior OS.
TLS
Laboratory TLS occurred in 10 patients (12.3%) including 3 (3.7%) with clinical TLS. Variables related to TLS for the overall cohort and for patients who developed TLS are shown in Table 6. For the overall cohort, most patients had low (61%) or intermediate (32%) risk for TLS, 53% were admitted at least once for TLS monitoring, and the majority received prophylactic allopurinol (90%) and/or IV fluids (67%). For patients who developed TLS, the median age was 66 years (range, 57-79), 3 had low risk and 5 had intermediate risk for TLS (missing data for 2 patients), 7 received venetoclax monotherapy and 3 received venetoclax in combination with other agents, and 7 were admitted for TLS monitoring at least once. The venetoclax dose at which the first TLS event occurred was 20 mg in 7 patients and 50 mg in 2 patients (missing data for 1 patient). In terms of TLS-related biochemical abnormalities (data available for all 10 patients with TLS), phosphate levels of ≥4.5 mg/dL occurred in 8 patients, uric acid ≥8 mg/dL in 4 patients, calcium ≤7 mg/dL in 4 patients, potassium ≥6 mmol/L in 3 patients, and creatinine ≥2 mg/dL in 3 patients. Six patients required rasburicase. Six patients developed TLS and had data available for response assessment, of whom 3 responded (2 CR, 1 PR, 1 SD, and 2 PD).
Variables related to TLS for the overall cohort and patients who developed TLS
| Variable (before venetoclax) . | Overall cohort, N = 81 (%) . | Patients with TLS, n = 10 (%) . |
|---|---|---|
| ALC (cells per uL), ≥25 000 | 5 (6.2) | 2 (20.0) |
| Any lymph node >5 cm | 26 (35.6) | 3 (42.9) |
| Any lymph node >10 cm | 6 (8.3) | 0 |
| Spleen size, median (range), cm | 16 (12-30) | 18 (15-27) |
| TLS risk | ||
| Low | 45 (60.8) | 3 (30.0) |
| Intermediate | 24 (32.4) | 5 (50.0) |
| High | 5 (6.8) | 0 |
| Venetoclax starting dose, mg | ||
| 20 | 66 (84.6) | 8 (88.9) |
| 50 | 7 (9.0) | 1 (11.1) |
| 100 | 2 (2.6) | 0 |
| ≥200 | 3 (3.8) | 0 |
| Inpatient monitoring for TLS | ||
| Yes, for the first dose level only | 14 (17.7) | 2 (20.0) |
| Yes, for the first 2 dose levels only | 12 (15.2) | 1 (10.0) |
| Yes, for ≥3 dose levels | 16 (20.3) | 4 (40.0) |
| No | 37 (46.8) | 3 (30.0) |
| Prophylactic allopurinol administered | 71 (89.9) | 9 (90.0) |
| Prophylactic IV fluids administered | 51 (67.1) | 9 (90.0) |
| Venetoclax dose at which TLS occurred, mg | ||
| 20 | — | 7 (77.8) |
| 50 | 2 (22.2) | |
| Venetoclax combined with other agent(s) | ||
| Combination | 31 (38.2) | 3 (30.0) |
| Venetoclax monotherapy | 50 (61.7) | 7 (70.0) |
| TLS-related biochemical abnormalities | ||
| Peak potassium, mmol/L | ||
| Median (range) | — | 4.8 (4.4-7.1) |
| ≥6 | 3 (30.0) | |
| Peak phosphorus, mg/dL | ||
| Median (range) | 5.6 (3.2-11.0) | |
| ≥4.5 | 8 (80.0) | |
| Peak uric acid, mg/dL | ||
| Median (range) | 6.8 (3.5-12.3) | |
| ≥8 | 4 (40.0) | |
| Nadir calcium, mg/dL | ||
| Median (range) | 7.8 (6.6-8.7) | |
| ≤7 | 4 (40.0) | |
| Peak creatinine, mg/dL | ||
| Median (range) | 1.5 (0.7-6.2) | |
| ≥2 | 3 (30.0) | |
| Received rasburicase | — | 6 (60) |
| Variable (before venetoclax) . | Overall cohort, N = 81 (%) . | Patients with TLS, n = 10 (%) . |
|---|---|---|
| ALC (cells per uL), ≥25 000 | 5 (6.2) | 2 (20.0) |
| Any lymph node >5 cm | 26 (35.6) | 3 (42.9) |
| Any lymph node >10 cm | 6 (8.3) | 0 |
| Spleen size, median (range), cm | 16 (12-30) | 18 (15-27) |
| TLS risk | ||
| Low | 45 (60.8) | 3 (30.0) |
| Intermediate | 24 (32.4) | 5 (50.0) |
| High | 5 (6.8) | 0 |
| Venetoclax starting dose, mg | ||
| 20 | 66 (84.6) | 8 (88.9) |
| 50 | 7 (9.0) | 1 (11.1) |
| 100 | 2 (2.6) | 0 |
| ≥200 | 3 (3.8) | 0 |
| Inpatient monitoring for TLS | ||
| Yes, for the first dose level only | 14 (17.7) | 2 (20.0) |
| Yes, for the first 2 dose levels only | 12 (15.2) | 1 (10.0) |
| Yes, for ≥3 dose levels | 16 (20.3) | 4 (40.0) |
| No | 37 (46.8) | 3 (30.0) |
| Prophylactic allopurinol administered | 71 (89.9) | 9 (90.0) |
| Prophylactic IV fluids administered | 51 (67.1) | 9 (90.0) |
| Venetoclax dose at which TLS occurred, mg | ||
| 20 | — | 7 (77.8) |
| 50 | 2 (22.2) | |
| Venetoclax combined with other agent(s) | ||
| Combination | 31 (38.2) | 3 (30.0) |
| Venetoclax monotherapy | 50 (61.7) | 7 (70.0) |
| TLS-related biochemical abnormalities | ||
| Peak potassium, mmol/L | ||
| Median (range) | — | 4.8 (4.4-7.1) |
| ≥6 | 3 (30.0) | |
| Peak phosphorus, mg/dL | ||
| Median (range) | 5.6 (3.2-11.0) | |
| ≥4.5 | 8 (80.0) | |
| Peak uric acid, mg/dL | ||
| Median (range) | 6.8 (3.5-12.3) | |
| ≥8 | 4 (40.0) | |
| Nadir calcium, mg/dL | ||
| Median (range) | 7.8 (6.6-8.7) | |
| ≤7 | 4 (40.0) | |
| Peak creatinine, mg/dL | ||
| Median (range) | 1.5 (0.7-6.2) | |
| ≥2 | 3 (30.0) | |
| Received rasburicase | — | 6 (60) |
Missing data for overall cohort: lymph node >5 cm, n = 8; lymph node >10 cm, n = 9; spleen size, n = 54; TLS risk, n = 7; venetoclax starting dose, n = 3; inpatient monitoring for TLS, n = 2; prophylactic allopurinol given, n = 2; and prophylactic IV fluids given, n = 5.
Missing data for patients with TLS: lymph node >5 cm, n = 3; lymph node >10 cm, n = 3; spleen size, n = 6; TLS risk, n = 2; venetoclax starting dose, n = 1; and venetoclax dose at which TLS occurred, n = 1.
Discussion
Our study cohort is enriched with patients with high-risk disease features including a high proportion of patients with high-risk MIPI scores, high Ki-67, blastoid/pleomorphic histology, complex karyotype, and TP53 alterations. Patients were heavily pretreated (median of 3 prior treatments) including previous BTKi therapy in the majority (91%). Furthermore, the short remission with first-line treatment (median, 1.6 years; POD24 in 50% of patients) and duration of treatment with BTKis (median, 6 months) reflect aggressive disease biology. In these patients with high-risk MCL, venetoclax resulted in an ORR of 40% and median PFS and OS of 3.7 and 12.5 months, respectively. Our results are in line with 2 smaller retrospective studies by Eyre et al (n = 20) and Zhao et al (n = 24) of venetoclax in patients previously treated with BTKis, showing ORR ranging from 50% to 53% and median PFS ranging from 3 to 8 months.15,16
Our cohort included 31 patients (38%) treated with venetoclax in combination with other agents, most commonly a BTKi (n = 16, 20%) or an anti-CD20 monoclonal antibody (n = 11, 14%). Combination therapy was not associated with a statistically significant improvement in ORR or PFS but was associated with superior OS in MVA. Notably, of the 16 patients in our study treated with venetoclax in combination with a BTKi, 10 (63%) had received prior treatment with a BTKi and had poor responses to the BTKi (ORR = 40%; median duration of treatment, 7 months). Continuing treatment with BTKis beyond progression is commonly done in MCL to avoid disease flare, which can be particularly important during the dose ramp-up of venetoclax. It is unclear whether continuing BTKi treatment after the venetoclax target dose has been reached adds benefit in patients whose MCL did not respond to, or progressed on, a BTKi. The synergistic effect of dual inhibition of BCL-2 and BTK seen in preclinical studies,21,22 minor overlapping toxicities between venetoclax and BTKis, and promising early clinical data of venetoclax and BTKi combinations may provide a rationale for this approach.23,24 In our study, the low ORR (33%) with the combination of venetoclax and a BTKi in the 10 patients previously treated with a BTKi, of whom 6 had SD/PD as the best response to the BTKi, does not support this practice, although we acknowledge the very small sample size. However, we are unable to comment on the relative efficacy of BTKi plus venetoclax in patients not previously treated with BTKi as compared with venetoclax monotherapy. Other factors associated with outcomes in MVA in our study were having a high-risk MIPI score at diagnosis, which was associated with inferior PFS, and a high-risk MIPI score before venetoclax therapy, being associated with inferior OS. As reported in other studies,20,25,26 shorter time to first relapse/progression (POD24) identifies a group of patients with MCL with worse outcomes and was associated with inferior OS in our study. In addition, receipt of fewer prior treatments was associated with higher odds of response to venetoclax in a univariable analysis. A similar observation was reported in a pooled analysis of patients with relapsed MCL treated with ibrutinib in which the receipt of fewer prior lines of treatment was associated with better outcomes with ibrutinib.27 Overall, our data support the evaluation of venetoclax in earlier lines of treatment in MCL, possibly in combination with other agents. Two nonrandomized clinical trials of venetoclax in combination with ibrutinib with or without obinutuzumab in patients with MCL not, previously treated with BTKis showed superior outcomes with combination therapy compared with historical controls of patients treated with BTKi monotherapy.23,24 The ongoing SYMPATICO (#NCT03112174) phase 3 clinical trial is evaluating ibrutinib plus venetoclax combination vs ibrutinib monotherapy in patients with relapsed MCL. In addition, venetoclax has shown encouraging early results when combined with lenalidomide and rituximab in the frontline and relapsed settings in MCL.28,29
A 5- to 6-week stepwise dose ramp-up starting at 20 mg has been proposed for patients with MCL who initiate treatment with venetoclax to mitigate the risk of TLS after reports of clinically significant TLS in patients with MCL who initiated venetoclax at doses ranging from 50 to 100 mg.14 We report laboratory TLS in 12.3% of our patients, including 3.7% with clinical TLS, despite several mitigation strategies including stepwise dose ramp-up (89% of patients who developed TLS started with 20 mg dose), prophylactic IV fluids and allopurinol in most patients, and inpatient monitoring for TLS in more than half of the patients (53%). Notably, even patients at low risk developed TLS, highlighting the limitations of the criteria currently used to define TLS risk categories in MCL and the importance of close monitoring of all patients with MCL who initiate treatment with venetoclax. The rapid disease progression on venetoclax in our study, as evident from the short duration of treatment (median, 2.8 months) and that the highest dose received of venetoclax was ≤200 mg in 22% of patients, raises the question of whether delays in reaching effective venetoclax doses contributed to the poor outcomes observed. These data highlight the challenge of achieving disease control in relapsed MCL, which typically has high proliferative capacity, particularly after BTKi therapy, and at the same time minimizing the risk of TLS by following a stepwise dose ramp-up schedule of venetoclax. Combining venetoclax with other active agents can be of use in this setting to achieve disease control that allows gradual venetoclax dose escalation.
In addition to its retrospective nature, our study has several limitations. Chart review was limited by missing data. Treatment with venetoclax was not uniform and varied in terms of dose ramp-up schedule, target dose, and its use as monotherapy vs a combination therapy. MVA might have been limited by the relatively small sample size and missing data.
In summary, in this cohort of patients with MCL at high risk and who were heavily pretreated, of whom most had received previous BTKi treatment, venetoclax resulted in a good ORR but short PFS. Venetoclax may have a better role in MCL in earlier lines of treatment and possibly in combination with other agents. However, effective treatment options following BTKi treatment failure in MCL are limited, and these outcomes with venetoclax compare favorably with those achieved with other currently available treatments (lenalidomide: ORR, 29%; phosphoinositide 3-kinase inhibitors: ORR, 25%),30,31 which make venetoclax a reasonable option as a bridge to CAR T cells or allogeneic HCT in eligible patients or for those who cannot receive intensive treatments. Results of ongoing clinical trials of novel agents in relapsed MCL such as the bispecific antibodies, noncovalent BTKis, antibody-drug conjugates, and others are eagerly awaited.32-34 Finally, TLS remains an important risk with venetoclax in relapsed MCL.
Acknowledgments
This work was supported, in part, by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute, Emory University, the National Institutes of Health, National Cancer Institute (P30CA138292), and by the Center for Clinical and Translational Science Bioinformatics Shared Resource, The Ohio State University (UL1TR002733).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: Y.S. and J.B.C. conceived and designed the study, collected and analyzed data, and wrote the manuscript; S. Goyal and J.M.S. performed statistical analyses and wrote the manuscript; J.T.R., M.K., I.B.G., B.T.H., K.M.I., C.A.P., A.M., S. Goldsmith, N.S.G., P.A.R., R.K., M. Burkart, M. Buege, O.A., P.T., A.K., B.T.H., and B.S.K. collected and analyzed the data and wrote and reviewed the manuscript.
Conflict-of-interest disclosure: A.K. has received research funding from AbbVie, Adaptive Biotechnologies, Celgene, Pharmacyclics, Loxo/Lily Pharmaceuticals, and Seattle Genetics; has received honoraria from AstraZeneca, Kite Pharmaceuticals, Janssen, Genentech, and Loxo/Lily Pharmaceuticals; and has consulted for Genentech. B.S.K. has received research funding from AbbVie, Genentech, Celgene, AstraZeneca, ADC Therapeutics (ADCT) and consulting fees from AbbVie, Bristol Myers Squibb (BMS), BeiGene, ADCT, Genentech, MEI, Janssen, Kite, and TG Therapeutics. B.T.H. has received research funding from, and consulted for, AbbVie and Genentech. C.A.P. has received research funding from AbbVie, TG Therapeutics, Acerta/AstraZeneca, BeiGene, and Merck and has consulted for AbbVie, BeiGene, Jansen, Pharmacyclics, Kite/Gilead, TG Therapeutics, and Merck. J.B.C. has received research funding from AstraZeneca, BMS/Celgene, Novartis, Takeda, Genentech, M2Gen and has consulted for Kite/Gilead, Loxo/Lilly, Hutch-Med, Adaptive, BeiGene, Pharmacyclics, AbbVie, and AstraZeneca. M.K. has received research funding from Novartis; consulted for AbbVie, AstraZeneca, Celgene/BMS, Adaptive Biotechnologies, ADCT, BeiGene, Genentech, ImpactBio, and Syncopation; served as a member of a speaker’s bureau for SeaGen; and served as a member of data monitoring committee for Celgene and Genentech. N.S.G. has received research funding from Genentech and has consulted for ADCT, KITE, Novartis, and Tessa Therapeutics. R.K. has received research funding from Celgene Corporation/Juno Therapeutics/BMS, Takeda, BeiGene, and Gilead Sciences/Kite; served as a member of an advisory board for Celgene Corporation, Gilead Sciences, Juno Therapeutics, Kite Pharma, Janssen, Karyopharm, Pharmacyclics, MorphoSys, and Epizyme; and served as a member of a speaker’s bureau for AstraZeneca, BeiGene, and MorphoSys. P.A.R. has received research funding from BMS, Kite Pharma, Inc/Gilead, MorphoSys, Calibr, Tessa Therapeutics, Fate Therapeutics, Xencor, and Novartis Pharmaceuticals Corporation; has served as a member of a speaker’s bureau for Kite Pharma, Inc/Gilead; has served as a member of an advisory board for AbbVie, Novartis Pharmaceuticals Corporation, BMS, Janssen, BeiGene, ADCT, Takeda Pharmaceutical Company, Kite Pharma, Inc/Gilead, Sana Biotechnology, Nektar Therapeutics, Nurix Therapeutics, Intellia Therapeutics, CVC Caremark, and Genmab; and has received honoraria from Novartis Pharmaceuticals Corporation. P.T. has consulted for Genentech, Genmab, TG Therapeutics, ADCT, and Seagen and Lilly USA. Y.S. has received research funding from BMS, Celgene, TG Therapeutics, and BeiGene and has consulted for TG Therapeutics and Epizyme. The remaining authors declare no competing financial interests.
Correspondence: Yazeed Sawalha, Division of Hematology, Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, 1140B Lincoln Tower, 1800 Cannon Dr, Columbus, OH 43210; e-mail: yazeed.sawalha@osumc.edu.
References
Author notes
Data are available on request from the corresponding author, Yazeed Sawalha (yazeed.sawalha@osumc.edu).
The full-text version of this article contains a data supplement.