TO THE EDITOR:
Regulatory T cells (Tregs) characterized by cell surface expression of CD4+, CD25+, CD127low, and high expression of intracellular FOXP3+ compromise 1% to 2% of total lymphocytes.1 Tregs can suppress exuberant immune responses as observed in coronavirus disease 2019 (COVID-19) associated acute respiratory distress syndrome (ARDS),2 and clear residual inflammatory cells in the lung.3,4 Lyu et al demonstrated that multiple injections of allogeneic Treg cells derived from umbilical cord blood (UCB) can decrease CD8+ pathogenic T cells in vivo leading to resolution of pulmonary inflammation.5 Gladstone et al reported that multiple infusions of allogeneic UCB Tregs at a fixed dose of 100 million cells6 was associated with clinical improvement which correlated with decrease in inflammatory burden in 2 patients with COVID-19 ARDS who had multiorgan failure requiring vasopressors and hemodialysis.7
Based on these data, we performed a phase 1, randomized, multicenter, double-blinded, placebo-controlled clinical trial (www.clinicaltrials.gov #NCT04468971) in patients with COVID-19 ARDS to examine the safety and early efficacy of CK0802, an off-the-shelf, cryopreserved, allogeneic UCB Treg cell product that does not require human leukocyte antigen matching. Eligibility criteria included diagnosis of SARS-CoV-2 infection, moderate-to-severe ARDS,8 intubated <120 hours, age ≥18 years, and ability to obtain informed consent. Patients were randomized in a 1:1:1 ratio to placebo, 100 million Tregs (CK0802-100), or 300 million Tregs (CK0802-300) per infusion on day 0 (ie, day of first infusion), day 3(±1), and day 7(±1), constrained to 15 patients in each arm (total n = 45). The trial was approved by the institutional review board and conducted in accordance with the Declaration of Helsinki.
The CK0802 product was generated from 8 manufacturing campaigns (average 4429 × 106 Treg cells per run [range, 1137-11 349 ×106]), which were subsequently cryopreserved. All products met the release criteria of CD4+CD25+>60% and CD4-CD8+<10%. The placebo consisted of an excipient, a DMSO-containing solution. Blinded, thawed product bags were hung by qualified hospital staff and administered intravenously by gravity for 15 to 20 minutes.
Based on the request of the US FDA during IND filing, we included an efficacy end point in the study. Thus, the 2 coprimary outcomes were: (1) TOX: grade ≥3 regimen-related NCI-CTCAE V4.0 toxicity within 48 hours of infusion; and (2) S28: patient alive and not intubated 28 days after the first infusion.
We recruited 45 critically ill patients during the peak of the pandemic (September 2020 to April 2021), with the majority on vasopressors, and previously treated with remdesivir and/or corticosteroids.9 Patients in the CK0802-300 arm had higher baseline serum IL-6, CRP, and body mass index, and lower PaO2:FiO2 and Glasgow Coma scores (Table 1).
Summary of baseline characteristics, overall, and by treatment arm of the safety population
| Variable . | Overall (N = 45) . | Placebo (N = 15) . | CK0802-100 (N = 15) . | CK0802-300 (N = 15) . |
|---|---|---|---|---|
| Age (y), median (min-max) | 60 (21-85) | 63 (21-77) | 64 (29-85) | 55 (27-71) |
| Age (y), n (%) | ||||
| <60 | 24 (53.3) | 8 (53.3) | 7 (46.7) | 9 (60.0) |
| ≥60 | 21 (46.7) | 7 (46.7) | 8 (53.3) | 6 (40.0) |
| Sex, n (%) | ||||
| Female | 18 (40.0) | 7 (46.7) | 5 (33.3) | 6 (40.0) |
| Male | 27 (60.0) | 8 (53.3) | 10 (66.7) | 9 (60.0) |
| Race, n (%) | ||||
| Black | 10 (22.2) | 4 (26.7) | 4 (26.7) | 2 (13.3) |
| Hispanic | 8 (17.8) | 4 (26.7) | 3 (20.0) | 1 (6.7) |
| Other | 5 (11.1) | 2 (13.3) | 0 (0.0) | 3 (20.0) |
| White | 22 (48.9) | 5 (33.3) | 8 (53.3) | 9 (60.0) |
| Hemodialysis, n (%) | ||||
| Yes | 4 (8.9) | 2 (13.3) | 1 (6.7) | 1 (6.7) |
| Vasopressor, n (%) | ||||
| Yes | 27 (60.0) | 7 (46.7) | 12 (80.0) | 8 (53.3) |
| Full anticoagulation, n (%) | ||||
| Yes | 22 (48.9) | 8 (53.3) | 6 (40) | 8 (53.3) |
| Time from diagnosis to intubation, d, median (range) | 7 (0-18) | 8 (0-16) | 5 (0-15) | 6 (0-18) |
| Time from intubation to first infusion, h, median (range) | 72 (0-144) | 72 (0-120) | 48 (24-144) | 72 (24-120) |
| Body mass index, median, (min-max) | 30 (20-74) | 30 (22-42) | 28 (21-51) | 34 (20-74) |
| PaO2:FiO2 ratio, median, (min-max) | 142 (66-248) | 174 (90-230) | 142 (66-248) | 114 (77-200) |
| Comorbidities, n (%) | ||||
| Hypertension | 25 (56.8) | 11 (73.3) | 6 (42.9) | 8 (53.3) |
| Coronary artery disease | 13 (28.9) | 4 (26.7) | 6 (40.0) | 3 (20.0) |
| Diabetes | 13 (28.9) | 4 (26.7) | 5 (33.3) | 4 (26.7) |
| Chronic kidney disease | 4 (8.9) | 1 (6.7) | 1 (6.7) | 2 (13.3) |
| GERD | 12 (26.7) | 2 (13.3) | 6 (40.0) | 4 (26.7) |
| Hyperlipidemia | 10 (22.2) | 3 (20.0) | 4 (26.7) | 3 (20.0) |
| COPD/asthma | 6 (13.3) | 3 (20.0) | 1 (6.7) | 2 (13.3) |
| WHO ordinal score median, (min-max) | 7 (6-8) | 7 (6-8) | 7 (6-8) | 7 (6-8) |
| SOFA score median, (min-max) | 9 (6-16) | 8 (6-16) | 10 (6-15) | 8 (6-14) |
| Mean arterial pressure, (mmHg) median, (min-max) | 74 (14-107) | 74 (14-89) | 74 (56-103) | 72 (20-107) |
| Glasgow coma score, median, (min-max) | 5 (3-10) | 7 (3-10) | 6 (3-10) | 3 (3-10) |
| Measures of inflammation | ||||
| CRP, mg/L, median, (min-max), missing | 84.1 (14.7-317.8), 2 | 88.5 (27-317.8), 1 | 58.5 (14.7-212.0), 1 | 105.5 (23.9-313.0), 0 |
| Ferritin, ng/mL, median, (min-max), missing | 692 (42-21068), 6 | 767 (216-21068), 1 | 572 (42-2220), 4 | 626 (179-21021), 1 |
| D-dimer, mg/L, median, (min-max), missing | 2.2 (0.32-30), 3 | 1.7 (0.53-20.82), 1 | 2.78 (0.32-30), 2 | 2.3 (0.42-19.44), 0 |
| Procalcitonin, ng/mL, median, (min-max), missing | 0.26 (0.05-38.22), 7 | 0.57 (0.15-34.97), 3 | 0.2 (0.1-1.90) | 0.22 (0.05-38.22), 1 |
| IL-6, pg/mL, median, (min-max), missing | 43.9 (2.7-3409), 12 | 70.3 (8.5-669), 3 | 38.2 (2.7-992), 4 | 427.32 (4.3-3409), 5 |
| Immunogenicity | ||||
| HLA antibodies-type 1, positive, n (%), missing | 16 (39), 4 | 6 (40), 0 | 5 (38.4), 2 | 5 (38.4), 2 |
| HLA antibodies-type 2, positive, n (%), missing | 21 (51.2), 4 | 8 (53.3), 0 | 7 (46.7), 2 | 6 (40), 2 |
| COVID treatments (before first infusion) | ||||
| Dexamethasone, n (%) | 42 (93.3) | 15 (100) | 14 (93.3) | 13 (86.7) |
| Dexamethasone duration, d, median, (min-max), missing | 4.5, (0-16), 2 | 5, (1-16), 0 | 4.5, (1-14), 1 | 4 (0-14), 10 |
| Dexamethasone duration > 5 d before first infusion, n (%) | 16 (35.6) | 6 (40) | 6 (40) | 4 (26.7) |
| Remdesivir, n (%) | 40 (88.9) | 14 (93.3) | 12 (80) | 14 (93.3) |
| Remdesivir duration, d, median, (min-max), missing | 4 (0-15), 5 | 4 (0-15), 1 | 4.5 (1-14), 3 | 4.5 (1-15), 1 |
| Remdesivir duration >5 d before first infusion, n (%), missing | 13 (32.5), 5 | 4 (28.6), 1 | 4 (33.3), 3 | 5 (35.7), 1 |
| Dexamethasone and remdesivir, n (%) | 38 (84.4) | 14 (93.3%) | 12 (80) | 12 (80) |
| Convalescent plasma, n (%) | 4 (8.9) | 2 (13.3) | 0 | 2 (13.3) |
| Tocilizumab, n (%) | 7 (15.6) | 1 (6.7) | 2 (13.3) | 4 (26.7) |
| Variable . | Overall (N = 45) . | Placebo (N = 15) . | CK0802-100 (N = 15) . | CK0802-300 (N = 15) . |
|---|---|---|---|---|
| Age (y), median (min-max) | 60 (21-85) | 63 (21-77) | 64 (29-85) | 55 (27-71) |
| Age (y), n (%) | ||||
| <60 | 24 (53.3) | 8 (53.3) | 7 (46.7) | 9 (60.0) |
| ≥60 | 21 (46.7) | 7 (46.7) | 8 (53.3) | 6 (40.0) |
| Sex, n (%) | ||||
| Female | 18 (40.0) | 7 (46.7) | 5 (33.3) | 6 (40.0) |
| Male | 27 (60.0) | 8 (53.3) | 10 (66.7) | 9 (60.0) |
| Race, n (%) | ||||
| Black | 10 (22.2) | 4 (26.7) | 4 (26.7) | 2 (13.3) |
| Hispanic | 8 (17.8) | 4 (26.7) | 3 (20.0) | 1 (6.7) |
| Other | 5 (11.1) | 2 (13.3) | 0 (0.0) | 3 (20.0) |
| White | 22 (48.9) | 5 (33.3) | 8 (53.3) | 9 (60.0) |
| Hemodialysis, n (%) | ||||
| Yes | 4 (8.9) | 2 (13.3) | 1 (6.7) | 1 (6.7) |
| Vasopressor, n (%) | ||||
| Yes | 27 (60.0) | 7 (46.7) | 12 (80.0) | 8 (53.3) |
| Full anticoagulation, n (%) | ||||
| Yes | 22 (48.9) | 8 (53.3) | 6 (40) | 8 (53.3) |
| Time from diagnosis to intubation, d, median (range) | 7 (0-18) | 8 (0-16) | 5 (0-15) | 6 (0-18) |
| Time from intubation to first infusion, h, median (range) | 72 (0-144) | 72 (0-120) | 48 (24-144) | 72 (24-120) |
| Body mass index, median, (min-max) | 30 (20-74) | 30 (22-42) | 28 (21-51) | 34 (20-74) |
| PaO2:FiO2 ratio, median, (min-max) | 142 (66-248) | 174 (90-230) | 142 (66-248) | 114 (77-200) |
| Comorbidities, n (%) | ||||
| Hypertension | 25 (56.8) | 11 (73.3) | 6 (42.9) | 8 (53.3) |
| Coronary artery disease | 13 (28.9) | 4 (26.7) | 6 (40.0) | 3 (20.0) |
| Diabetes | 13 (28.9) | 4 (26.7) | 5 (33.3) | 4 (26.7) |
| Chronic kidney disease | 4 (8.9) | 1 (6.7) | 1 (6.7) | 2 (13.3) |
| GERD | 12 (26.7) | 2 (13.3) | 6 (40.0) | 4 (26.7) |
| Hyperlipidemia | 10 (22.2) | 3 (20.0) | 4 (26.7) | 3 (20.0) |
| COPD/asthma | 6 (13.3) | 3 (20.0) | 1 (6.7) | 2 (13.3) |
| WHO ordinal score median, (min-max) | 7 (6-8) | 7 (6-8) | 7 (6-8) | 7 (6-8) |
| SOFA score median, (min-max) | 9 (6-16) | 8 (6-16) | 10 (6-15) | 8 (6-14) |
| Mean arterial pressure, (mmHg) median, (min-max) | 74 (14-107) | 74 (14-89) | 74 (56-103) | 72 (20-107) |
| Glasgow coma score, median, (min-max) | 5 (3-10) | 7 (3-10) | 6 (3-10) | 3 (3-10) |
| Measures of inflammation | ||||
| CRP, mg/L, median, (min-max), missing | 84.1 (14.7-317.8), 2 | 88.5 (27-317.8), 1 | 58.5 (14.7-212.0), 1 | 105.5 (23.9-313.0), 0 |
| Ferritin, ng/mL, median, (min-max), missing | 692 (42-21068), 6 | 767 (216-21068), 1 | 572 (42-2220), 4 | 626 (179-21021), 1 |
| D-dimer, mg/L, median, (min-max), missing | 2.2 (0.32-30), 3 | 1.7 (0.53-20.82), 1 | 2.78 (0.32-30), 2 | 2.3 (0.42-19.44), 0 |
| Procalcitonin, ng/mL, median, (min-max), missing | 0.26 (0.05-38.22), 7 | 0.57 (0.15-34.97), 3 | 0.2 (0.1-1.90) | 0.22 (0.05-38.22), 1 |
| IL-6, pg/mL, median, (min-max), missing | 43.9 (2.7-3409), 12 | 70.3 (8.5-669), 3 | 38.2 (2.7-992), 4 | 427.32 (4.3-3409), 5 |
| Immunogenicity | ||||
| HLA antibodies-type 1, positive, n (%), missing | 16 (39), 4 | 6 (40), 0 | 5 (38.4), 2 | 5 (38.4), 2 |
| HLA antibodies-type 2, positive, n (%), missing | 21 (51.2), 4 | 8 (53.3), 0 | 7 (46.7), 2 | 6 (40), 2 |
| COVID treatments (before first infusion) | ||||
| Dexamethasone, n (%) | 42 (93.3) | 15 (100) | 14 (93.3) | 13 (86.7) |
| Dexamethasone duration, d, median, (min-max), missing | 4.5, (0-16), 2 | 5, (1-16), 0 | 4.5, (1-14), 1 | 4 (0-14), 10 |
| Dexamethasone duration > 5 d before first infusion, n (%) | 16 (35.6) | 6 (40) | 6 (40) | 4 (26.7) |
| Remdesivir, n (%) | 40 (88.9) | 14 (93.3) | 12 (80) | 14 (93.3) |
| Remdesivir duration, d, median, (min-max), missing | 4 (0-15), 5 | 4 (0-15), 1 | 4.5 (1-14), 3 | 4.5 (1-15), 1 |
| Remdesivir duration >5 d before first infusion, n (%), missing | 13 (32.5), 5 | 4 (28.6), 1 | 4 (33.3), 3 | 5 (35.7), 1 |
| Dexamethasone and remdesivir, n (%) | 38 (84.4) | 14 (93.3%) | 12 (80) | 12 (80) |
| Convalescent plasma, n (%) | 4 (8.9) | 2 (13.3) | 0 | 2 (13.3) |
| Tocilizumab, n (%) | 7 (15.6) | 1 (6.7) | 2 (13.3) | 4 (26.7) |
None of the patients in any arm had a grade ≥3 toxicity related to the investigational agent. Assuming beta(.4, .6) before Pr(toxicity) in each arm, the mean posterior probability (95% posterior credible interval [CI]) of toxicity was 2.5% (95% posterior CI, 0.0-13.5). No increase in infection rates was reported in CK0802 recipients compared with placebo. The absence of dose-limiting toxicities associated with 85 allogeneic Treg cell infusions in a very ill population suggests an acceptable safety profile.
Baseline human leukocyte antigens (HLA) antibodies and day 28 HLA antibody screens using panel reactive antibodies were evaluated by flow cytometry using the solid phase assay.10 At baseline, positive HLA1 and HLA2 antibodies were detected in 16 and 21 patients, respectively. Positive seroconversions were seen in 4 for HLA1 antibodies (placebo = 2; CK0802-100 = 2) and 1 with HLA2 antibodies in the CK0802 to 300 arm. Seroconversions for cross-matched donor-specific antibodies were reported in only 3 patients, all of whom had received products derived from the same donor; on day 28, all patients were alive and 1 was extubated. Therefore, our data support the feasibility of multiple infusions of “off-the-shelf,” non-HLA matched, CB Treg cells without any significant induction of immunogenicity or impact on clinical outcome.
Among the 30 patients who received >2 infusions of CK0802, 26 received all products derived from the same donor and 4 received products derived from >2 donors. HLA chimerism analysis performed on CD3+-sorted cells from peripheral blood on days 3, 7, 11, and 28 did not detect any contribution from donor haplotypes, regardless of the number or source of infused products. This is not surprising, as Tregs are known to leave the peripheral circulation and traffic to areas of active inflammation,11 based partly on the survival signal of surplus IL-2 at the site of tissue destruction, which is largely the lung in patients with ARDS. Because of COVID restrictions, we were unable to obtain bronchoalveolar samples to pursue donor Treg cell detection.
S28 was achieved in 9, 9, and 6 patients in the placebo, CK0802-100, and CK0803-300 arms, respectively. Bayesian regression analyses with covariates (age, gender, race, vasopressors, oxygenation, and duration of intubation) defined before unblinding demonstrated 89.7% probability of beneficial effect (PBE) for S28 for CK0802-100 vs placebo (95% CI, −0.71 to 3.54), and 28.4% for CK0802-300 vs placebo (95% CI, −2.7 to 1.5). The magnitude of PBE that is considered to be meaningfully large is subjective when a noninformative prior is used, as in this study. In this situation, a cutoff of 99% is often chosen to indicate a meaningfully large effect. Our results were lower than this value and should not be viewed as demonstrating the efficacy of the 100 million cell dose.
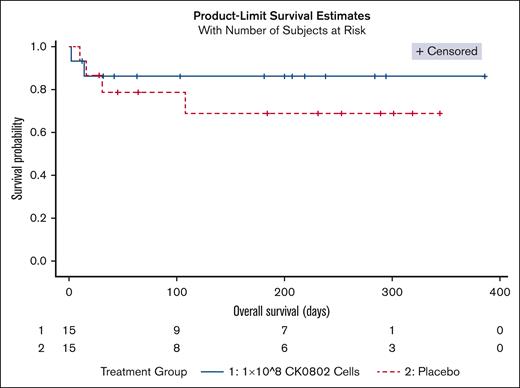
There were 13 deaths: placebo = 4; CK0802-100 = 2; CK0802-300 = 7. The median follow-up period from the first infusion to the last follow-up was 45-days (IQR, 23-235). Fitted Bayesian Weibull regression model for overall survival demonstrated a covariate adjusted PBE of 98.6% for CK0802-100 vs placebo (Figure 1), and PBE of 27.9% for CK0802-300 vs placebo. The lower PBE in the CK0802-300 arm may be attributed to patients having a number of poor prognostic factors including higher IL-6 levels12 and CRP levels,13 more obesity,14 greater hypoxia,15 and lower GCS scores16; and longer duration of steroids.17 None of these factors were incorporated into the preplanned regression models. Similar discordant findings regarding cell dose have been reported in other cellular therapy trials.18,19 For example, mesenchymal stem cell (MSC) therapy trials have suggested a minimal effective dose range between 100 and 150 million cells, whereas doses of <70 million and doses of >200 million were less or not effective.20 In a diabetic neuropathy double blind trial, 150 million MSCs, but not 300 million cells, significantly improved estimated glomerular filtration rate.21 In a hip arthroplasty double blind, placebo-controlled trial, 150 million, but not 300 million, placenta-derived MSCs significantly improved gluteus medius strength and weight.22 The small size of our pilot study makes it impossible to draw any definitive conclusions with respect to efficacy.
The ability to infuse unmatched allogeneic product cell products from different donors into the same patient without generating an overwhelming immune response provides a proof-of-concept to the “off-the-shelf,” on-demand cell therapy approach. These cells are ideal from a logistical perspective to treat inflammatory diseases for the following reasons: feasibility of administering cryopreserved cell therapy products in the intensive care unit setting, no requirement for onsite complex reconstitution of cell products, infusion of cell products from ready to use CryoStore CS50N freezer bags that can be thawed at the bedside, and the possibility of onsite drug inventory for immediate access.
Our trial, as well as the recent MSC cell therapy trial,23 set the stage for potential future studies of cell therapy in the field of ARDS. With a multiprong mechanism of action including secretion of the anti-inflammatory cytokine, IL-10, and acting as a cytokine sink for the proinflammatory cytokine, IL-2, the balance-restorative intervention of allogeneic, off-the-shelf, Treg cells should not be affected by different SARS-CoV-2 variants, and therefore could find wider application for treatment of virus-induced ARDS.24
In conclusion, these results suggest that infusion of multiple doses of cryopreserved, allogeneic, and non–HLA-matched regulatory T cells is safe in critically ill patients with COVID-19 associated ARDS. Given the imbalances at the baseline and the small sample size, CK0802 at a fixed dose of 100 million cells should be examined in a larger confirmatory study.
Acknowledgments: The authors thank members of the Data Safety and Monitoring Board: Hari Parmesawarn, Hematologist/Oncologist/Stem Cell Transplant/Cellular Therapy, Medical College Wisconsin; Marcos Delima, Hematologist/Oncologist/Stem Cell Transplant/Cellular Therapy, University Hospital Cleveland; Huifang Linda Lu, Rheumatologist and Clinical Immunologist, MD Anderson Cancer Center; Ali Filali-Mouhim, Biostatistician, University of Montreal; and Nisha Rathi, Pulmonologist, MD Anderson Cancer Center, for their diligent oversight of the conduct of this trial. The authors thank the Society for Interventional Radiology and Clifford Weiss for their support, as well as Hari Parmeswaran for critical review of the manuscript. This was a company-sponsored trial and was funded by Cellenkos Inc., Houston, TX.
Contribution: C.H., J.P.H., S.T.C., D.A., F.D., J.R.M., K.W.G., and T.S. performed the clinical trial; S.M., M.-A.L., K.Z., and M.H. performed the research; D.E.G. performed the clinical trial, analyzed the data, and wrote the manuscript; S.P. and C.R.F. designed research, analyzed data, and wrote the manuscript; P.F.T. designed the trial, analyzed the data, and wrote the manuscript; and D.S., R.B., and A.S.S. analyzed the data and wrote the manuscript.
Conflict-of-interest disclosure: S.P. is a scientific founder, has equity interest, reports board membership, and has received sponsored research funding from Cellenkos Inc. A.S.S. is a paid scientific consultant for Cellenkos Inc. D.S. is a paid scientific consultant for Cellenkos Inc. T.S. is employed by Cellenkos Inc. The remaining authors declare that the clinical trial was sponsored by Cellenkos Inc., and their relevant institutions received funding for the clinical trial. There is no support from any other organization for the submitted work, no financial relationships with any other organizations that might have an interest in the submitted work in the previous 3 years, and no other relationships or activities that could appear to have influenced the submitted work except as stated above.
Correspondence: Arthur S. Slutsky, St. Michael’s Hospital, 30 Bond St, Toronto, ON Canada M5B 1T8; e-mail: Arthur.slutsky@unityhealth.to.
References
Author notes
∗D.E.G., F.D., and C.H. contributed equally to this manuscript.
Data are available on request from the corresponding author, Arthur S. Slutsky (Arthur.slutsky@unityhealth.to).