TO THE EDITOR:
Cardiovascular events are the second most frequent cause of death in myelodysplastic syndromes (MDSs).1-3 Furthermore, the management of acute coronary syndrome (ACS), which includes both type I and type II ACS, is more difficult in patients with MDS because of the increased risk of bleeding owing to thrombocytopenia and platelet dysfunction.4 Type I ACS includes type I myocardial infarction (MI), comprising ST-elevation MI and non–ST-elevation MI, and unstable angina; and type II ACS comprises type II MI owing to a mismatch between myocardial oxygen supply and demand.5 Currently, no guidelines exist for the appropriate management of ACS in MDS.
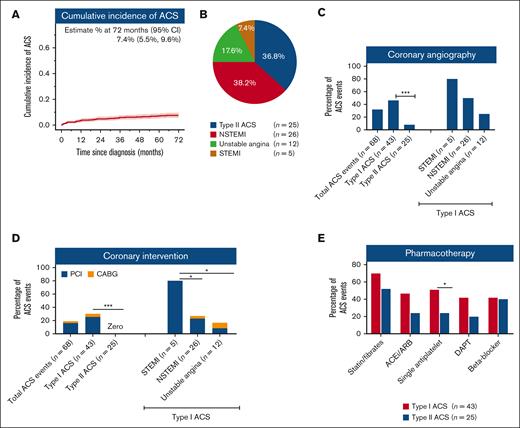
To assess the burdens and challenges in the management of ACS in MDS, we analyzed 687 patients consecutively enrolled into the South Australian MDS registry between 1998 and 2017 with a median survival of 31.1 (95% confidence interval [CI], 28.6-37.3) months. Based on case-mix data, 542 (78.9%) patients required hospitalization during the study period, including 191 patients with a total of 287 separate cardiac events. However, careful review of the medical records for each hospitalization revealed that 60 (31%) patients had an actual diagnosis different from their presumptive cardiac diagnosis, often in the context of complex presentations. This resulted in a final study cohort of 131 patients who required 218 hospitalizations for, or conditions that were complicated by, cardiac events. Of these 131 patients, 51 (38.9%) had 68 ACS events (supplemental Figure 1). Clinical variables in cases with and without ACS events are summarized in supplemental Table 1. The cumulative incidence of ACS was 7.4% (95% CI, 5.5-9.6) (Figure 1A). Furthermore, during follow-up, 10 (20%) patients had recurrent ACS. Of the total 68 ACS events, the majority were type I (n = 43, 63.2%) whereas 36.7% (n = 25) were type II (Figure 1B). The type I events comprised 26 NSTEMI, 5 STEMI, and 12 unstable angina events.
Incidence, risk factors, type, and management of ACS in patients with MDS. (A) The cumulative incidence of ACS in the whole cohort. (B) Distribution of cases according to type of ACS. Type I ACS include ST-elevation MI (STEMI), non–ST-elevation MI (NSTEMI), and unstable angina; whereas type II ACS include type II MI. (C) Utilization of coronary angiography for ACS. (D) Utilization of percutaneous coronary intervention (PCI) and coronary artery bypass graft (CABG) for ACS. (E) Guideline-recommended pharmacotherapy utilization in patients with type I and II ACS. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blockers; DAPT, dual antiplatelet therapy.
Incidence, risk factors, type, and management of ACS in patients with MDS. (A) The cumulative incidence of ACS in the whole cohort. (B) Distribution of cases according to type of ACS. Type I ACS include ST-elevation MI (STEMI), non–ST-elevation MI (NSTEMI), and unstable angina; whereas type II ACS include type II MI. (C) Utilization of coronary angiography for ACS. (D) Utilization of percutaneous coronary intervention (PCI) and coronary artery bypass graft (CABG) for ACS. (E) Guideline-recommended pharmacotherapy utilization in patients with type I and II ACS. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-receptor blockers; DAPT, dual antiplatelet therapy.
Of all 43 admissions for type I ACS, only 46.5% (n = 20) had coronary angiography, 25.6% (n = 11) had percutaneous coronary intervention, and 4.6% (n = 2) had coronary artery bypass surgery (Figure 1C-D; supplemental Table 2). Guideline-recommended pharmacotherapy was also under prescribed. Only 41.8% and 51.1% ACS events were treated with dual- or single-agent antiplatelet (aspirin or P2Y12 inhibitor) therapy, respectively. The low rates of angiography, revascularization, and dual antiplatelet therapy could not be explained by thrombocytopenia, as platelet counts at the time of ACS were ≥50 × 109/L in 85.4% cases and only 1 patient had platelet count <30 × 109/L. Apart from older age and higher creatinine, other MDS-related factors, such as Revised International Prognostic Scoring System risk groups and treatment, were not significantly different in patients who underwent coronary angiography compared with that in those who did not (supplemental Table 3). Lipid-lowering drugs, beta-blockers, and angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers were also prescribed for only 69.7%, 42.9%, and 46.5% type I ACS events, respectively (Figure 1E). This highlights lower than expected use of interventional and pharmacological therapy for ACS in patients with MDS.
The majority of type II ACS events occurred in the setting of infection or anemia (23/25, 92%) (supplemental Table 2). Coronary angiography was performed for only 2 (7.4%) type II ACS events and did not result in revascularization (Figure 1C-D). Only 25.9% and 18.5% of patients with type II ACS received dual or single antiplatelet therapy, respectively; although prescribing rates were also low for lipid-lowering medication (55.6%), beta-blockers (44.4%), and angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers (29.6%) (Figure 1E). Anemia is one of the main precipitating factors for ACS, especially for type II ACS (supplemental Table 2). Currently, there are no uniformly agreed hemoglobin (Hb) trigger thresholds for red blood cell transfusions in MDS. In patients with ACS, 73%, 35%, and 9.5% of red blood cell transfusion episodes were not triggered until Hb dropped to <90 g/L, <80 g/L, and <70 g/L, respectively. At the time of ACS, Hb was <100 g/L in 78.4% and <80 g/L in 31.3% of cases.
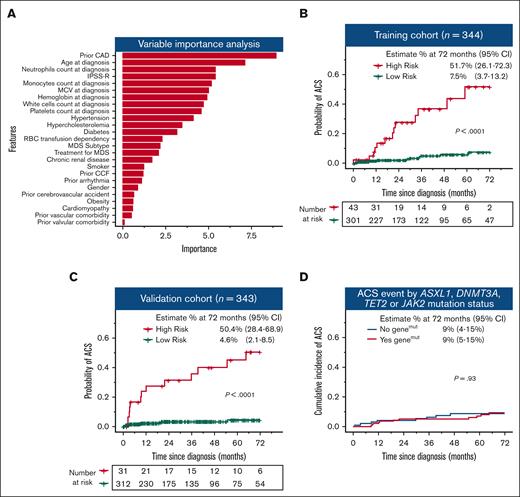
Identifying patients with MDS at high risk of ACS is crucial to optimize risk factor management; however, there are no dedicated prediction models for MDS. We used a random forest algorithm to identify patients at high risk of ACS using 25 variables at the time of MDS diagnosis. A total of 687 patients were randomly allocated 1:1 into a training (n = 344) and independent validation cohort (n = 343) based on an equal proportion of ACS event and year of diagnosis (supplemental Figure 2A). The patient characteristics for these cohorts are shown in supplemental Table 4. Strikingly, after a previous history of coronary artery disease and age, MDS-related parameters were among the most influential predictors for ACS (Figure 2A; supplemental Figure 3). The model predicted ACS cases with high probability in both training and validation cohort (supplemental Figure 2B-C; supplemental Table 5). Of the 344 patients in the training cohort, the model identified 43 (12.5%) patients at a high risk for ACS. Of these high-risk patients, 52% (95% CI, 26-72) developed ACS events during the follow-up. Meanwhile, in the low-risk group, the probability of developing an ACS was only 8% (95% CI, 4-13; P < .0001) (Figure 2B). Similarly, in the independent validation cohort, 50% (95% CI, 28-69) of high-risk patients developed ACS whereas in the low-risk group, ACS occurred in only 5% of the cases (95% CI, 2-8; P < .0001) (Figure 2C). Supplemental Figure 4 provides case examples of patients classified as high- or low-risk with and without ACS events.
Machine learning model identified patients at high risk of ACS. (A) Variable importance analysis showing the variables from the most to the least important to influence risk of ACS. The risk of ACS was significantly higher in the high-risk group compared with that in the low-risk group in both training (B) and validation (C) cohorts. (D) Cumulative incidence of ACS is not significantly different in patients with and without ASXL1, DNMT3A, TET2, and JAK2 somatic mutations. CAD, coronary artery disease; CCF, congestive cardiac failure; MCV, mean corpuscular volume; IPSS-R, Revised International Prognostic Scoring System.
Machine learning model identified patients at high risk of ACS. (A) Variable importance analysis showing the variables from the most to the least important to influence risk of ACS. The risk of ACS was significantly higher in the high-risk group compared with that in the low-risk group in both training (B) and validation (C) cohorts. (D) Cumulative incidence of ACS is not significantly different in patients with and without ASXL1, DNMT3A, TET2, and JAK2 somatic mutations. CAD, coronary artery disease; CCF, congestive cardiac failure; MCV, mean corpuscular volume; IPSS-R, Revised International Prognostic Scoring System.
Recent studies reported an association between the presence of clonal hematopoiesis of indeterminate potential and cardiovascular events.6-8 Clonal hematopoiesis of indeterminate potential mutations involving TET2, ASXL1, or DNMT3A carry an approximately twofold increased risk of coronary artery disease, whereas individuals with JAK2 mutations have an over 10-fold increased risk. In our cohort, 228 patients had available mutation data at MDS diagnosis and of these, 58.8% had at least 1 mutation in ASXL1, TET2, DNMT3A, or JAK2. We did not observe a difference in the incidence of ACS in patients with or without these mutations (Figure 2D). These findings need further validation in an independent cohort. Secondly, our study spans over 2 decades and guidelines for the management of MDS and ACS have changed over the time. However, this would have minimal impact on the prediction model as patients were stratified for diagnosis period and ACS events.
The findings of this study are important. Firstly, the reliance on administrative case-mix data can overestimate the prevalence of cardiac admissions in MDS and needs to be considered when reviewing peer-reviewed literature collected from this source. Specifically, previously published registries using Medicare databases have reported a 5-year cumulative incidence of MI and cardiovascular disease of 12.5% and 17%, respectively,9 which is higher than our experience. Secondly, there appears a reluctance to offer invasive coronary angiography and revascularization for patients with both MDS and ACS. Moreover, there is an alarming practice not to use guideline-recommended therapies including dual antiplatelet therapy in many patients with type I ACS, whereas type II ACS is managed even more conservatively. This is often in the absence of absolute contraindications. Machine-learning algorithms utilizing routinely available parameters may help clinicians identify patients at higher risk of ACS and optimize their risk factor management. Finally, our findings should prompt review of the guidelines for managing type I and II ACS in patients with MDS and how to best ensure their implementation.
Acknowledgments: The authors thank the patients, their families, and South Australia Cancer Research Biobank.
D.K.H. was supported by a National Health and Medical Research Council/Medical Research Future Fund investigator grant MRF1195517 and Cancer Australia and Leukemia Foundation Australia. P.J.P. is supported by research fellowships from the National Heart Foundation of Australia (Future Leader Fellowship FLF102056) and National Health and Medical Research Council of Australia (CDF1161506).
Contribution: N.M. collected and analyzed the data and wrote the manuscript; A.L., R.C., O.F., and C.H.K. collected and analyzed the data and edited the manuscript; D.R., D.Y., and D.S. recruited the patients and edited the manuscript; and P.J.P. and D.K.H. designed the project, directed the study, analyzed the data, and edited the final version of the manuscript.
Conflict-of-interest disclosure: P.J.P. has received research support from Abbott Vascular; consulting fees from Amgen and Esperion; and speaker honoraria from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Merck Schering-Plough, Novartis, Pfizer, and Sanofi. D.K.H. has received research grant and honoraria from Novartis. The remaining authors declare no competing financial interests.
Correspondence: Devendra K. Hiwase, Department of Haematology, Royal Adelaide Hospital, Central Adelaide Local Health Network, Port Rd, Adelaide 5000, SA, Australia; e-mail: devendra.hiwase@sa.gov.au.
References
Author notes
Data are available on request from the corresponding author, Devendra K. Hiwase (devendra.hiwase@sa.gov.au).
The full-text version of this article contains a data supplement.