TO THE EDITOR:
TP53 is the most frequently mutated gene in cancer also playing important roles in pathogenesis of hematologic malignancies.1,2 In patients with de novo acute myeloid leukemia (AML) and with myelodysplastic syndrome (MDS), TP53 alterations (TP53alt) occur in ∼5% to 15% of the patients; in patients with therapy-related AML (t-AML) and MDS and in those with relapses, they are detected at higher frequencies (∼25% to 40%).3-8TP53 mutations (TP53mut) are generally associated with advanced stages of the disease,a complex karyotype, resistance to conventional (chemo-)therapies, and dismal prognosis.5,8-11 Several targeted drugs and novel therapeutic options have been developed in recent years; however, the optimal treatment strategy for patients with AML and MDS harboring TP53alt remains a critical area of unmet need. TP53alts comprise not only gene mutations but also allelic imbalances, including deletions (dels) in TP53 and regions with copy-neutral loss of heterozygosity (cnLOH) comprising 17p/TP53. Frequently, both alleles are altered either by biallelic mutations or TP53muts, with accompanying allelic imbalance of the other allele (dels or cnLOH).2,12-15 Although monoallelic mutations in TP53 (without accompanying dels or cnLOH; single hit [sh]) already show a negative impact on survival, biallelic alterations in TP53 (double hit [dh]) lead to a dismal outcome both in patients with AML and MDS.14,15
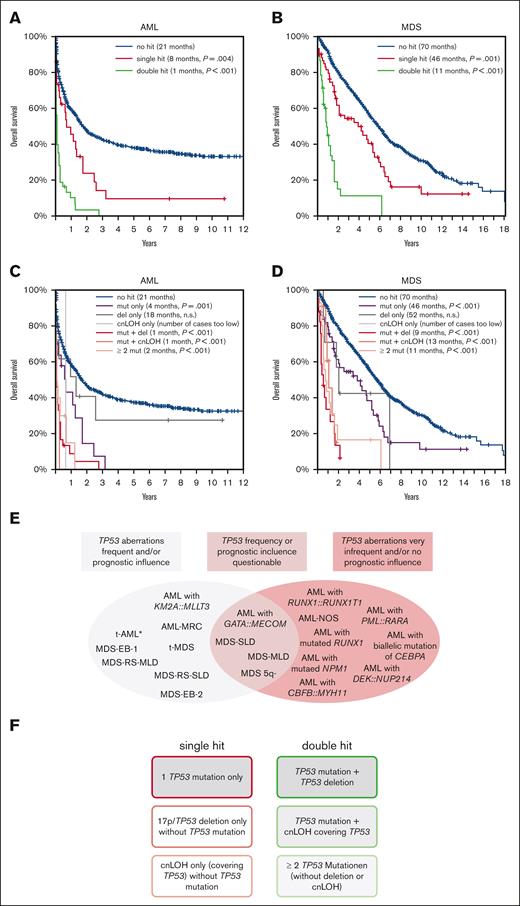
The focus of this study is to analyze TP53sh (single TP53muts, dels in 17p comprising TP53, or cnLOH in 17p including TP53) and dh events (≥2 TP53mut, TP53mut + del, and TP53mut + cnLOH) in more detail, with a focus on the distribution, type of aberration, and impact on survival in AML and MDS subgroups (classification in accordance with the World Health Organization 2017).16 A total of 1519 samples were used for analysis, comprising 772 newly diagnosed AML and 747 MDS cases (Table 1). Whole genome sequencing was performed for all 1519 patients at the time of diagnosis (median coverage, 100×). For this, 151bp paired-end reads were generated on NovaSeq 6000 and HiSeq X machines (Illumina, San Diego, CA; for further information, see supplemental Data). Cases were categorized into (1) one TP53mut without accompanying del or cnLOH (mut-only), (2) TP53 del-only, (3) cnLOH-only (1-3; sh events), (4) TP53mut and accompanying del in TP53 (mut + del; might include 1 or more TP53muts), (5) TP53mut and accompanying cnLOH (mut + cnLOH, might include 1 or more TP53muts), and (6) ≥2 TP53muts (≥2 mut-only; without accompanying del or cnLOH; 4-6; dh events). For AML, in 84 of 772 cases at least 1 TP53alt was detected (11%); for MDS, 96 of 747 cases showed TP53alt (13%). Regarding AML, the highest frequency of TP53alt were detected in the group having AML with myelodysplasia-related changes (AML-MRC; 65 of 152 cases, 42%; supplemental Figure 1A; Table 1). In addition, TP53alt were recurrently found in AML with KMT2A::MLLT3 (3 of 26, 12%), AML with GATA2::MECOM (3 of 32, 9%), AML with PML::RARA (4 of 50, 8%), AML with maturation (2 of 52, 4%) and AML with mutated NPM1 (2 of 160, 1%). In the remaining AML subgroups, TP53alt were only found in 1 patient each or were even completely absent (Table 1). These results were validated using classical diagnostic methods performed on 11 796 unselected AML cases, in which very low TP53alt frequencies were also detected for the respective subentities (supplemental Table 1). Regarding MDS, TP53alt were detected in all analyzed subentities, with the highest frequencies detected in therapy-related MDS (6 of 22, 27%), followed by MDS 5q– (21 of 104, 20%), MDS with excess blasts 1 (MDS-EB1) (25 of 151, 17%), and MDS-EB2 (21 of 149, 14%) (supplemental Figure 1B; Table 1). In the total AML cohort, 34 of 84 patients (40%) showed sh (mut-only, 24%; del-only 14%; and cnLOH-only, 2%), whereas TP53dh was found in 50 of 84 patients (60%; mut + del, 38%; mut + cnLOH, 6%; and ≥2 mut, 16%). In MDS, sh was found in 56 of 96 patients (58%) and dh in 40 of 96 patients (42%) (mut-only, 50%; del-only, 7%; cnLOH-only 1%; and mut + del, 20%; mut + cnLOH, 9%; and ≥2 mut, 13%). For analysis of TP53alt types in the subentities, only subgroups in which TP53alt was found in >10 cases were selected (AML-MRC, MDS with multilineage dysplasia with ring sideroblasts, MDS-EB1, MDS-EB2, and MDS 5q; supplemental Table 2). Although patients with MDS 5q– predominantly showed a TP53sh caused by a TP53mut (81%), those with AML-MRC and MDS-EB2 predominantly showed dh (68% for each type; supplemental Figure 2). In addition, dels comprising TP53 were frequently found in MDS with multilineage dysplasia with ring sideroblasts (17%) and MDS-EB1 (14%), whereas mut + cnLOH, causing a dh, was detected in 14% of MDS-EB1 cases. Regarding cnLOH, 5 of 50 cases (19%) of all dh cases in AML and 9 of 40 cases (23%) in MDS were caused by mut + cnLOH; hence, this alteration significantly contributes to the formation of TP53dh. Overall survival (OS) was significantly less in patients with a TP53sh than in patients without TP53alt, both for patients with AML and MDS (sh vs no hit; AML: 8 months vs 21 months, P = .004; MDS: 46 vs 70 months, P = .001). However, in both entities, the presence of a dh worsened the prognosis drastically (dh vs no hit; AML: 1 vs 21 months, P < .001; MDS: 11 vs 70 months, P < .001) (Figure 1A,B). Moreover, when the cohorts were split per the sh and dh events, a significant negative impact on OS compared with cases without TP53alt was observed for all dh events and mut-only sh events, in both patients with AML and MDS. For cases with del-only, a trend toward a lower OS was detected, which was, however, not statistically significant; whereas for cnLOH-only, the number of cases was not sufficient for meaningful analysis (Figure 1C,D). Moreover, as cnLOH results merely in a duplication of the TP53 wild-type allele, no clinical impact is assumed. Influence of TP53alt on OS was also investigated in the selected subgroups (>10 cases with TP53alt), the remaining AML cases were summarized and grouped as AML–non-MRC. Interestingly, a significant negative impact of TP53alt on OS was found for all subgroups, except for the MDS 5q– subgroup (supplemental Figure 3).
TP53 alteration frequencies in subgroups of AML and MDS
| . | Specific subgroup according to WHO 2017 . | Number of cases . | Cases without TP53 aberration [n] . | Cases with TP53 aberration [n] . | Frequency of TP53 aberration [%] . |
|---|---|---|---|---|---|
| AML | AML-MRC∗ | 152 | 87 | 65 | 43 |
| AML with t(9;11)(p21;q23); KMT2A::MLLT3 | 26 | 23 | 3 | 12 | |
| AML with inv(3)(q21q26); GATA2::MECOM | 32 | 29 | 3 | 9 | |
| Acute promyelocytic leukemia with PML::RARA | 50 | 46 | 4 | 8 | |
| Acute monoblastic and monocytic leukemia | 14 | 13 | 1 | 7 | |
| Therapy-related AML | 19 | 18 | 1 | 5 | |
| AML with maturation | 52 | 50 | 2 | 4 | |
| AML without maturation | 30 | 29 | 1 | 3 | |
| Acute myelomonocytic leukemia | 33 | 32 | 1 | 3 | |
| AML with mutated RUNX1 | 43 | 42 | 1 | 2 | |
| AML with mutated NPM1 | 160 | 158 | 2 | 1 | |
| AML with biallelic mutation of CEBPA | 47 | 47 | 0 | 0 | |
| AML with inv(16)(p13q22); CBFB::MYH11 | 47 | 47 | 0 | 0 | |
| AML with t(6;9)(p23;q34); DEK::NUP214 | 10 | 10 | 0 | 0 | |
| AML with minimal differentiation | 14 | 14 | 0 | 0 | |
| AML with t(8;21)(q22;q22); RUNX1::RUNX1T1 | 43 | 43 | 0 | 0 | |
| Total in AML | 772 | 688 | 84 | 11 | |
| MDS | Therapy-related MDS | 22 | 16 | 6 | 27 |
| MDS with isolated del(5q) (MDS 5q–)∗ | 104 | 83 | 21 | 20 | |
| MDS-EB-2∗ | 151 | 126 | 25 | 17 | |
| MDS-EB-1∗ | 149 | 128 | 21 | 14 | |
| MDS-RS-SLD | 42 | 38 | 4 | 10 | |
| MDS-RS-MLD∗ | 148 | 136 | 12 | 8 | |
| MDS-SLD | 18 | 17 | 1 | 6 | |
| MDS-MLD | 113 | 107 | 6 | 5 | |
| Total in MDS | 747 | 651 | 96 | 13 | |
| In total cohort | 1519 | 1339 | 180 | 12 |
| . | Specific subgroup according to WHO 2017 . | Number of cases . | Cases without TP53 aberration [n] . | Cases with TP53 aberration [n] . | Frequency of TP53 aberration [%] . |
|---|---|---|---|---|---|
| AML | AML-MRC∗ | 152 | 87 | 65 | 43 |
| AML with t(9;11)(p21;q23); KMT2A::MLLT3 | 26 | 23 | 3 | 12 | |
| AML with inv(3)(q21q26); GATA2::MECOM | 32 | 29 | 3 | 9 | |
| Acute promyelocytic leukemia with PML::RARA | 50 | 46 | 4 | 8 | |
| Acute monoblastic and monocytic leukemia | 14 | 13 | 1 | 7 | |
| Therapy-related AML | 19 | 18 | 1 | 5 | |
| AML with maturation | 52 | 50 | 2 | 4 | |
| AML without maturation | 30 | 29 | 1 | 3 | |
| Acute myelomonocytic leukemia | 33 | 32 | 1 | 3 | |
| AML with mutated RUNX1 | 43 | 42 | 1 | 2 | |
| AML with mutated NPM1 | 160 | 158 | 2 | 1 | |
| AML with biallelic mutation of CEBPA | 47 | 47 | 0 | 0 | |
| AML with inv(16)(p13q22); CBFB::MYH11 | 47 | 47 | 0 | 0 | |
| AML with t(6;9)(p23;q34); DEK::NUP214 | 10 | 10 | 0 | 0 | |
| AML with minimal differentiation | 14 | 14 | 0 | 0 | |
| AML with t(8;21)(q22;q22); RUNX1::RUNX1T1 | 43 | 43 | 0 | 0 | |
| Total in AML | 772 | 688 | 84 | 11 | |
| MDS | Therapy-related MDS | 22 | 16 | 6 | 27 |
| MDS with isolated del(5q) (MDS 5q–)∗ | 104 | 83 | 21 | 20 | |
| MDS-EB-2∗ | 151 | 126 | 25 | 17 | |
| MDS-EB-1∗ | 149 | 128 | 21 | 14 | |
| MDS-RS-SLD | 42 | 38 | 4 | 10 | |
| MDS-RS-MLD∗ | 148 | 136 | 12 | 8 | |
| MDS-SLD | 18 | 17 | 1 | 6 | |
| MDS-MLD | 113 | 107 | 6 | 5 | |
| Total in MDS | 747 | 651 | 96 | 13 | |
| In total cohort | 1519 | 1339 | 180 | 12 |
AML and MDS subgroups are sorted according to frequency of TP53 alteration, respectively.
MDS-MLD, MDS with multilineage dysplasia; MDS-RS-MLD, MDS with multilineage dysplasia with ring sideroblasts; MDS-RS-SLD, MDS with single lineage dysplasia with ring sideroblasts; MDS-SLD, MDS with single lineage dysplasia.
The subgroups in which more than 10 cases had TP53 alterations are highlighted in gray. Those were thus selected for further detailed analysis of the alteration type and OS.
OS of patients with TP53 alteration in the total AML and MDS cohorts, and suggestion of a diagnostic algorithm. (A-B) In patients with (A) AML and (B) MDS, the OS was analyzed for patients with TP53 sh (red line), TP53 dh (green line), and without TP53alts (blue line; no hit). In (C) and (D), OS was analyzed in more detail for patients with TP53 mut-only (purple line), with TP53 del-only (dark gray line), with cnLOH-only (light gray line), with mut + del (red line), with mut + cnLOH (light red line), with ≥2 mut-only (orange line), and in patients with no hit (blue line). The P values denote the significance of the respective alteration in comparison to no hit. Clinical data were available for 717 patients with AML and 737 patients with MDS. (E,F) Recommendation of TP53 analysis for AML and MDS subgroups and suggestion of a diagnostic algorithm. (E) The analyzed entities were categorized into (1) entities in which TP53 aberrations were frequently detected and/or showed a prognostic influence (marked in gray; left), (2) cases for which the TP53 alteration frequency was very low or not detectable and/or did not show prognostic relevance (marked in red; right), and (3) entities for which the TP53 alteration frequency or prognostic influence is questionable (middle). AML without defining genetic abnormalities were summarized as AML-NOS (not otherwise specified). ∗, TP53 alterations in t-AML cases were rare in our analysis because of the low number of t-AML cases included in our cohort; however, t-AMLs are known to frequently harbor TP53 alterations, hence they were included into category 1. (F) Proposed categories for TP53 sh (left) and TP53 dh events (right). Marked with gray background are events that have impact on the prognosis in patients with AML and MDS.
OS of patients with TP53 alteration in the total AML and MDS cohorts, and suggestion of a diagnostic algorithm. (A-B) In patients with (A) AML and (B) MDS, the OS was analyzed for patients with TP53 sh (red line), TP53 dh (green line), and without TP53alts (blue line; no hit). In (C) and (D), OS was analyzed in more detail for patients with TP53 mut-only (purple line), with TP53 del-only (dark gray line), with cnLOH-only (light gray line), with mut + del (red line), with mut + cnLOH (light red line), with ≥2 mut-only (orange line), and in patients with no hit (blue line). The P values denote the significance of the respective alteration in comparison to no hit. Clinical data were available for 717 patients with AML and 737 patients with MDS. (E,F) Recommendation of TP53 analysis for AML and MDS subgroups and suggestion of a diagnostic algorithm. (E) The analyzed entities were categorized into (1) entities in which TP53 aberrations were frequently detected and/or showed a prognostic influence (marked in gray; left), (2) cases for which the TP53 alteration frequency was very low or not detectable and/or did not show prognostic relevance (marked in red; right), and (3) entities for which the TP53 alteration frequency or prognostic influence is questionable (middle). AML without defining genetic abnormalities were summarized as AML-NOS (not otherwise specified). ∗, TP53 alterations in t-AML cases were rare in our analysis because of the low number of t-AML cases included in our cohort; however, t-AMLs are known to frequently harbor TP53 alterations, hence they were included into category 1. (F) Proposed categories for TP53 sh (left) and TP53 dh events (right). Marked with gray background are events that have impact on the prognosis in patients with AML and MDS.
Generally, our results corroborate previous findings that TP53alt play an important role in prognosis and risk stratification of patients with AML and MDS.3,4,17-22 Recently, for MDS, both the World Health Organization classification 2022 and the International Consensus Classification recognized TP53 mutated cases as a distinct disease entity, although the inclusion criteria vary.23,24 Our data show that although presence of a single TP53mut already influences OS, and a TP53dh worsens OS dramatically in both patients with AML and MDS.14,15,17 In addition, our results demonstrate that the presence of TP53alt and influence on prognosis differs markedly within the analyzed AML and MDS subtypes, some depicting low frequencies and/or only minor or no prognostic relevance (see supplemental Data). Moreover, we postulate that for a correct definition of TP53dh in a diagnostic setting and for proper risk stratification, (1) the number of TP53muts (none, 1, and ≥2), (2) the presence of a 17p/TP53del, and (3) the presence of cnLOH involving TP53 should be investigated (Figure 1E,F). Because of the dismal outcome of both patients with AML and MDS with biallelic TP53alts with an urgent need for novel therapeutic options, it can be suggested that these cases should be considered as separate entities in future classification systems. Moreover, as the frequency of TP53mut is known to be enriched among patients with relapsed AML and MDS because of the selective advantage of the TP53 mutated cells caused by their resistance to chemotherapy,3-8 it raises concerns regarding the use of this treatment option for patients with monoallelic TP53muts.
Acknowledgments: The authors thank all coworkers at the MLL Munich Leukemia Laboratory for their dedicated work. They also thank all physicians for providing samples, caring for patients, and collecting data.
Contribution: A.S. designed the study, interpreted the data, and wrote the manuscript; A.S. and C.H. were responsible for cytogenetic analyses; M.M., C.B., and S.H. for molecular and bioinformatic analyses; W.K. for immunophenotyping; T.H. for cytomorphologic analyses; and all authors read and contributed to the final version of the manuscript.
Conflict-of-interest disclosure: C.H., W.K., and T.H. declare part ownership of MLL Münchner Leukämielabor GmbH. A.S., M.M., C.B., and S.H. are employed by the MLL.
Correspondence: Anna Stengel, MLL Munich Leukemia Laboratory, Max-Lebsche-Platz 31, 81377 München, Germany; e-mail: anna.stengel@mll.com.
References
Author notes
For original data, please contact the corresponding author, Anna Stengel (anna.stengel@mll.com).
The full-text version of this article contains a data supplement.