Key Points
Bridging therapy (BT) is safe, and complete/partial response to BT confers a 42% reduction in risk of progression/death after CD19CAR-T therapy.
Good response to BT is twice as likely with polatuzumab than with other modalities and is particularly important for Tisa-cel outcomes.
Visual Abstract
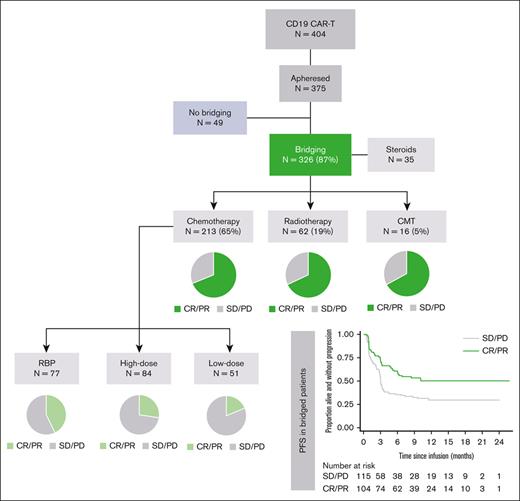
Summary of BT modality, BT response and PFS post-CAR-T in all apheresed LBCL patients.
Summary of BT modality, BT response and PFS post-CAR-T in all apheresed LBCL patients.
Abstract
The impact of bridging therapy (BT) on CD19-directed chimeric antigen receptor T-cell (CD19CAR-T) outcomes in large B-cell lymphoma (LBCL) is poorly characterized. Current practice is guided through physician preference rather than established evidence. Identification of effective BT modalities and factors predictive of response could improve both CAR-T intention to treat and clinical outcomes. We assessed BT modality and response in 375 adult patients with LBCL in relation to outcomes after axicabtagene ciloleucel (Axi-cel) or tisagenlecleucel (Tisa-cel) administration. The majority of patients received BT with chemotherapy (57%) or radiotherapy (17%). We observed that BT was safe for patients, with minimal morbidity or mortality. We showed that complete or partial response to BT conferred a 42% reduction in disease progression and death after CD19CAR-T therapy. Multivariate analysis identified several factors associated with likelihood of response to BT, including response to last line therapy, the absence of bulky disease, and the use of polatuzumab-containing chemotherapy regimens. Our data suggested that complete or partial response to BT may be more important for Tisa-cel than for Axi-cel, because all patients receiving Tisa-cel with less than partial response to BT experienced frank relapse within 12 months of CD19CAR-T infusion. In summary, BT in LBCL should be carefully planned toward optimal response and disease debulking, to improve patient outcomes associated with CD19CAR-T. Polatuzumab-containing regimens should be strongly considered for all suitable patients, and failure to achieve complete or partial response to BT before Tisa-cel administration may prompt consideration of further lines of BT where possible.
Introduction
Chimeric antigen receptor T-cell (CAR-T) therapy confers durable responses in 30% to 40% of patients with relapsed/refractory (r/r) large B-cell lymphoma (LBCL),1-5 leading to Food and Drug Administration approval of tisagenlecleucel (Tisa-cel), axicabtagene ciloleucel (Axi-cel), and lisocabtagene maraleucel.
Bridging therapy (BT) is the anticancer therapy administered to patients during the CAR-T manufacture period. Intention to treat with CAR-T is compromised owing to poor disease control during this period, and dismal prognosis is reported for patients failing to reach CAR-T infusion.1 In the Juliet study, 92% of the patients2 and ∼80% of the real-world cohorts received BT,6-9 but current practice is guided through patient and physician preferences rather than through published evidence.2,6,10 Algorithms to identify which patients are likely to benefit from BT and what strategies11-13 confer best CAR-T therapy outcomes would be clinically valuable.
Retrospective analyses suggesting poor CAR-T therapy outcomes in patients undergone BT14-17 also found an association between BT and high-risk baseline disease factors, indicating a selection bias toward more intensive BT for patients with aggressive disease. More recently, several groups have described the deleterious impact of high tumor burden before CAR-T infusion18-20 with the result that many clinicians are moving toward more intensive BT practices for tumor debulking purposes before CAR-T therapy.
In some patients, BT unexpectedly leads to complete response (CR).21 Limited published data on CAR-T efficacy and toxicity in the absence of measurable disease, and a lack of clarity around minimum tumor burden or antigen threshold requirements for effective CAR-T therapy means that physicians frequently defer CAR-T infusion in this setting. However, emerging evidence suggests good outcomes in patients proceeding to CAR-T therapy in CR.22
Here, we report outcomes of BT in 375 adult patients with r/r LBCL undergoing leukapheresis for Axi-cel or Tisa-cel. The objectives of the study were to identify which patients respond to BT (including those who achieve CR to BT and proceed to CAR-T infusion), and a comparison of the impact of BT on CAR-T therapy safety and efficacy outcomes between patient groups treated with Axi-cel and Tisa-cel.
Methods
Patients
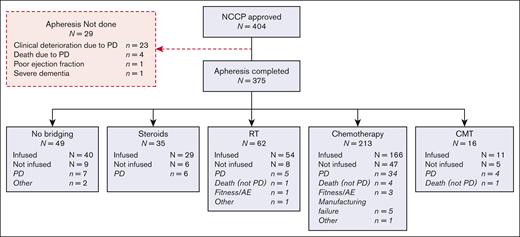
As part of a National Service Evaluation, data were collected retrospectively from electronic medical records for consecutive patients with r/r LBCL submitted to the United Kingdom National CAR Clinical Panel (NCCP) for approval of treatment with licensed CD19CAR-T at commissioned CAR-T centers. The NCCP equivalent Scottish CAR-T center approved additional 12 patients using similar eligibility criteria, were included.1
BT
BT was defined as lymphoma-directed therapy administered between leukapheresis and lymphodepletion (LD). BT was subdivided into none (no BT), corticosteroids alone, chemotherapy (CT), radiotherapy (RT), and combined-modality therapy (CMT), ie, CT + RT. CT was further categorized into high-dose chemotherapy (HDT), low-dose chemotherapy (LDT) and rituxamab-bendamustine-polatuzumab (RBP).13 HDT is defined as regimens that are delivered IV, many of which are conventionally used in patients with LBCL fit for autologous stem cell transplant and are associated with periods of neutropenia and in some cases with a requirement for hospital admission. HDT was subdivided into gemcitabine-based (HDT-Gem), ifosphamide-based (HDT-Ifos), and other (HDT-Other).
LDT bridging was defined as either oral treatments or IV regimens not considered to be conventional LBCL salvage (eg, single agent rituximab and rituximab-bendamustine). Definitions and details of BT modalities are listed in supplemental Table 1. If patients received ≥1 line of BT (N = 9), the final regimen was used for this analysis. Patients achieving CR to BT could proceed to CAR-T infusion at the discretion of the CAR-T center.
Statistics
Pretreatment factors were compared using Wilcoxon Mann-Whitney or Kruskal-Wallis (nonnormally distributed continuous variables) or χ2 or Fisher exact tests (discreate variables). Progression-free survival (PFS) and overall survival (OS) were analyzed using Kaplan-Meier survival analysis, Cox regression, and the log rank tests. Time was measured from the date of infusion until the occurrence of first event. Nonrelapse mortality was analyzed using the method developed by Fine and Grey with relapse treated as a competing event. Logistic regression was used to compare baseline characteristics and response to bridging. All analyses were performed using STATA version 16.1 (StataCorp, TX).
Results
Demographics and BT for all patients who underwent leukapheresis
Between December 2018 and November 2020, 375 patients from the United Kingdom with r/r LBCL underwent leukapheresis for CD19CAR-T, and 87% (326/375) received BT (Figure 1). The majority received CT bridging and of these, most (148/194; 76.3%) received a single cycle (supplemental Table 1).
Baseline demographics for all patients who underwent leukapheresis and who received infusion according to BT modality are illustrated in Table 1 and supplemental Table 2. Patients who underwent CT-BT were significantly more likely than who did not undergo BT to have stage III/IV disease (84.4% vs 69.9%; P = .033), more extranodal disease (≥1 site, 72.3% vs 41%; P < .001), and have an Eastern Cooperative Oncology Group of 1 rather than 0 (62.4% vs 36.7%; P = .001). Patients who underwent RT-BT had significantly less stage III/IV disease (P = .024), lower level of lactate dehydrogenase (LDH) (P = .039), and less extranodal disease (P = .041) than those who underwent CT. Only 16 patients received CMT bridging, but baseline characteristics were similar to the CT group.
Demographics for all patients who underwent leukapheresis at submission
| . | No bridging . | Bridging therapy . | P-value none vs RT . | P-value none vs CT . | P-value steroids vs none . | P-value steroids vs RT . | P-value steroids vs CT . | P-value CT vs RT . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Steroids . | RT . | CT . | CMT . | ||||||||
| N = 49 . | N = 35 . | N = 62 . | N = 213 . | N = 16 . | |||||||
| Age, y | 63.0 (58-68) | 58.0(51-65) | 57.0(49-66) | 60.0(51-68) | 63.0(47.5-66.5) | .0082 | .080 | .069 | .54 | .78 | .20 |
| Sex, N (%) | |||||||||||
| Male | 30 (61.2) | 21 (60.0) | 37 (59.7) | 134 (62.9) | 8 (50.0) | >.99 | .87 | >.99 | >.99 | .85 | .66 |
| Female | 19 (38.8) | 14 (40.0) | 25 (40.3) | 79 (37.1) | 8 (50.0) | ||||||
| Disease type, N (%) | |||||||||||
| De novo DLBCL | 31 (63.3) | 24 (68.6) | 40 (64.5) | 148 (69.5) | 9 (56.3) | .95 | .72 | .53 | .58 | .30 | .44 |
| PMBL | 3 (6.1) | 0 | 4 (6.5) | 11 (5.2) | 2 (12.5) | ||||||
| tFL | 12 (24.5) | 10 (28.6) | 16 (25.8) | 39 (18.3) | 4 (25.0) | ||||||
| t-Other | 3 (6.1) | 1 (2.9) | 2 (3.2) | 15 (7.0) | 1 (6.3) | ||||||
| DHL/THL, N (%) | |||||||||||
| No | 28 (75.7) | 24 (75.0) | 38 (69.1) | 128 (68.8) | 8 (61.5) | .84 | .78 | >.99 | .88 | .87 | .84 |
| Yes | 4 (10.8) | 3 (9.4) | 8 (14.5) | 23 (12.4) | 2 (15.4) | ||||||
| DE/TE | 5 (13.5) | 5 (15.6) | 9 (16.4) | 35 (18.8) | 3 (23.1) | ||||||
| Missing/unknown | 12 | 3 | 7 | 27 | 3 | ||||||
| Stage, N (%) | |||||||||||
| Stage 1-2 | 14 (30.4) | 9 (25.7) | 18 (29.5) | 33 (15.6) | 2 (13.3) | >.99 | .033 | .80 | .82 | .15 | .024 |
| Stage 3-4 | 32 (69.6) | 26 (74.3) | 43 (70.5) | 179 (84.4) | 13 (86.7) | ||||||
| Missing/unknown | 3 | 0 | 1 | 1 | 1 | ||||||
| ECOG, N (%) | |||||||||||
| 0 | 31 (63.3) | 18 (51.4) | 32 (51.6) | 80 (37.6) | 3 (18.8) | .25 | .001 | .37 | >.99 | .14 | .056 |
| 1 | 18 (36.7) | 17 (48.6) | 30 (48.4) | 133 (62.4) | 13 (81.3) | ||||||
| Bulk>7.5cm, N (%) | |||||||||||
| No | 39 (79.6) | 26 (74.3) | 41 (66.1) | 149 (70.0) | 8 (53.3) | .14 | .22 | .61 | .50 | .69 | .64 |
| Yes | 10 (20.4) | 9 (25.7) | 21 (33.9) | 64 (30.0) | 7 (46.7) | ||||||
| Missing/unknown | 0 | 0 | 0 | 0 | 1 | ||||||
| No. of extra nodal sites, N (%) | |||||||||||
| None | 29 (59.2) | 15 (44.1) | 25 (40.3) | 59 (27.7) | 4 (25.0) | .039 | <.001 | .083 | .90 | .19 | .041 |
| 1-2 | 19 (38.8) | 14 (41.2) | 33 (53.2) | 129 (60.6) | 9 (56.3) | ||||||
| 3+ | 1 (2.0) | 5 (14.7) | 4 (6.5) | 25 (11.7) | 3 (18.8) | ||||||
| Missing/unknown | 0 | 1 | 0 | 0 | 0 | ||||||
| LDH, N (%) | |||||||||||
| <ULN | 12 (25.0) | 10 (28.6) | 19 (33.3) | 40 (19.6) | 0 | .83 | .062 | .79 | .67 | .23 | .039 |
| >ULN | 29 (60.4) | 17 (48.6) | 26 (45.6) | 104 (51.0) | 10 (71.4) | ||||||
| >2ULN | 7 (14.6) | 8 (22.9) | 12 (21.1) | 60 (29.4) | 4 (28.6) | ||||||
| Missing/unknown | 1 | 0 | 5 | 9 | 2 | ||||||
| IPI, N (%) | |||||||||||
| 0-2 | 26 (60.5) | 20 (57.1) | 32 (56.1) | 93 (45.1) | 8 (57.1) | .69 | .093 | .82 | >.99 | .20 | .18 |
| 3+ | 17 (39.5) | 15 (42.9) | 25 (43.9) | 113 (54.9) | 6 (42.9) | ||||||
| Missing/unknown | 6 | 0 | 5 | 7 | 2 | ||||||
| Fit for SCT?, N (%) | |||||||||||
| No | 11 (22.4) | 7 (20.0) | 6 (9.7) | 43 (20.2) | 5 (31.3) | .11 | .70 | >.99 | .21 | >.99 | .061 |
| Yes | 38 (77.6) | 28 (80.0) | 56 (90.3) | 170 (79.8) | 11 (68.8) | ||||||
| HCT-CI score, N (%) | |||||||||||
| <3 | 47 (95.9) | 30 (85.7) | 59 (96.7) | 194 (91.9) | 13 (81.3) | >.99 | .54 | .12 | .22 | .22 | .26 |
| ≥3 | 2 (4.1) | 5 (14.3) | 2 (3.3) | 17 (8.1) | 3 (18.8) | ||||||
| Missing/unknown | 0 | 0 | 1 | 2 | 0 | ||||||
| Life expectancy of <3 mo if no response to bridging, N (%) | |||||||||||
| No | 42 (85.7) | 20 (58.8) | 42 (67.7) | 120 (56.3) | 11 (68.8) | .044 | <.001 | .009 | .50 | .85 | .14 |
| Yes | 7 (14.3) | 14 (41.2) | 20 (32.3) | 93 (43.7) | 5 (31.3) | ||||||
| Missing/unknown | 0 | 1 | 0 | 0 | 0 | ||||||
| >2 previous lines, N (%) | |||||||||||
| No | 29 (59.2) | 19 (54.3) | 43 (69.4) | 123 (57.7) | 12 (75.0) | .32 | .87 | .66 | .19 | .72 | .11 |
| Yes | 20 (40.8) | 16 (45.7) | 19 (30.6) | 90 (42.3) | 4 (25.0) | ||||||
| Previous transplant, N (%) | |||||||||||
| No | 41 (83.7) | 30 (85.7) | 51 (82.3) | 173 (81.2) | 13 (81.3) | .60 | .93 | >.99 | .36 | .70 | .69 |
| Auto | 7 (14.3) | 4 (11.4) | 11 (17.7) | 35 (16.4) | 3 (18.8) | ||||||
| Allo | 1 (2.0) | 1 (2.9) | 0 | 5 (2.3) | 0 | ||||||
| Refractory, N (%) | |||||||||||
| No | 24 (50.0) | 21 (60.0) | 34 (55.7) | 117 (57.6) | 9 (60.0) | .57 | .42 | .38 | .83 | .85 | .88 |
| Yes | 24 (50.0) | 14 (40.0) | 27 (44.3) | 86 (42.4) | 6 (40.0) | ||||||
| Missing/unknown | 1 | 0 | 1 | 10 | 1 | ||||||
| Response to last line, N (%) | |||||||||||
| CMR/PR | 18 (36.7) | 13 (37.1) | 15 (24.2) | 50 (23.5) | 5 (31.3) | .21 | .070 | >.99 | .24 | .096 | >.99 |
| SD/PD | 31 (63.3) | 22 (62.9) | 47 (75.8) | 163 (76.5) | 11 (68.8) | ||||||
| . | No bridging . | Bridging therapy . | P-value none vs RT . | P-value none vs CT . | P-value steroids vs none . | P-value steroids vs RT . | P-value steroids vs CT . | P-value CT vs RT . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Steroids . | RT . | CT . | CMT . | ||||||||
| N = 49 . | N = 35 . | N = 62 . | N = 213 . | N = 16 . | |||||||
| Age, y | 63.0 (58-68) | 58.0(51-65) | 57.0(49-66) | 60.0(51-68) | 63.0(47.5-66.5) | .0082 | .080 | .069 | .54 | .78 | .20 |
| Sex, N (%) | |||||||||||
| Male | 30 (61.2) | 21 (60.0) | 37 (59.7) | 134 (62.9) | 8 (50.0) | >.99 | .87 | >.99 | >.99 | .85 | .66 |
| Female | 19 (38.8) | 14 (40.0) | 25 (40.3) | 79 (37.1) | 8 (50.0) | ||||||
| Disease type, N (%) | |||||||||||
| De novo DLBCL | 31 (63.3) | 24 (68.6) | 40 (64.5) | 148 (69.5) | 9 (56.3) | .95 | .72 | .53 | .58 | .30 | .44 |
| PMBL | 3 (6.1) | 0 | 4 (6.5) | 11 (5.2) | 2 (12.5) | ||||||
| tFL | 12 (24.5) | 10 (28.6) | 16 (25.8) | 39 (18.3) | 4 (25.0) | ||||||
| t-Other | 3 (6.1) | 1 (2.9) | 2 (3.2) | 15 (7.0) | 1 (6.3) | ||||||
| DHL/THL, N (%) | |||||||||||
| No | 28 (75.7) | 24 (75.0) | 38 (69.1) | 128 (68.8) | 8 (61.5) | .84 | .78 | >.99 | .88 | .87 | .84 |
| Yes | 4 (10.8) | 3 (9.4) | 8 (14.5) | 23 (12.4) | 2 (15.4) | ||||||
| DE/TE | 5 (13.5) | 5 (15.6) | 9 (16.4) | 35 (18.8) | 3 (23.1) | ||||||
| Missing/unknown | 12 | 3 | 7 | 27 | 3 | ||||||
| Stage, N (%) | |||||||||||
| Stage 1-2 | 14 (30.4) | 9 (25.7) | 18 (29.5) | 33 (15.6) | 2 (13.3) | >.99 | .033 | .80 | .82 | .15 | .024 |
| Stage 3-4 | 32 (69.6) | 26 (74.3) | 43 (70.5) | 179 (84.4) | 13 (86.7) | ||||||
| Missing/unknown | 3 | 0 | 1 | 1 | 1 | ||||||
| ECOG, N (%) | |||||||||||
| 0 | 31 (63.3) | 18 (51.4) | 32 (51.6) | 80 (37.6) | 3 (18.8) | .25 | .001 | .37 | >.99 | .14 | .056 |
| 1 | 18 (36.7) | 17 (48.6) | 30 (48.4) | 133 (62.4) | 13 (81.3) | ||||||
| Bulk>7.5cm, N (%) | |||||||||||
| No | 39 (79.6) | 26 (74.3) | 41 (66.1) | 149 (70.0) | 8 (53.3) | .14 | .22 | .61 | .50 | .69 | .64 |
| Yes | 10 (20.4) | 9 (25.7) | 21 (33.9) | 64 (30.0) | 7 (46.7) | ||||||
| Missing/unknown | 0 | 0 | 0 | 0 | 1 | ||||||
| No. of extra nodal sites, N (%) | |||||||||||
| None | 29 (59.2) | 15 (44.1) | 25 (40.3) | 59 (27.7) | 4 (25.0) | .039 | <.001 | .083 | .90 | .19 | .041 |
| 1-2 | 19 (38.8) | 14 (41.2) | 33 (53.2) | 129 (60.6) | 9 (56.3) | ||||||
| 3+ | 1 (2.0) | 5 (14.7) | 4 (6.5) | 25 (11.7) | 3 (18.8) | ||||||
| Missing/unknown | 0 | 1 | 0 | 0 | 0 | ||||||
| LDH, N (%) | |||||||||||
| <ULN | 12 (25.0) | 10 (28.6) | 19 (33.3) | 40 (19.6) | 0 | .83 | .062 | .79 | .67 | .23 | .039 |
| >ULN | 29 (60.4) | 17 (48.6) | 26 (45.6) | 104 (51.0) | 10 (71.4) | ||||||
| >2ULN | 7 (14.6) | 8 (22.9) | 12 (21.1) | 60 (29.4) | 4 (28.6) | ||||||
| Missing/unknown | 1 | 0 | 5 | 9 | 2 | ||||||
| IPI, N (%) | |||||||||||
| 0-2 | 26 (60.5) | 20 (57.1) | 32 (56.1) | 93 (45.1) | 8 (57.1) | .69 | .093 | .82 | >.99 | .20 | .18 |
| 3+ | 17 (39.5) | 15 (42.9) | 25 (43.9) | 113 (54.9) | 6 (42.9) | ||||||
| Missing/unknown | 6 | 0 | 5 | 7 | 2 | ||||||
| Fit for SCT?, N (%) | |||||||||||
| No | 11 (22.4) | 7 (20.0) | 6 (9.7) | 43 (20.2) | 5 (31.3) | .11 | .70 | >.99 | .21 | >.99 | .061 |
| Yes | 38 (77.6) | 28 (80.0) | 56 (90.3) | 170 (79.8) | 11 (68.8) | ||||||
| HCT-CI score, N (%) | |||||||||||
| <3 | 47 (95.9) | 30 (85.7) | 59 (96.7) | 194 (91.9) | 13 (81.3) | >.99 | .54 | .12 | .22 | .22 | .26 |
| ≥3 | 2 (4.1) | 5 (14.3) | 2 (3.3) | 17 (8.1) | 3 (18.8) | ||||||
| Missing/unknown | 0 | 0 | 1 | 2 | 0 | ||||||
| Life expectancy of <3 mo if no response to bridging, N (%) | |||||||||||
| No | 42 (85.7) | 20 (58.8) | 42 (67.7) | 120 (56.3) | 11 (68.8) | .044 | <.001 | .009 | .50 | .85 | .14 |
| Yes | 7 (14.3) | 14 (41.2) | 20 (32.3) | 93 (43.7) | 5 (31.3) | ||||||
| Missing/unknown | 0 | 1 | 0 | 0 | 0 | ||||||
| >2 previous lines, N (%) | |||||||||||
| No | 29 (59.2) | 19 (54.3) | 43 (69.4) | 123 (57.7) | 12 (75.0) | .32 | .87 | .66 | .19 | .72 | .11 |
| Yes | 20 (40.8) | 16 (45.7) | 19 (30.6) | 90 (42.3) | 4 (25.0) | ||||||
| Previous transplant, N (%) | |||||||||||
| No | 41 (83.7) | 30 (85.7) | 51 (82.3) | 173 (81.2) | 13 (81.3) | .60 | .93 | >.99 | .36 | .70 | .69 |
| Auto | 7 (14.3) | 4 (11.4) | 11 (17.7) | 35 (16.4) | 3 (18.8) | ||||||
| Allo | 1 (2.0) | 1 (2.9) | 0 | 5 (2.3) | 0 | ||||||
| Refractory, N (%) | |||||||||||
| No | 24 (50.0) | 21 (60.0) | 34 (55.7) | 117 (57.6) | 9 (60.0) | .57 | .42 | .38 | .83 | .85 | .88 |
| Yes | 24 (50.0) | 14 (40.0) | 27 (44.3) | 86 (42.4) | 6 (40.0) | ||||||
| Missing/unknown | 1 | 0 | 1 | 10 | 1 | ||||||
| Response to last line, N (%) | |||||||||||
| CMR/PR | 18 (36.7) | 13 (37.1) | 15 (24.2) | 50 (23.5) | 5 (31.3) | .21 | .070 | >.99 | .24 | .096 | >.99 |
| SD/PD | 31 (63.3) | 22 (62.9) | 47 (75.8) | 163 (76.5) | 11 (68.8) | ||||||
Compares RT/CT/CMT. P-values are χ2 or Fisher exact test (discreate variables) or Kruskal-Wallis (continuous).
CMR, complete metabolic response; DHL, double hit lymphoma; DE, double expressor; HCT-CI, Hematopoietic Cell Transplantation-specific Comorbidity Index; PMBL, primary mediastinal B-cell lymphoma; SCT, stem cell transplant; SD, stable disease; tFL, transformed follicular lymphoma; THL, triple hit lymphoma; TE, triple expressor; ULN, upper limit of normal.
HDT (compared with LDT) was delivered to younger patients (median age, 53 years vs 65 years; P = .0016), to those with advanced stage disease (stage III/IV, 91.7% vs 78.4%; P = .029) and to “rapid progressors,” ie, patients in whom life expectancy without response to BT was predicted to be <3 months by clinicians (74.5% vs 41.7%; P < .001). Patients who received RBP were also older and had higher HCT-CI scores (15.8% vs 4.8%, ≥3; P = .015) compared to those in HDT group (supplemental Table 3).
BT selection evolved toward more intensive approaches in between 2019 and 2020 compared with that between 2018 and 2019. CT (64.0% vs 51.2%) and RT (19.9% vs 14.7%) were increasingly used, with a concomitant reduction in corticosteroids alone (1.8% vs 15.2%) and no BT (10.4% vs 15.2%) (supplemental Table 4). RBP became the CT regimen of choice (68.1% vs 6.1%) through the early access to medicines scheme from June 2019 onwards. Patient selection also evolved between 2019 and 2020 to increasingly include older patients (median age, 62.5 years vs 58 years; P = .011), those unfit for autostem cell transplantation (23.8% vs 15.6%; P = .047), and patients with primary refractory disease (ie, refractory to rituximab-cyclophosphamide-hydroxydaunorubicin-oncovin-prednisone) or “never responders” (ie, refractory to all lines of therapy before CAR-T therapy) (49.7% vs 38.6%; P = .036), likely reflecting access to RBP as a tolerable and effective BT, and clinicians growing confidence and familiarity with delivery of CAR-T.
Response to BT and impact on CAR-T infusion rates
Overall response rates (ORR) to BT were higher with RT and RBP (65% and 42%, respectively) than with LDT (18%), HDT (29%), and CMT (33%) (Table 2). Overall, 23 patients achieved CR to BT: 4 after RT and 19 after CT (RBP in 11/19 cases), and 13 and 8 patients were infused with Axi-cel and Tisa-cel, respectively. Failure to reach CAR-T infusion was higher in patients who underwent HDT than in those in other cohorts (30% vs 13%-18%), largely owing to progressive disease (PD), central nervous system relapse, or death. Only 6 patients in total (RT, N = 1; CT, N = 4; CMT, N = 1) died of non-PD causes, mostly infection (5/6 cases). The interval between leukapheresis and infusion was not significantly different among BT cohorts, but significant delays of ≥8 weeks were most commonly ascribed to adverse events (N = 11) and COVID-19 (N = 10) and were more frequent in patients who underwent BT (Table 2).
Response to bridging and feasibility of CAR-T infusion
| Apheresed patients . | No bridging . | Bridging therapy . | P-value|| . | CT bridging . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Steroids . | RT . | CT . | CMT . | Low dose . | High dose§ . | RBP . | |||
| N = 49 . | N = 35 . | N = 62 . | N = 213 . | N = 16 . | N = 51 . | N = 84 . | N = 77 . | ||
| Response to bridging, N (%) | |||||||||
| CR/PR | - | - | 40 (67.8) | 64 (31.4) | 5 (33.3) | <.001 | 8 (15.7) | 23 (27.4) | 33 (42.9) |
| SD/PD/death before infusion | - | - | 19 (32.2) | 140 (68.6) | 10 (66.7) | 35 (68.3) | 60 (71.4) | 43 (57.1) | |
| Unknown | - | - | 3 | 9 | 1 | 8 | 1 | 0 | |
| CR | - | - | 4 (6.8) | 19 (9.3) | 0 | <.001 | 1 (2.0) | 7 (8.3) | 11 (14.3) |
| Infused, N (%) | |||||||||
| Infused | 40 (81.6) | 29 (82.9) | 54 (87.1) | 166 (77.9) | 11 (68.8) | .39 | 42 (82.4) | 58 (69.1) | 66 (85.7) |
| Not infused | 9 (18.4) | 6 (17.1) | 8 (12.9) | 47 (22.1) | 5 (31.3) | 9 (17.7) | 26 (31.0) | 11 (14.3) | |
| PD/CNS relapse/death due to PD | 7 | 6 | 5 | 34 | 4 | 7 | 21 | 5 | |
| Death (not PD)∗ | 0 | 0 | 1 | 4 | 1 | 0 | 2 | 2 | |
| Patient fitness/AE† | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 2 | |
| Manufacturing failure | 0 | 0 | 0 | 5 | 0 | 2 | 2 | 1 | |
| Other‡ | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | |
| Apheresed patients . | No bridging . | Bridging therapy . | P-value|| . | CT bridging . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Steroids . | RT . | CT . | CMT . | Low dose . | High dose§ . | RBP . | |||
| N = 49 . | N = 35 . | N = 62 . | N = 213 . | N = 16 . | N = 51 . | N = 84 . | N = 77 . | ||
| Response to bridging, N (%) | |||||||||
| CR/PR | - | - | 40 (67.8) | 64 (31.4) | 5 (33.3) | <.001 | 8 (15.7) | 23 (27.4) | 33 (42.9) |
| SD/PD/death before infusion | - | - | 19 (32.2) | 140 (68.6) | 10 (66.7) | 35 (68.3) | 60 (71.4) | 43 (57.1) | |
| Unknown | - | - | 3 | 9 | 1 | 8 | 1 | 0 | |
| CR | - | - | 4 (6.8) | 19 (9.3) | 0 | <.001 | 1 (2.0) | 7 (8.3) | 11 (14.3) |
| Infused, N (%) | |||||||||
| Infused | 40 (81.6) | 29 (82.9) | 54 (87.1) | 166 (77.9) | 11 (68.8) | .39 | 42 (82.4) | 58 (69.1) | 66 (85.7) |
| Not infused | 9 (18.4) | 6 (17.1) | 8 (12.9) | 47 (22.1) | 5 (31.3) | 9 (17.7) | 26 (31.0) | 11 (14.3) | |
| PD/CNS relapse/death due to PD | 7 | 6 | 5 | 34 | 4 | 7 | 21 | 5 | |
| Death (not PD)∗ | 0 | 0 | 1 | 4 | 1 | 0 | 2 | 2 | |
| Patient fitness/AE† | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 2 | |
| Manufacturing failure | 0 | 0 | 0 | 5 | 0 | 2 | 2 | 1 | |
| Other‡ | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | |
| Infused patients . | No bridging . | Bridging therapy . | P-value . | CT bridging . | |||||
|---|---|---|---|---|---|---|---|---|---|
| . | Steroids . | RT . | CT . | CMT . | Low dose . | High dose . | RBP . | ||
| N = 40 . | N = 29 . | N = 54 . | N = 166 . | N = 11 . | N = 43 . | N = 64 . | N = 70 . | ||
| Response, N (%) | |||||||||
| CR/PR | - | - | 37 (72.6) | 63 (39.9) | 4 (40.0) | <.001 | 8 (18.6) | 24 (37.5) | 35 (50.0) |
| SD/PD/death before infusion | - | - | 14 (27.5) | 95 (60.1) | 6 (60.0) | 28 (65.1) | 38 (59.4) | 35 (50.0) | |
| Unknown | 3 | 8 | 1 | 7 | 1 | 0 | |||
| CR | - | - | 2 (3.9) | 19 (12.0) | 0 | <.001 | 1 (2.4) | 7 (12.1) | 11 (16.7) |
| Time to infusion (d), median (IQR) range | 45 (37.5-49) 33 - 189 | 42 (35-47.5) 35 - 105 | 42 (36-55) 32 - 118 | 42 (37-54) 12 - 264 | 54 (48-62) 34-74 | .17 | 40 (34-48) 12-116 | 43 (39-53) 32-159 | 41.5 (37-57) 16 - 264 |
| Number delayed >8 wk | 5 (12.5) | 2 (6.9) | 13 (24.1) | 33 (19.9) | 5 (45.5) | .049 | 7 (16.7) | 9 (15.5) | 17 (25.8) |
| Delay reasons (patients could have multiple reasons) | |||||||||
| AE | 2 | 0 | 4 | 5 | 0 | 1 | 3 | 10 | |
| PD/disease requiring further bridging | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | |
| Apheresis/manufacturing capacity | 1 | 1 | 3 | 0 | 0 | 1 | 1 | 1 | |
| Bridging | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 0 | |
| CMR | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | |
| COVID | 0 | 0 | 2 | 8 | 0 | 1 | 2 | 5 | |
| Patient choice | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | |
| Awaiting biopsy results | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | |
| Unknown | 1 | 1 | 4 | 14 | 4 | 1 | 3 | 10 | |
| Preinfusion characteristics | |||||||||
| Low platelets (<50) | 1 (2.5) | 4 (13.8) | 1 (1.9) | 18 (10.8) | 0 | .075 | 3 (7.1) | 10 (17.2) | 5 (7.6) |
| Low lymphocytes (<0.5) | 7 (17.5) | 12 (41.4) | 26 (48.2) | 76 (46.6) | 6 (54.6) | .008 | 23 (57.5) | 20 (34.5) | 33 (50.8) |
| LDH | |||||||||
| Normal | 12 (30.8) | 9 (31/90) | 15 (30.6) | 32 (20.0) | 0 | .057 | 8 (19.1) | 8 (13.8) | 16 (24.2) |
| >ULN | 24 (61.5) | 16 (55.2) | 23 (46.9) | 85 (53.1) | 8 (80.0) | 244 (57.1) | 27 (46.6) | 24 (51.5) | |
| >2ULN | 3 (7.7) | 4 (13.8) | 11 (22.5) | 43 (26.9) | 2 (20.0) | 8 (19.1) | 19 (32.8) | 16 (24.2) | |
| Missing | 1 | 0 | 5 | 6 | 1 | 2 | 4 | 0 | |
| Infused patients . | No bridging . | Bridging therapy . | P-value . | CT bridging . | |||||
|---|---|---|---|---|---|---|---|---|---|
| . | Steroids . | RT . | CT . | CMT . | Low dose . | High dose . | RBP . | ||
| N = 40 . | N = 29 . | N = 54 . | N = 166 . | N = 11 . | N = 43 . | N = 64 . | N = 70 . | ||
| Response, N (%) | |||||||||
| CR/PR | - | - | 37 (72.6) | 63 (39.9) | 4 (40.0) | <.001 | 8 (18.6) | 24 (37.5) | 35 (50.0) |
| SD/PD/death before infusion | - | - | 14 (27.5) | 95 (60.1) | 6 (60.0) | 28 (65.1) | 38 (59.4) | 35 (50.0) | |
| Unknown | 3 | 8 | 1 | 7 | 1 | 0 | |||
| CR | - | - | 2 (3.9) | 19 (12.0) | 0 | <.001 | 1 (2.4) | 7 (12.1) | 11 (16.7) |
| Time to infusion (d), median (IQR) range | 45 (37.5-49) 33 - 189 | 42 (35-47.5) 35 - 105 | 42 (36-55) 32 - 118 | 42 (37-54) 12 - 264 | 54 (48-62) 34-74 | .17 | 40 (34-48) 12-116 | 43 (39-53) 32-159 | 41.5 (37-57) 16 - 264 |
| Number delayed >8 wk | 5 (12.5) | 2 (6.9) | 13 (24.1) | 33 (19.9) | 5 (45.5) | .049 | 7 (16.7) | 9 (15.5) | 17 (25.8) |
| Delay reasons (patients could have multiple reasons) | |||||||||
| AE | 2 | 0 | 4 | 5 | 0 | 1 | 3 | 10 | |
| PD/disease requiring further bridging | 2 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | |
| Apheresis/manufacturing capacity | 1 | 1 | 3 | 0 | 0 | 1 | 1 | 1 | |
| Bridging | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 0 | |
| CMR | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | |
| COVID | 0 | 0 | 2 | 8 | 0 | 1 | 2 | 5 | |
| Patient choice | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 1 | |
| Awaiting biopsy results | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | |
| Unknown | 1 | 1 | 4 | 14 | 4 | 1 | 3 | 10 | |
| Preinfusion characteristics | |||||||||
| Low platelets (<50) | 1 (2.5) | 4 (13.8) | 1 (1.9) | 18 (10.8) | 0 | .075 | 3 (7.1) | 10 (17.2) | 5 (7.6) |
| Low lymphocytes (<0.5) | 7 (17.5) | 12 (41.4) | 26 (48.2) | 76 (46.6) | 6 (54.6) | .008 | 23 (57.5) | 20 (34.5) | 33 (50.8) |
| LDH | |||||||||
| Normal | 12 (30.8) | 9 (31/90) | 15 (30.6) | 32 (20.0) | 0 | .057 | 8 (19.1) | 8 (13.8) | 16 (24.2) |
| >ULN | 24 (61.5) | 16 (55.2) | 23 (46.9) | 85 (53.1) | 8 (80.0) | 244 (57.1) | 27 (46.6) | 24 (51.5) | |
| >2ULN | 3 (7.7) | 4 (13.8) | 11 (22.5) | 43 (26.9) | 2 (20.0) | 8 (19.1) | 19 (32.8) | 16 (24.2) | |
| Missing | 1 | 0 | 5 | 6 | 1 | 2 | 4 | 0 | |
RT (pneumonia), CT (Covid 19, neutropenic sepsis/PCP, sepsis, sudden death/PE), CMT (neutropenic sepsis/ischemic bowel); †RT (Clinical deterioration owing to LRTI/influenza requiring intubation), CT ( clinical deterioration–perforation, inflammatory pneumonitis, MI); ‡No bridging (patient was in CMR, decided not to proceed, MDS diagnosis), RT (patient was in CMR, decided not to proceed), CT (Patient choice), §ORR/CR rate by HDT group, all apheresed patients: HDT-Ifos, 8(26.7%)/1(3.3%); HDT-Gem, 9(25.0%)/2(5.6%); HDT-other, 9(39.1%)/4(17.4%). ORR/CR rate by HDT group, all infused patients: HDT-Ifos, 7(33.3%)/1(44.8%); HDT-Gem, 8(30.8%)/2(7.7%); HDT-other, 9(60.0%)/4(26.7%).
P-values are χ2 or Fisher exact test (discreate variables) or Kruskal-Wallis (continuous) and compare all 5 groups (except response which compares RT/CT/CMT only).
CAR-T toxicity according to BT and response to BT
No difference in cytokine release syndrome (CRS), immune effector cell–associated neurotoxicity syndrome (ICANS), use of tocilizumab/corticosteroids or intensive care unit admission was observed across BT cohorts (Table 3; supplemental Table 5). Rates of ≥G3 thrombocytopenia at 1-month after CAR-T therapy differed by BT type (P < .001), with the highest incidence observed in patients who underwent CT-bridged therapy (56.0%) compared to those with no BT (20.6%) and other BT groups (10%-31.9%). There was a higher incidence of ≥G3 neutropenia at 1-month in BT vs no BT groups (40%-45% vs 27.3%; P = .051), and within the CT-bridged group, rates of both were highest in patients who underwent HDT-bridged therapy compared with those who underwent LDT and RBP-BT (P = .012; and P = .002). There were no significant differences at month 3.
Toxicity from CAR-T according to bridging strategy
| . | No bridging . | Bridging therapy . | P-value . | |||
|---|---|---|---|---|---|---|
| Steroids . | RT . | CT . | CMT . | |||
| N = 40 . | N = 29 . | N = 54 . | N = 166 . | N = 11 . | ||
| CAR-T toxicities | ||||||
| CRS (grade 3+), N (%) | 1 (2.5) | 4 (13.8) | 5 (9.3) | 11 (6.6) | 2 (18.2) | .19 |
| ICANS (grade 3+), N (%) | 7 (17.5) | 6 (20.7) | 8 (14.8) | 26 (15.7) | 0 | .64 |
| Grade 3+ Neutropenia (1 mo)∗ | 9/33 (27.3) | 9/20 (45.0) | 20/45 (44.4) | 64/114 (44.4) | 4/10 (40.0) | .051 |
| Grade 3+ Neutropenia (3 mo)∗ | 3/20 (15.0) | 2/8 (25.0) | 7/31 (22.6) | 13/67 (19.4) | 1/5 (20.0) | .94 |
| Grade 3+ Thrombocytopenia (1 mo)∗ | 7/34 (20.6) | 6/30 (30.0) | 15/47 (31.9) | 65/116 (56.0) | 1/10 (10.0) | <.001 |
| Grade 3+ Thrombocytopenia (3 mo)∗ | 1/20 (5.0) | 0/8 (0) | 2/31 (6.5) | 15/67 (22.4) | 1/5 (20) | .11 |
| Toxicity therapies | ||||||
| Steroids given, N (%) | 15 (37.5) | 13 (44.8) | 23 (45.6) | 61 (36.8) | 4 (36.4) | .88 |
| Tocilizumab used, N (%) | 22 (55.0) | 30 (69.0) | 39 (72.2) | 114 (68.7) | 5 (45.5) | .22 |
| ITU required, N (%) | 9 (22.5) | 11 (37.9) | 14 (25.9) | 46 (27.7) | 3 (27.3) | .72 |
| Observation only | 1 (2.5) | 3 (10.3) | 6 (11.1) | 15 (9.1) | 0 | |
| Inotropes | 4 (10.0) | 5 (17.2) | 5 (9.3) | 16 (9.7) | 3 (26.3) | |
| Organ support/Intubation | 4 (10.0) | 3 (10.3) | 3 (5.6) | 14 (8.5) | 0 | |
| Cumulative incidence of NRM at 1 y | 8.0% (2.6-23.0) | 3.5% (0.5-22.1) | 3.7% (0.9-14.0) | 9.4% (5.6-15.5) | 0% | |
| NRM events | 3 | 1 | 2 | 15 | 0 | |
| Infection† | 2 | 1 | 1 | 11 | 0 | |
| Cardiac | 1 | 0 | 0 | 0 | 0 | |
| Haematemesis | 0 | 0 | 1 | 0 | 0 | |
| Second malignancy | 0 | 0 | 0 | 1 | 0 | |
| HLH | 0 | 0 | 0 | 1 | 0 | |
| Bowel perforation | 0 | 0 | 0 | 2 | 0 | |
| . | No bridging . | Bridging therapy . | P-value . | |||
|---|---|---|---|---|---|---|
| Steroids . | RT . | CT . | CMT . | |||
| N = 40 . | N = 29 . | N = 54 . | N = 166 . | N = 11 . | ||
| CAR-T toxicities | ||||||
| CRS (grade 3+), N (%) | 1 (2.5) | 4 (13.8) | 5 (9.3) | 11 (6.6) | 2 (18.2) | .19 |
| ICANS (grade 3+), N (%) | 7 (17.5) | 6 (20.7) | 8 (14.8) | 26 (15.7) | 0 | .64 |
| Grade 3+ Neutropenia (1 mo)∗ | 9/33 (27.3) | 9/20 (45.0) | 20/45 (44.4) | 64/114 (44.4) | 4/10 (40.0) | .051 |
| Grade 3+ Neutropenia (3 mo)∗ | 3/20 (15.0) | 2/8 (25.0) | 7/31 (22.6) | 13/67 (19.4) | 1/5 (20.0) | .94 |
| Grade 3+ Thrombocytopenia (1 mo)∗ | 7/34 (20.6) | 6/30 (30.0) | 15/47 (31.9) | 65/116 (56.0) | 1/10 (10.0) | <.001 |
| Grade 3+ Thrombocytopenia (3 mo)∗ | 1/20 (5.0) | 0/8 (0) | 2/31 (6.5) | 15/67 (22.4) | 1/5 (20) | .11 |
| Toxicity therapies | ||||||
| Steroids given, N (%) | 15 (37.5) | 13 (44.8) | 23 (45.6) | 61 (36.8) | 4 (36.4) | .88 |
| Tocilizumab used, N (%) | 22 (55.0) | 30 (69.0) | 39 (72.2) | 114 (68.7) | 5 (45.5) | .22 |
| ITU required, N (%) | 9 (22.5) | 11 (37.9) | 14 (25.9) | 46 (27.7) | 3 (27.3) | .72 |
| Observation only | 1 (2.5) | 3 (10.3) | 6 (11.1) | 15 (9.1) | 0 | |
| Inotropes | 4 (10.0) | 5 (17.2) | 5 (9.3) | 16 (9.7) | 3 (26.3) | |
| Organ support/Intubation | 4 (10.0) | 3 (10.3) | 3 (5.6) | 14 (8.5) | 0 | |
| Cumulative incidence of NRM at 1 y | 8.0% (2.6-23.0) | 3.5% (0.5-22.1) | 3.7% (0.9-14.0) | 9.4% (5.6-15.5) | 0% | |
| NRM events | 3 | 1 | 2 | 15 | 0 | |
| Infection† | 2 | 1 | 1 | 11 | 0 | |
| Cardiac | 1 | 0 | 0 | 0 | 0 | |
| Haematemesis | 0 | 0 | 1 | 0 | 0 | |
| Second malignancy | 0 | 0 | 0 | 1 | 0 | |
| HLH | 0 | 0 | 0 | 1 | 0 | |
| Bowel perforation | 0 | 0 | 0 | 2 | 0 | |
Patients with PD are excluded, †Infection details: None; Covid-19, steroids; RSV pneumonia, RT; Sepsis (NOS), Systemic; Covid-19 (N = 5), Fungal chest infection/HLH (N = 1), Necrotizing fasciitis (N = 1), sepsis (NOS) (N = 3), fungal sepsis (N =1) Notes: 1 ITU level missing (CT bridging).
BT was not associated with increased nonrelapse mortality (NRM) (supplemental Figures 1A-B; Table 3). In particular, patients who underwent CT-bridged therapy had similar NRM rates at 1-year to those who did not receive BT (9.4% vs 8.0%). Landmark analyses showed a significant association between thrombocytopenia at 1-month and higher NRM, but not inferior PFS (supplemental Figures 1C-D). No significant difference in NRM was observed for neutropenia at 1- or 3-month.
Despite a reduction in tumor burden, there was no significant difference in the incidence of ≥G3 CRS, the use of tocilizumab or intensive care unit admission in patients with CR or partial response (PR) after BT compared with nonresponders. In contrast, the incidence of ≥G3 ICANS was significantly higher in BT-nonresponders than in patients achieving CR/PR (21.7% vs 9.5%/6%; P = .005), but corticosteroid use was similar between these groups (CR = 45.2%; nonresponder = 42.9%) (supplemental Table 6).
CAR-T efficacy according to BT and response to BT
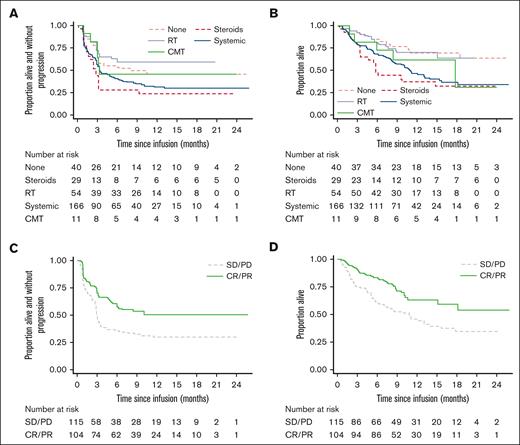
ORR to CAR-T at 3-months according to BT modality appears to show differences as follows: RT, 64.8%; no BT, 55%; CMT, 45.5%; CT, 44.6%, and corticosteroids-alone, 27.6% The same pattern was also observed in PFS and OS (Figure 2A and B; supplemental Figures 2A-B).
PFS and OS post-CAR-T according to BT modality and response. (A) PFS: comparing BT groups 1-year rates; no BT 46.0% (29.7-60.9), steroids 23.7% (10.2-40.2), RT 59.1% (44.8-70.9), CT 31.3% (23.9-38.9), CMT 45.5% (16.7-70.7). (B) OS: comparing BT groups 1-year rates; no BT 69.7% (51.4-82.2), steroids 37.4% (20.2-54.5), RT 70.3% (55.4-81.0), CT 46.9% (38.1-55.2), CMT 62.3% (27.8-84.0). (C) PFS: comparing BT responder vs nonresponder: HR 0.55 (0.39-0.79), P = .001. 1-year rates: Responder: 50.1% (39.6-59.7); Nonresponder: 29.7% (21.3-38.6). (D) OS: comparing BT responder vs nonresponder: HR 0.51 (0.33-0.77), P = .001. 1-year rates: Responder: 63.2% (51.5-72.8); Nonresponder: 45.9% (35.9-55.3).
PFS and OS post-CAR-T according to BT modality and response. (A) PFS: comparing BT groups 1-year rates; no BT 46.0% (29.7-60.9), steroids 23.7% (10.2-40.2), RT 59.1% (44.8-70.9), CT 31.3% (23.9-38.9), CMT 45.5% (16.7-70.7). (B) OS: comparing BT groups 1-year rates; no BT 69.7% (51.4-82.2), steroids 37.4% (20.2-54.5), RT 70.3% (55.4-81.0), CT 46.9% (38.1-55.2), CMT 62.3% (27.8-84.0). (C) PFS: comparing BT responder vs nonresponder: HR 0.55 (0.39-0.79), P = .001. 1-year rates: Responder: 50.1% (39.6-59.7); Nonresponder: 29.7% (21.3-38.6). (D) OS: comparing BT responder vs nonresponder: HR 0.51 (0.33-0.77), P = .001. 1-year rates: Responder: 63.2% (51.5-72.8); Nonresponder: 45.9% (35.9-55.3).
Among patients receiving BT (excluding corticosteroids-only) post-BT CR/PR vs nonresponse was associated with a 1-year PFS of 50.1% vs 29.7% (hazard ratio [HR], 0.55; 95% confidence interval [CI], 0.39-0.79; P = .001), and a 1-year OS of 63.2% vs 45.9% (HR, 0.51; 95% CI, 0.33-0.77; P = .001) (Figure 2C-D). PFS and OS benefit were similar between patients achieving CR and PR (data not shown).
Response to bridging was included in a multivariable analysis along with other pretreatment factors to investigate its association with post-CAR-T PFS. This identified a 42% reduction in the risk of progression of disease or death for patients with CR or PR to BT compared with nonresponders (Table 4). Although response to BT was prognostic, BT modality was not, because responders did well regardless of how the response was achieved (interaction P = .44 for RT/CT/CMT; P = .70 for CT type). Although a reasonable effect size was still seen in multivariate analysis (MVA) for OS (0.61 vs 0.51 in univariate analysis), this did not reach significance.
Multivariable analysis of baseline factors (at submission) influencing outcome to systemic BT and factors associated with PFS after CAR-T infusion
| Factors affecting response to BT . | Responder/N . | OR (95% CI) . | P-value . |
|---|---|---|---|
| RBP bridging | |||
| No | 34/134 | 1.00 | .010 |
| Yes | 35/83 | 2.21 (1.21-4.05) | |
| Response last line | |||
| SD/PD | 45/165 | 1.00 | .023 |
| CR/PR | 24/52 | 2.16 (1.11-4.22) | |
| Bulky disease | |||
| No | 55/149 | 1.00 | .045 |
| Yes | 14/68 | 0.49 (0.25-0.98) |
| Factors affecting response to BT . | Responder/N . | OR (95% CI) . | P-value . |
|---|---|---|---|
| RBP bridging | |||
| No | 34/134 | 1.00 | .010 |
| Yes | 35/83 | 2.21 (1.21-4.05) | |
| Response last line | |||
| SD/PD | 45/165 | 1.00 | .023 |
| CR/PR | 24/52 | 2.16 (1.11-4.22) | |
| Bulky disease | |||
| No | 55/149 | 1.00 | .045 |
| Yes | 14/68 | 0.49 (0.25-0.98) |
| Factors affecting response to CAR-T . | Events/N . | HR (95% CI) . | P-value . |
|---|---|---|---|
| LDH at LD | |||
| ≤2ULN | 74/141 | 1.00 | .001 |
| >2ULN | 34/41 | 2.06 (1.34-3.16) | |
| Extra nodal sites | |||
| <3 | 91.160 | 1.00 | .001 |
| ≥3 | 17/22 | 2.51 (1.46-4.32) | |
| BT response | |||
| SD/PD | 68/100 | 1.00 | .012 |
| CR/PR | 40/82 | 0.58 (0.38-0.89) |
| Factors affecting response to CAR-T . | Events/N . | HR (95% CI) . | P-value . |
|---|---|---|---|
| LDH at LD | |||
| ≤2ULN | 74/141 | 1.00 | .001 |
| >2ULN | 34/41 | 2.06 (1.34-3.16) | |
| Extra nodal sites | |||
| <3 | 91.160 | 1.00 | .001 |
| ≥3 | 17/22 | 2.51 (1.46-4.32) | |
| BT response | |||
| SD/PD | 68/100 | 1.00 | .012 |
| CR/PR | 40/82 | 0.58 (0.38-0.89) |
MVA baseline factors associated with response BT: (responder vs nonresponder; CR/PR vs SD/PD). ∗Logistic regression, using backward selection (P = .05 inclusion) incorporating the following variables all measured at submission: bridging chemotherapy type, age, sex, ECOG, stage, bulky disease, extra nodal sites, LDH, lymphoma subtype, DHL, refractoriness to previous therapies, response to last line, and ≥3 lines of previous therapy. MVA factors associated with PFS after infusion including BT response (only patients receiving RT/CT/CMT). Variables that remain significant in MVA (backward selection, P = .05 for rejection). Variables included in the MVA: age, sex, ECOG, stage (submission), bulky disease, extra nodal sites (submission), LDH (pre-LD), CRP, low platelets, low lymphocytes, lymphoma subtype, DHL, refractory to previous therapies, response last line, >2 lines previous therapy and response to bridging.
Impact of response to BT on Axi-cel vs Tisa-cel outcomes
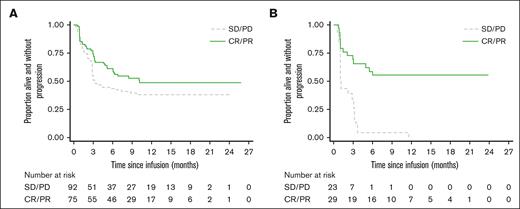
Of the patients infused with CAR-T, 224 of 300 (74.7%) received Axi-cel and 76 of 300 (25.3%) received Tisa-cel. Tisa-cel patients were significantly older and were significantly less likely to have bulky disease and/or low lymphocytes.1 Evaluation of PFS in patients treated with Axi-cel vs those treated with Tisa-cel shows a significant interaction (P = .006) between product and response to BT (Figure 3A-B). For Axi-cel, the effect was smaller, and did not reach significance (HR, 0.68 [0.45-1.03]; P = .071), but for Tisa-cel, nonresponse to BT (N = 23) was associated with disease relapse within 12 months in all patients (HR, 0.22 [0.11-0.44]; P < .001). Analysis of BT nonresponders showed that patients receiving Tisa-cel were significantly less likely than those receiving Axi-cel to achieve a response to CAR-T at 1, 3, and 6 months after infusion (supplemental Table 7). A comparison of baseline patient and disease characteristics of BT nonresponders infused with CAR-T shows a trend toward patients receiving Tisa-cel being older and less likely to have bulky disease, but without clear identifiers for heightened risk of relapse (supplemental Table 8).
PFS following Axi-cel and Tisa-cel according to BT response. (A) PFS after Axi-Cel administration: comparing BT responders vs nonresponders: HR (response vs no response): 0.68 (0.45-1.03), P = .071. (B) PFS after Tisa-Cel administration: comparing BT responders vs nonresponders: HR (response vs no response): 0.22 (0.11-0.44), P = <.001.
PFS following Axi-cel and Tisa-cel according to BT response. (A) PFS after Axi-Cel administration: comparing BT responders vs nonresponders: HR (response vs no response): 0.68 (0.45-1.03), P = .071. (B) PFS after Tisa-Cel administration: comparing BT responders vs nonresponders: HR (response vs no response): 0.22 (0.11-0.44), P = <.001.
Factors associated with response to BT
Factors that were independently associated with higher likelihood of response to BT in MVA were RBP-bridging, in which patients were twice as likely to achieve a response (overall response [OR] compared to LDT/HDT, 2.21 [1.21-4.05]; P = .010); response to last line of therapy (OR, 2.17 [1.11-4.22]; P = .023), and the absence of bulky disease (>7.5cm diameter, OR, 0.49 [0.25-0.98]; P = .045) (Table 4).
Discussion
The development of novel bridging approaches for patients with r/r LBCL referred for CAR-T therapy is a clinical research priority. Data from trials and real-world analyses show that the majority of patients who underwent CAR-T therapy currently receive conventional BT, but in chemo-refractory patients, conventional BT approaches are often ineffective and a significant proportion of bridged patients fail to reach CAR-T infusion.1,2,17 Some studies also show that there is heightened CAR-T toxicity15 and inferior outcomes associated with CAR-T15,16 for patients receiving BT, but this may be confounded through the limited use of BT in some centers, where only patients with high-risk disease features (ie, those who are already at risk of worse toxicity and outcomes after CAR-T therapy) receive BT.
In this analysis, the majority (87%) of patients (not just those with high-risk features), received BT after leukapheresis. BT selection by clinicians was based on perception of patient fitness and pace of disease progression. As expected, clinicians elected for CT-based bridging in patients with high-risk disease features (extra nodal sites, advanced stage, higher LDH, ECOG 1, and risk of “rapid progression”), hence comparison of outcomes to CT may be skewed and should be interpreted with caution.
BT was broadly safe. There was no specific association between BT modality and delayed CAR-T infusion. There was no excess toxicity observed between patients who received BT and those who did not, and specifically no difference between BT modalities. Very few patients died before CAR-T therapy from adverse events associated with BT. Rather, patients were more likely to die from PD after failure of BT to control disease.
BT modality did not affect the incidence of CAR-T toxicity, except an increased rate of G3-4 thrombocytopenia at 1-month in patients bridged with CT (highest in HDT). Protracted cytopenias are associated with increased morbidity and mortality after CAR-T therapy,23,24 and caution should be exercised in the use of BT which confers a heightened risk of hematotoxicity, albeit no evidence of a difference in NRM was seen in our cohort.
Here, we describe the largest cohort of patients treated with CAR-T in CR in the literature.21 In line with the study by Bishop et al who reported outcomes from 7 patients treated with Tisa-cel in CR after BT in the Juliet study,21 we conclude that this approach is both safe and effective. We observed similar CRS rates between patients with CR and BT-nonresponders, implying CAR-T expansion in the absence of measurable disease. Further, durable remissions were observed in the majority of patients with CR after BT. This would be unexpected after a single cycle of BT in multiple relapsed, chemo-refractory LBCL, and implies that CAR-T as “consolidation” after BT may be an effective strategy, providing evidence to clinicians that this is a valid approach.
We provide evidence for the selection of more intensive BT modalities for patients. Our data clearly show that response to BT significantly increases the likelihood of durable remission after CAR-T therapy, regardless of the bridging modality used, with a substantial 42% reduction in the risk of progression of disease or death in those with CR/PR after BT compared to that in nonresponders. These data do not allow us to definitively state that disease reduction before CAR-T, irrespective of biology, is what confers durable remissions after CAR-T therapy, or whether response to BT is simply a marker of “better disease” which may in and of itself be more CAR-T responsive.
We observed that RT or RBP was associated with the highest rates of CR/PR before CAR-T therapy and an MVA for systemically bridged patients, also suggested that RBP bridging, absence of bulky disease, and response to last line therapy were associated with higher rates of response.
Although this gives clinicians a suggestion of which patients are most likely to respond to BT, the only modifiable risk factor identified is the choice of bridging modality. With the caveat that this is not a randomized comparison, we show here in this exploratory analysis what we have observed in practice, that RBP is a safe and potent bridging option, and is twice as likely to deliver a response compared to LDT or HDT so should be strongly considered in all suitable patients.
Why RBP appeared to be more effective in delivering CR/PR than other modalities is likely because of the immunotherapeutic targeting of CD79b in patients with no prior polatuzumab exposure, whose disease was to that point “naïve” to this mode of targeting. In contrast, all patients were multiple chemotherapy-exposed and chemo-relapsed/refractory, likely increasing the futility of conventional chemotherapy-based salvage or HDT. For RBP nonresponders, alternative BT approaches are urgently required, and agents such as lenalidomide, Bruton's tyrosine kinase inhibitor25 and bispecific antibodies targeting B-cell antigens26,27 hold significant promise in this space.
Interestingly, our data suggested that BT nonresponse may have a larger prognostic impact in patients scheduled for Tisa-cel vs Axi-cel therapy. All patients infused with Tisa-cel with nonresponse to BT relapsed within 12 months of infusion. This has potentially important clinical management implications and physicians may want to carefully consider CAR-T infusion (and/or consider alternative lines of BT) in this scenario. Although there were no overt differences in baseline demographics for BT nonresponders receiving Tisa-cel vs Axi-cel therapy, we recognize that this is a subgroup analysis with relatively small patient numbers, and the potential for unmeasured confounding variables, and therefore this finding needs to be confirmed in other datasets.
We acknowledge several limitations of this retrospective data analysis. We performed multiple comparisons, had small numbers in some treatment groups, and analyzed the impact of nonrandomized treatment modalities and regimens in which there was clearly a treatment selection bias, which may not be overcome with the use of multivariable analyses. However, despite these caveats, these data suggest that BT is safe, and that a reduction in disease burden before CAR-T therapy can lead to better outcomes.
The median time from apheresis to infusion in the United Kingdom was 42 days (interquartile range, 37-53).1 Although this interval between apheresis and infusion may appear long, we found no association between PFS after CAR-T and time to infusion,1 and the interval is in keeping with other real world datasets.8,28 In the United States, this interval can be shorter and may deter physicians from giving BT16 owing to time constraints. However, it is becoming increasingly apparent that BT may be more than just a “holding measure” during the manufacture period. We and others have shown that CR or PR after effective BT is associated with lower rates of immunotoxicity and better PFS after CAR-T therapy.22
As we begin to treat older, frailer patients in whom the minimization of immunotoxicity is paramount, the role of BT in disease burden reduction before CAR-T therapy becomes increasingly important. Further, the clear association of BT response and improved PFS illustrates that better BT approaches may help to improve CAR-T therapy outcomes, independently of advances in CAR-T design or targeting. Optimized BT toward increased CR or PR and improved CAR-T intention to treat may also affect health economics and quality of life measures.
Here, we identify pretreatment factors predictive of response to BT and highlight the transformative impact of RBP-bridging as a safe and effective strategy to improve CAR-T delivery and ITT.
Acknowledgments
The authors thank the patients, their relatives and caregivers, and investigators and staff involved in this analysis. C.R. and W.T. received funding from the National Institute for Health and Care Research (NIHR) University College London Hospitals NHS Foundation Trust Biomedical Research Centre.
Authorship
Contribution: C.R., A.K., A.A.K., and L.N. conceived of the project, collected the data, and wrote the manuscript; and all remaining authors collected data and wrote and reviewed the manuscript.
Conflict-of-interest disclosure: A.K., S.C., C.B, S.I., and C.R. have served on advisory boards and received honoraria from Kite/Gilead, Novartis, and Bristol Myers Squibb. A.A.K. received honoraria from Kite/Gilead. R.S., D.I., B.U., E.T., C.J., and M.O. have served on advisory boards and received honoraria from Kite/Gilead and Novartis. W.O. has served on advisory boards and received honoraria from Kite/Gilead, Novartis, Bristol Myers Squibb, Janssen, Roche, Servier, and Pfizer. W.T. has received honoraria and consultancy fees from Kite, Bristol Myers Squibb, and Roche. The remaining authors declare no competing financial interests.
Correspondence: Claire Roddie, University College London Cancer Institute, 72 Huntley St, London, United Kingdom, WC1E 6DD; e-mail: c.roddie@ucl.ac.uk.
References
Author notes
Data are available on request from the corresponding author, Claire Roddie (c.roddie@ucl.ac.uk).
The full-text version of this article contains a data supplement.