TO THE EDITOR:
Multiple myeloma (MM) is a plasma cell malignancy characterized by the clonal proliferation of plasma cells and the presence of monoclonal immunoglobulins (M-protein) in the tumor cells. MM accounts for ∼1% to 2% of all malignancies and has a distinctly late age of onset with a median age at diagnosis of ∼70 years.1-4
The life span study (LSS) is a cohort study of 120 321 atomic-bomb (A-bomb) survivors, followed up for investigating the effects of radiation exposure on malignant and other diseases.5,6 In general, epidemiological findings on the relationship between exposure to ionizing radiation and MM are inconsistent.7 Some studies of occupational populations have shown an increased risk of MM associated with radiation exposure,8-10 whereas the LSS has not shown any evidence of radiation-associated excess risk for MM to date.6,11 Monoclonal gammopathy of undetermined significance (MGUS) is a condition characterized by the presence of M-protein and low plasma cell content in the bone marrow. It is generally believed that almost all MM cases are preceded by first MGUS and then smoldering MM, which shares the same morphologic features as symptomatic MM, but lacks evidence of end-organ damage.12-14 A study of >50 000 Nagasaki A-bomb survivors found significantly elevated risks of MGUS among the survivors exposed to the bomb at younger ages.15 Other studies of fewer numbers of A-bomb survivors did not find a significant effect of radiation on MGUS.16,17
Previously, we reported on the radiation-related risks for incidence of lymphoid neoplasms by histological type in the LSS for the period of 1950 to 1994.18 In that study, lymphoid tumor types were determined primarily by histological verification (70% overall). For MM, however, less than half of the cases were histologically verified, whereas the remaining cases were diagnosed based on medical record reviews. We were concerned about diagnostic certainty for the large number of cases without histological verification. Therefore, we conducted a standardized hemato-pathological review of all MM cases in the previously reported study and assessed diagnostic certainty based on histological and clinical features. Briefly, we classified the possible 166 MM cases in the previous study into 3 categories based on diagnostic certainty (supplemental Table 1). Detailed methods are available in supplemental Methods. This study was approved by the Institutional Review Board of the Radiation Effects Research Foundation (RP3-94) and was conducted in accordance with the Declaration of Helsinki.
Among the 166 potential MM cases, 164 were classified by the hemato-pathology review as MM with varying diagnostic certainty and 2 were rejected as misclassified lymphoma. There were 122 primary MM cases diagnosed in Hiroshima and Nagasaki prefectures between 1950 and 1994 with radiation dose estimates, and they were used in the radiation dose–response analyses. These included 67 definite cases, 23 probable cases, and 32 undetermined cases (supplemental Figure 1). Histological materials were reviewed for 64%, 52%, and 9%, of definite, probable, and undetermined cases, respectively (supplemental Table 2). The availability of histological materials varied little among different dose categories. A majority (81%) of 54 histologically verified cases in the previous study18 were classified as definite in the present study.
The demographic distribution of MM cases and crude incidence rates (per 100 000 person-years) by diagnostic certainty are presented (Table 1). For definite MM, the rates increased with attained age (age at diagnosis) and the highest rate was observed in the oldest age group (70 years or older). The lowest rates among those exposed at age <20 need to be interpreted with caution because, at the end of the current follow-up, a majority of these young survivors had not reached the ages at which one sees the largest MM rates (ie, 70 years and older). The highest rate during the period of 1980 to 1989 corresponds to cross-sectional M-protein examinations offered to Adult Health Study (AHS) participants between 1979 and 1987 (shown in Table 1). Probable and undetermined cases presented similar demographic distributions and rate patterns.
Distribution and crude rates of definite and probable MM cases by demographic and radiation dose categories
| . | Subjects . | Total MM (n) . | PYR (×105) . | Definite . | Probable . | Definite and probable . | Undetermined . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of cases . | Rate∗ . | Number of cases . | Rate∗ . | Number of cases . | Rate∗ . | Number of cases . | Rate∗ . | ||||
| City | |||||||||||
| Hiroshima | 78 562 | 84 | 23 | 46 (68.7%) | 2.0 | 12 (52.2%) | 0.5 | 58 (81.3%) | 2.5 | 26 (81.3%) | 1.1 |
| Nagasaki | 34 489 | 38 | 9.3 | 21 (31.3%) | 2.2 | 11 (47.9%) | 1.2 | 32 (18.8%) | 3.4 | 6 (18.8%) | 0.6 |
| Sex | |||||||||||
| Female | 66 251 | 72 | 20 | 44 (65.7%) | 2.2 | 11 (47.8%) | 0.6 | 55 (53.1%) | 2.8 | 17 (53.1%) | 0.9 |
| Male | 46 800 | 50 | 12.4 | 23 (34.3%) | 1.9 | 12 (52.2%) | 1 | 35 (46.9%) | 2.8 | 15 (46.9%) | 1.2 |
| Age at exposure, y | |||||||||||
| <20 | 46 420 | 15 | 15.1 | 9 (13.4%) | 0.6 | 5 (21.7%) | 0.3 | 14 (3.1%) | 0.9 | 1 (3.1%) | 0.1 |
| 20-39 | 31 041 | 59 | 10.5 | 31 (46.3%) | 2.9 | 10 (43.5%) | 0.9 | 41 (56.3%) | 3.9 | 18 (56.3%) | 1.7 |
| 40+ | 35 590 | 48 | 6.7 | 27 (40.3%) | 4.0 | 8 (34.8%) | 1.2 | 35 (40.6%) | 5.2 | 13 (40.6%) | 1.9 |
| Age at diagnosis, y | |||||||||||
| Median age (min, max) | 71 (47-96) | 69 (41-83) | 69 (41-96) | 78 (39-88) | |||||||
| <40 | 68 099 | 1 | 10.3 | 0 | 0 | 0 | 0 | 0 (0.0%) | 0 | 1 (3.1%) | 0.1 |
| 40-49 | 82 557 | 7 | 6.0 | 3 (4.5%) | 0.5 | 4 (17.4%) | 0.7 | 7 (7.8%) | 1.2 | 0 (0.0%) | 0.0 |
| 50-59 | 94 464 | 12 | 6.3 | 9 (13.4%) | 1.4 | 2 (8.7%) | 0.3 | 11 (12.2%) | 1.7 | 1 (3.1%) | 0.2 |
| 60-64 | 91 599 | 9 | 2.9 | 7 (10.4%) | 2.4 | 1 (4.3%) | 0.3 | 8 (8.9%) | 2.8 | 1 (3.1%) | 0.3 |
| 65-69 | 88 284 | 20 | 2.4 | 12 (17.9%) | 5.0 | 6 (26.1%) | 2.5 | 18 (20.0%) | 7.6 | 2 (6.2%) | 0.8 |
| >70 | 82 991 | 73 | 4.5 | 36 (53.7%) | 8.0 | 10 (43.5%) | 2.2 | 46 (51.1%) | 10.3 | 27 (84.4%) | 6.0 |
| Calendar period, y | |||||||||||
| 1950-59 | 113 051 | 3 | 9 | 1 (1.5%) | 0.1 | 1 (4.3%) | 0.1 | 2 (2.2%) | 0.2 | 1 (3.1%) | 0.1 |
| 1960-69 | 103 034 | 9 | 8.3 | 4 (6.0%) | 0.5 | 3 (13.0%) | 0.4 | 7 (7.8%) | 0.8 | 2 (6.3%) | 0.2 |
| 1970-79 | 90 362 | 33 | 6.9 | 20 (29.9%) | 2.9 | 5 (21.7%) | 0.7 | 25 (27.8%) | 3.6 | 8 (25.0%) | 1.2 |
| 1980-89 | 76 861 | 61 | 5.7 | 34 (50.7%) | 5.9 | 12 (52.2%) | 2.1 | 46 (51.1%) | 8.1 | 15 (46.9%) | 2.6 |
| 1990-94 | 62 603 | 16 | 2.4 | 8 (11.9%) | 3.3 | 2 (8.7%) | 0.8 | 10 (11.1%) | 4.2 | 6 (18.8%) | 2.5 |
| Bone marrow dose, Gy | |||||||||||
| NIC† | 26 498 | 21 | 7.7 | 15 | 2.0 | 2 | 0.3 | 17 | 2.2 | 4 | 0.5 |
| <0.005 | 37 864 | 43 | 10.7 | 22 (42.3%) | 2.1 | 7 (33.3%) | 0.7 | 29 (39.7%) | 2.7 | 14 (50.0%) | 1.3 |
| 0.005 to <0.1 | 29 539 | 37 | 8.5 | 19 (36.5%) | 2.2 | 7 (33.3%) | 0.8 | 26 (36.6%) | 3.1 | 11 (39.3%) | 1.3 |
| 0.1 to <0.3 | 9186 | 11 | 2.7 | 5 (9.6%) | 1.9 | 5 (23.8%) | 1.9 | 10 (13.6%) | 3.7 | 1 (7.1%) | 0.4 |
| 0.3 to <1.0 | 7226 | 7 | 2.1 | 3 (5.8%) | 1.4 | 2 (9.6%) | 1 | 5 (6.8%) | 2.4 | 2 (3.6%) | 1 |
| 1.0 to <2.0 | 1938 | 0 | 0.5 | 0 | 0 | 0 | 0 | 0 (0.0%) | 0.0 | 0 | 0 |
| 2.0+ | 800 | 3 | 0.2 | 3 (5.8%) | 13.8 | 0 | 0 | 3 (4.1%) | 15.0 | 0 | 0 |
| Total | 113 051 | 122 | 32.3 | 67 (100%) | 2.1 | 23 (100%) | 0.7 | 90 (100%) | 2.8 | 32 (100%) | 1 |
| . | Subjects . | Total MM (n) . | PYR (×105) . | Definite . | Probable . | Definite and probable . | Undetermined . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of cases . | Rate∗ . | Number of cases . | Rate∗ . | Number of cases . | Rate∗ . | Number of cases . | Rate∗ . | ||||
| City | |||||||||||
| Hiroshima | 78 562 | 84 | 23 | 46 (68.7%) | 2.0 | 12 (52.2%) | 0.5 | 58 (81.3%) | 2.5 | 26 (81.3%) | 1.1 |
| Nagasaki | 34 489 | 38 | 9.3 | 21 (31.3%) | 2.2 | 11 (47.9%) | 1.2 | 32 (18.8%) | 3.4 | 6 (18.8%) | 0.6 |
| Sex | |||||||||||
| Female | 66 251 | 72 | 20 | 44 (65.7%) | 2.2 | 11 (47.8%) | 0.6 | 55 (53.1%) | 2.8 | 17 (53.1%) | 0.9 |
| Male | 46 800 | 50 | 12.4 | 23 (34.3%) | 1.9 | 12 (52.2%) | 1 | 35 (46.9%) | 2.8 | 15 (46.9%) | 1.2 |
| Age at exposure, y | |||||||||||
| <20 | 46 420 | 15 | 15.1 | 9 (13.4%) | 0.6 | 5 (21.7%) | 0.3 | 14 (3.1%) | 0.9 | 1 (3.1%) | 0.1 |
| 20-39 | 31 041 | 59 | 10.5 | 31 (46.3%) | 2.9 | 10 (43.5%) | 0.9 | 41 (56.3%) | 3.9 | 18 (56.3%) | 1.7 |
| 40+ | 35 590 | 48 | 6.7 | 27 (40.3%) | 4.0 | 8 (34.8%) | 1.2 | 35 (40.6%) | 5.2 | 13 (40.6%) | 1.9 |
| Age at diagnosis, y | |||||||||||
| Median age (min, max) | 71 (47-96) | 69 (41-83) | 69 (41-96) | 78 (39-88) | |||||||
| <40 | 68 099 | 1 | 10.3 | 0 | 0 | 0 | 0 | 0 (0.0%) | 0 | 1 (3.1%) | 0.1 |
| 40-49 | 82 557 | 7 | 6.0 | 3 (4.5%) | 0.5 | 4 (17.4%) | 0.7 | 7 (7.8%) | 1.2 | 0 (0.0%) | 0.0 |
| 50-59 | 94 464 | 12 | 6.3 | 9 (13.4%) | 1.4 | 2 (8.7%) | 0.3 | 11 (12.2%) | 1.7 | 1 (3.1%) | 0.2 |
| 60-64 | 91 599 | 9 | 2.9 | 7 (10.4%) | 2.4 | 1 (4.3%) | 0.3 | 8 (8.9%) | 2.8 | 1 (3.1%) | 0.3 |
| 65-69 | 88 284 | 20 | 2.4 | 12 (17.9%) | 5.0 | 6 (26.1%) | 2.5 | 18 (20.0%) | 7.6 | 2 (6.2%) | 0.8 |
| >70 | 82 991 | 73 | 4.5 | 36 (53.7%) | 8.0 | 10 (43.5%) | 2.2 | 46 (51.1%) | 10.3 | 27 (84.4%) | 6.0 |
| Calendar period, y | |||||||||||
| 1950-59 | 113 051 | 3 | 9 | 1 (1.5%) | 0.1 | 1 (4.3%) | 0.1 | 2 (2.2%) | 0.2 | 1 (3.1%) | 0.1 |
| 1960-69 | 103 034 | 9 | 8.3 | 4 (6.0%) | 0.5 | 3 (13.0%) | 0.4 | 7 (7.8%) | 0.8 | 2 (6.3%) | 0.2 |
| 1970-79 | 90 362 | 33 | 6.9 | 20 (29.9%) | 2.9 | 5 (21.7%) | 0.7 | 25 (27.8%) | 3.6 | 8 (25.0%) | 1.2 |
| 1980-89 | 76 861 | 61 | 5.7 | 34 (50.7%) | 5.9 | 12 (52.2%) | 2.1 | 46 (51.1%) | 8.1 | 15 (46.9%) | 2.6 |
| 1990-94 | 62 603 | 16 | 2.4 | 8 (11.9%) | 3.3 | 2 (8.7%) | 0.8 | 10 (11.1%) | 4.2 | 6 (18.8%) | 2.5 |
| Bone marrow dose, Gy | |||||||||||
| NIC† | 26 498 | 21 | 7.7 | 15 | 2.0 | 2 | 0.3 | 17 | 2.2 | 4 | 0.5 |
| <0.005 | 37 864 | 43 | 10.7 | 22 (42.3%) | 2.1 | 7 (33.3%) | 0.7 | 29 (39.7%) | 2.7 | 14 (50.0%) | 1.3 |
| 0.005 to <0.1 | 29 539 | 37 | 8.5 | 19 (36.5%) | 2.2 | 7 (33.3%) | 0.8 | 26 (36.6%) | 3.1 | 11 (39.3%) | 1.3 |
| 0.1 to <0.3 | 9186 | 11 | 2.7 | 5 (9.6%) | 1.9 | 5 (23.8%) | 1.9 | 10 (13.6%) | 3.7 | 1 (7.1%) | 0.4 |
| 0.3 to <1.0 | 7226 | 7 | 2.1 | 3 (5.8%) | 1.4 | 2 (9.6%) | 1 | 5 (6.8%) | 2.4 | 2 (3.6%) | 1 |
| 1.0 to <2.0 | 1938 | 0 | 0.5 | 0 | 0 | 0 | 0 | 0 (0.0%) | 0.0 | 0 | 0 |
| 2.0+ | 800 | 3 | 0.2 | 3 (5.8%) | 13.8 | 0 | 0 | 3 (4.1%) | 15.0 | 0 | 0 |
| Total | 113 051 | 122 | 32.3 | 67 (100%) | 2.1 | 23 (100%) | 0.7 | 90 (100%) | 2.8 | 32 (100%) | 1 |
MM, multiple myeloma; NIC, not in the cities at the bombing; PYR, person-years.
Crude rate per 100 000 PYR
NIC cases are excluded for the distribution of cases by bone marrow dose.
By bone marrow dose (Table 1), the MM rates showed no monotonic pattern in the low-to-medium dose range (<0.3 Gy) and 84% of the definite/probable cases were in that dose range compared with 93% of undetermined cases. The highest rate for definite MM in the highest dose range (2.0+ Gy) was based on 3 cases; there were no probable or undetermined MM cases in the 2 highest dose categories (>1.0 Gy).
During 1979 to 1981 and 1985 to 1987, M-protein screening was offered to a clinical subcohort of 24 358 AHS members as part of biennial health examinations.16 Overall, 24 survivors with MM had participated in the screening and they were not known to have had MM before the screening except for 1 case. Of these, 11 cases had definite MM;5 had probable; and 8 had undetermined. All but one of the 16 with definite/probable diagnoses were diagnosed between 1980 and 1989 (supplemental Table 3). Among unscreened participants, the proportion of MM cases increased steadily over the years and declined in the latest calendar period (supplemental Table 4). Therefore, the effect of M-protein screening on MM diagnosis appeared to be limited to the 1980 to 1989 period. We further examined the first opportunity for MM diagnosis among definite and probable MM cases, including those diagnosed after M-protein screenings outside the AHS (supplemental Figure 2). Nine (82%) of the 11 definite MM cases among participants of AHS M-protein screening were diagnosed with MM based on M-protein screening results. Among the 56 definite MM cases who were not screened at AHS, 5 (9%) of them were diagnosed by screening conducted outside AHS (supplemental Figure 2). However, the number of LSS subjects screened outside AHS is unknown but is likely small.
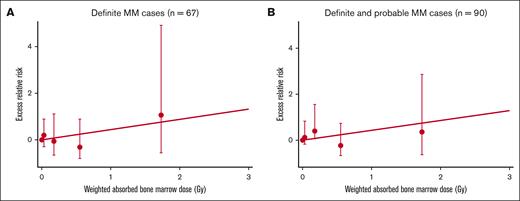
The primary focus of the dose-response analyses was definite MM. However, it seemed likely that many of the probable cases may have had MM but were classified as probable owing to the lack of bone marrow or M-protein data. Therefore, we also repeated the dose-response analysis by pooling definite and probable cases. In the analysis of definite cases, we found an elevated radiation risk with a sex-averaged ERR/Gy of 0.44 (95% confidence interval [CI], <–0.02 to 2.4) (Figure 1A), which was slightly higher than the previously-reported histologically verified case (0.36, [95% CI, –0.28 to 1.96]) and remarkably higher than all cases, including nonhistologically diagnosed ones (–0.02 [95% CI, –0.24 to 0.62])18 (supplemental Table 5). The ERR/Gy based on pooled definite/probable cases was almost identical (ERR/Gy = 0.43 [95% CI, <–0.01 to 2.1]) (Figure 1B). Neither estimate was statistically significant (P = .32). The difference in the risk estimates from the present and previous studies appeared to be in large part because of the rejection of substantial number of cases that are classified as undetermined in this study.
Radiation dose–response for definite MM cases and pooled definite and probable cases, the LSS, 1950 to 1994. Fitted linear-dose responses for (A) definite MM and (B) pooled definite and probable MM. Dots with bars show ERR estimates and a 95% confidence interval by categorical dose.
Radiation dose–response for definite MM cases and pooled definite and probable cases, the LSS, 1950 to 1994. Fitted linear-dose responses for (A) definite MM and (B) pooled definite and probable MM. Dots with bars show ERR estimates and a 95% confidence interval by categorical dose.
Although the LSS cohort members with higher doses were intentionally oversampled for inclusion in the AHS, the AHS includes LSS cohort members with doses covering the full dose range. Therefore, the radiation risk can reliably be estimated using data from AHS participants. However, because of the concern that screening-detected cases may have biased the dose-response relationship, we repeated the analysis excluding MM-screened cases. The estimated sex-averaged ERR/Gy for pooled definite/probable MM cases was 0.89 (95% CI, –0.05 to 2.9) and higher, though not statistically significant (P = .29) than all definite/probable MM cases including screened cases.
In this study, the large number of survivors (n = 46 220) exposed at ages younger than 20 years (41%) had not reached the age of 70 years at the end of follow-up in 1994. These young survivors will enter the oldest age group in the next few decades. Future follow-up will be informative of the long-term effects of radiation on MM in this cohort.
Acknowledgments: The Radiation Effects Research Foundation, Hiroshima and Nagasaki, Japan, is a public interest incorporated foundation funded by the Japanese Ministry of Health, Labour and Welfare, and the US Department of Energy. The views of the authors do not necessarily reflect those of the 2 governments. This research was also supported by the National Institutes of Health, National Cancer Institute contract N02-CO-2009-0005 with additional support from the Division of Cancer Epidemiology and Genetics, NCI Intramural Research Program. This study was also supported in part by the KAKENHI (19K16574) and Tsuchiya Memorial Medical Foundation (N.Y.). This publication was supported by Radiation Effects Research Foundation Research Protocol 3-94.
Contribution: N.Y. and M.F. designed and executed hemato-pathological review; D.L.P. and K.M. designed and analyzed data; R.S. designed and executed statistical analysis; K.O., A.H., and W.O. assisted with analysis; and N.Y., D.L.P., R.S., and K.M. cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Noriaki Yoshida, Department of Clinical Studies, Radiation Effects Research Foundation, 5-2 Hijiyama Park, Minami-ku, Hiroshima 7320815, Japan; e-mail: noriaki3@rerf.or.jp.
References
Author notes
Data are available on request from the corresponding author Noriaki Yoshida (noriaki3@rerf.or.jp).
The full-text version of this article contains a data supplement.