Key Points
One-third of patients with CLL relapsing on ibrutinib do not carry BTK/PLCG2 mutations, even with a 0.1% sensitivity.
Additional mechanisms, such as del(8p), EGR2 and NF-κB pathway mutations, may be cooperating in determining progression on ibrutinib.
Abstract
Patients with chronic lymphocytic leukemia (CLL) progressing on ibrutinib constitute an unmet need. Though Bruton tyrosine kinase (BTK) and PLCG2 mutations are associated with ibrutinib resistance, their frequency and relevance to progression are not fully understood. In this multicenter retrospective observational study, we analyzed 98 patients with CLL on ibrutinib (49 relapsing after an initial response and 49 still responding after ≥1 year of continuous treatment) using a next-generation sequencing (NGS) panel (1% sensitivity) comprising 13 CLL-relevant genes including BTK and PLCG2. BTK hotspot mutations were validated by droplet digital polymerase chain reaction (ddPCR) (0.1% sensitivity). By integrating NGS and ddPCR results, 32 of 49 relapsing cases (65%) carried at least 1 hotspot BTK and/or PLCG2 mutation(s); in 6 of 32, BTK mutations were only detected by ddPCR (variant allele frequency [VAF] 0.1% to 1.2%). BTK/PLCG2 mutations were also identified in 6 of 49 responding patients (12%; 5/6 VAF <10%), of whom 2 progressed later. Among the relapsing patients, the BTK-mutated (BTKmut) group was enriched for EGR2 mutations, whereas BTK-wildtype (BTKwt) cases more frequently displayed BIRC3 and NFKBIE mutations. Using an extended capture-based panel, only BRAF and IKZF3 mutations showed a predominance in relapsing cases, who were enriched for del(8p) (n = 11; 3 BTKwt). Finally, no difference in TP53 mutation burden was observed between BTKmut and BTKwt relapsing cases, and ibrutinib treatment did not favor selection of TP53-aberrant clones. In conclusion, we show that BTK/PLCG2 mutations were absent in a substantial fraction (35%) of a real-world cohort failing ibrutinib, and propose additional mechanisms contributing to resistance.
Introduction
The first-in-class Bruton tyrosine kinase (BTK) inhibitor ibrutinib covalently binds to BTK,1,2,3 and has demonstrated efficacy in both treatment-naïve and relapsed/refractory chronic lymphocytic leukemia (CLL).4-7 Although the majority of patients with CLL obtain long-lasting responses, the following 3 main reasons for ibrutinib discontinuation have emerged: intolerance (∼25% of patients), and, particularly among relapsed/refractory patients, Richter transformation (10%), and CLL progression (∼20%).8,9 Several studies have identified BTK and/or PLCG2 gene mutations in the majority (up to 100%) of patients with CLL relapsing on ibrutinib,10-13 even several months before clinical relapse.10,13 Mutations preferentially occurred at the cysteine 481 residue resulting in the replacement of cysteine by serine (p.C481S) or arginine (p.C481R). Mutations at this site lead to abrogation of the covalent binding of ibrutinib, with only transient inhibition of the mutant protein.10,11 In contrast, multiple, though less frequent, mutations within the downstream signaling molecule PLCG2 usually result in a gain-of-function, promoting B cell receptor (BcR) signaling despite BTK inhibition.10,11,14 Additional mechanisms of resistance to ibrutinib have been proposed such as the loss of TRAIL-R expression because of del(8p),15-17 whereas mutations of individual genes (eg, EIF2AK3, EP300, KMT2D)15 have been occasionally reported.
The proportion of CLL cells carrying mutations within the BTK/PLCG2 genes varies considerably, with some cases showing a very low clonal burden, hence challenging their proposed contribution to resistance.18 Thus, a comprehensive understanding of the prevalence and relevance of these mutations in relation to response to ibrutinib will help better refine the mechanisms driving resistance and identify other potential key driver mutations or pathways. In particular, it remains to be established whether these mutations also occur in patients who continue to respond to ibrutinib. Insight into these issues may aid in the validation of predictors of relapse to assist treatment decisions, and in the design of novel treatment modalities to ultimately prevent relapse and disease progression.
To this end, we designed a multicenter international retrospective study, coordinated by the European research initiative on CLL (ERIC), aimed at investigating, in a “real-world” setting, the presence of recurrent gene mutations in BTK/PLCG2 and other genes of interest by targeted next-generation sequencing (NGS) in patients with CLL failing ibrutinib, and in a cohort of patients who have maintained a response to ibrutinib and remain on therapy for at least 12 months after ibrutinib initiation.
Methods
Patient enrollment and sample collection
Ninety-eight patients with CLL treated with ibrutinib from 21 institutions were included and assigned to 1 of the 2 following groups: relapsed (n = 49; patients progressing after an initial response) and responders (n = 49; patients who maintained a response to ibrutinib for ≥1 year) (Table 1). Patients in both groups received full-dose ibrutinib without >14 days interruption. Progression and response were defined according to the international workshop on CLL 2008 criteria19; primary refractory cases and patients with Richter transformation were excluded. Paired samples at baseline (at the time of treatment initiation) and progression or ≥1 year after therapy initiation, were available for 50 patients (19 relapsed and 31 responders). Informed consent was obtained in accordance with the declaration of Helsinki and ethical approval was granted by local review committees.
Clinical characteristics of patients included in the study
| Characteristics . | Relapsed cases (n = 49) . | Responders (n = 49) . | Entire cohort (n = 98) . | P value . |
|---|---|---|---|---|
| Median age, y (range) | 66 (33-86) | 68 (46-85) | 67 (33-86) | ns |
| Male:female | 32:17 | 31:18 | 63:35 | ns |
| Median number of previous therapies | 2 | 1 | 2 | ns |
| Unmutated IGHV, n (%) | 28/35 (80) | 29/41 (70.7) | 57/76 (75) | ns |
| del(11q), n (%) | 14/45 (31) | 10/44 (22.7) | 24/89 (26.9) | ns |
| del(17p), n (%) | 21/46 (45.6) | 21/46 (45.6) | 42/92 (45.6) | ns |
| TP53 mutation, n (%) | 13/27 (48.1) | 8/38 (21) | 21/65 (32.3) | .03 |
| TP53 aberrations (del(17p) and/or TP53 mutations), n (%) | 25/46 (54.3) | 23/46 (50) | 48/92 (52.2) | ns |
| Best response to ibrutinib | ||||
| PR/PR-L | 44/49 | 35/49 | 79/98 | ns |
| CR | 5/49 | 14/49 | 19/98 | ns |
| Median duration of ibrutinib treatment, (range) (mo) | 36 (6-68) | 44 (18-87) | 40 (6-87) | ns |
| Follow-up | ||||
| Median follow-up, (range) (mo) | 43 (8-84) | 46 (18-87) | 44 (8-87) | ns |
| Median overall survival, (95% CI) (mo) | 58 (36-80) | NR | NR | P < .001 |
| Dead, n (%) | 25 (51) | 5 (10.2) | 30 (30.6) | P < .001 |
| Characteristics . | Relapsed cases (n = 49) . | Responders (n = 49) . | Entire cohort (n = 98) . | P value . |
|---|---|---|---|---|
| Median age, y (range) | 66 (33-86) | 68 (46-85) | 67 (33-86) | ns |
| Male:female | 32:17 | 31:18 | 63:35 | ns |
| Median number of previous therapies | 2 | 1 | 2 | ns |
| Unmutated IGHV, n (%) | 28/35 (80) | 29/41 (70.7) | 57/76 (75) | ns |
| del(11q), n (%) | 14/45 (31) | 10/44 (22.7) | 24/89 (26.9) | ns |
| del(17p), n (%) | 21/46 (45.6) | 21/46 (45.6) | 42/92 (45.6) | ns |
| TP53 mutation, n (%) | 13/27 (48.1) | 8/38 (21) | 21/65 (32.3) | .03 |
| TP53 aberrations (del(17p) and/or TP53 mutations), n (%) | 25/46 (54.3) | 23/46 (50) | 48/92 (52.2) | ns |
| Best response to ibrutinib | ||||
| PR/PR-L | 44/49 | 35/49 | 79/98 | ns |
| CR | 5/49 | 14/49 | 19/98 | ns |
| Median duration of ibrutinib treatment, (range) (mo) | 36 (6-68) | 44 (18-87) | 40 (6-87) | ns |
| Follow-up | ||||
| Median follow-up, (range) (mo) | 43 (8-84) | 46 (18-87) | 44 (8-87) | ns |
| Median overall survival, (95% CI) (mo) | 58 (36-80) | NR | NR | P < .001 |
| Dead, n (%) | 25 (51) | 5 (10.2) | 30 (30.6) | P < .001 |
CI, confidence interval; CR, complete response; ns, not significant; NR, not reached PR, partial response; PR-L, partial response with lymphocytosis.
A total of 151 samples were analyzed, obtained from peripheral blood mononuclear cells (PBMC) (n = 143), bone marrow (BM) (n = 7) and 1 baseline sample derived from formalin-fixed paraffin-embedded lymph node tissue. For 3 patients, both BM and PBMC samples obtained at relapse were analyzed.
The fraction of tumor cells by flow cytometry was ≥80% in 79% of all samples in the study. B cells were purified from peripheral blood using a negative-selection immunodensity method (RosetteSep Human B Cells, StemCell Technologies) or from viable frozen PBMCs using a positive-selection method (>95% purity) (EasySep Human CD19 Positive Selection Kit II, StemCell Technologies).
Genomic DNA (gDNA) was extracted using Maxwell 16 Blood DNA Purification kit (Promega) for samples with >1×106 cells; QIAamp DNA Micro kit (Qiagen) for cases with cells numbers ranging from 5 × 104 to 1 × 106; NucleoSpin Tissue XS kit (Macherey-Nagel) for cases with <5 × 104 cells. The gDNA concentration was determined using Qubit (ThermoFisher) and integrity was assessed on Agilent 4200 TapeStation (Agilent Technologies).
NGS
HaloPlex panel: a previously published custom Agilent HaloPlex high sensitivity panel design20 was modified using the Agilent SureDesign software (https://earray.chem.agilent.com/suredesign/). The custom probes were designed to target the coding exons or hotspot regions of 13 genes of interest in CLL (ATM, BIRC3, BTK, EGR2, FBXW7, MYD88, NFKBIE, NOTCH1, PLCG2, POT1, SF3B1, TP53, and XPO1) (supplemental Table 1). Libraries were prepared using 50 ng of high-quality gDNA input, following the manufacturer’s instructions. Paired-end sequencing (150 bp reads) was performed on a NextSeq instrument (Illumina, Hayward, CA).
Lymphoid panel: DNA samples were analyzed using a custom-designed, capture-based gene panel, GMS Lymphoid panel (Twist Bioscience), including 252 genes, selected based on their relevance in lymphoid malignancies.21,22 The panel also included genome-wide backbone probes for copy-number analysis. Library preparation and sequencing were performed as described in supplemental Data.
Bioinformatics analysis
HaloPlex panel (refer to supplemental Data): FASTQ files were preprocessed by Agilent SureCallTrimmer (v4.0.1) , aligned to the GRCh37 human reference genome using bwa-mem (v0.7.16) and postprocessed using Samtools (v1.8). The Agilent LocatIt tool (v4.0.1) was applied for processing of molecular barcode information. Pisces (v5.2.10.49) was used for detection of single nucleotide variants and small insertions/deletions (indels) (1% VAF). Variants were annotated with population variation databases and Cosmic (v85) using VEP23 (v91) and SnpEFF (v4.3). Pysamstats (v1.1.2) was used for detailed investigation of mutations at codon 481 of the BTK gene and at selected PLCG2 hotspots (“per base” analysis).
Lymphoid panel: BALSAMIC24 was applied to analyze the FASTQ files and for somatic variant calling (10% VAF) and copy-number aberration detection, as described in supplemental Data.
Droplet digital polymerase chain reaction (ddPCR)
One hundred sixteen samples included in the HaloPlex analysis were analyzed with ddPCR with a sensitivity of 0.1% at the BTK hotspot C481S (c.1442G>C and c.1441T>A), according to the manufacturer’s instructions, using Bio-Rad reagents and equipments (refer to supplemental Data).
Statistical analysis
Continuous variables were analyzed using the median and range (minimum, maximum). Categorical variables are presented as percentage of total number of patients with available information. Correlation of variables with disease outcome were evaluated in univariate analysis with nonparametric tests (Chi-square and Fisher Exact test in case of categorical variables, Mann-Whitney and Kruskal-Wallis test in case of continuous variables). Time-to-progression (TTP, time between ibrutinib initiation and documented progression or last follow-up) and overall survival (time between ibrutinib initiation and last follow-up or date of death) were estimated using the Kaplan-Meier Product Limit estimator and log-rank test.
Results
Patient clinical characteristics
Characteristics of the 98 eligible patients are described in Table 1. The study population was enriched for patients with high-risk features consistent with a heavily pretreated population (median number of previous therapies 2; range, 1-6), with only 17 patients receiving ibrutinib as first-line treatment (13 responders; 4 relapsed). No significant differences between relapsed and responders subgroups were identified, except for a higher percentage of TP53 mutations in relapsed cases (48% vs 21%, P = .03). All patients obtained at least a partial response with lymphocytosis (PR-L) on ibrutinib, with those in the relapsed group progressing after a median of 34 months (range, 5-66). The median duration of treatment was 44 months (range, 18-87) in the responders and 36 months (range, 6-68) in the relapsed cases.
Detection of hotspot BTK and PLCG2 mutations by HaloPlex NGS analysis and validation by ddPCR
By applying our standardized bioinformatics pipeline (1% sensitivity), a total of 38 hotspot BTK mutations were detected in 24 of 49 relapsed patients (49%), with 7 of 24 (29%) carrying ≥2 hotspot BTK mutations (Table 2). The VAF of the individual BTK mutations differed considerably (range, 1.8%-79.5%; median, 16.8%; not sex-normalized). In 6 of 24 patients (25%), BTK mutations were present at low-VAF only (<10%), whereas in 4 of 24 patients (16.7%), low- and high-VAF BTK mutations co-occurred (Table 2).
BTK and PLCG2 hotspot mutations in relapsed and responsive cohorts, assessed by both HaloPlex NGS and ddPCR analysis
| Patient ID . | Cohort . | % CD19+ in sample . | BTK hotspot mutations . | PLCG2 hotspot mutations . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coding DNA description . | Protein description . | Standard NGS VAF (%) . | Per base NGS VAF (%) . | ddPCR VAF (%) . | Coding DNA description . | Protein description . | Standard NGS VAF (%) . | Per base NGS VAF (%) . | Time on ibrutinib at sampling (months) . | |||
| 1 | Relapsed | 99 | c.1442G>C | p.C481S | 79.5 | 79.5 | 85.0 | c.3418G>A | p.D1140N | 3.9 | 3.9 | 46 |
| c.1441T>A | p.C481S | 2.6 | 2.6 | 11 | ||||||||
| c.1442G>A | p.C481Y | ND | 0.5 | not tested | ||||||||
| c.1441T>C | p.C481R | ND | 0.3 | not tested | c.2977G>T | p.D993Y | ND | 0.9 | ||||
| c.3422T>A | p.M1141K | ND | 0.4 | |||||||||
| 2 | Relapsed | >90 | c.1441T>A | p.C481S | 53.4 | 53.7 | 65 | c.2978A>G | p.D993G | 7.9 | 7.8 | 12 |
| c.1442G>C | p.C481S | 12.8 | 12.8 | 30.7 | c.2977G>T | p.D993Y | 4.8 | 4.8 | ||||
| 3 | Relapsed | 85 | c.1442G>C | p.C481S | 2.7 | 2.7 | 2.8 | c.3418G>A | p.D1140N | ND | 0.4 | 36 |
| c.1441T>A | p.C481S | 2.4 | 2.5 | 2.2 | c.3422T>G | p.M1141R | ND | 0.3 | ||||
| 4 | Relapsed | unknown | c.1441T>C | p.C481R | 40.3 | 40.1 | not tested | None | None | NA | NA | 17 |
| 5 | Relapsed | >90 | c.1442G>C | p.C481S | 32.0 | 32.0 | 32.7 | None | None | NA | NA | 47 |
| c.1441T>A | p.C481S | ND | ND | 0.9 | ||||||||
| 6 | Relapsed | unknown | c.1442G>C | p.C481S | 6.1 | 6.1 | 7.1 | c.3412_3414del | p.1138_1138del | 13.0 | 13.0 | 11 |
| c.1441T>A | p.C481S | ND | ND | 0.5 | c.2543T>G | p.L848R | 9.3 | 9.3 | ||||
| c.3422T>A | p.M1141K | 2.0 | 2.0 | |||||||||
| c.3418G>A | p.D1140N | ND | 1.0 | |||||||||
| c.3422T>G | p.M1141R | ND | 1.7 | |||||||||
| 7-BM | Relapsed | >95 | c.1442G>C | p.C481S | 7.1 | 7.1 | 8.0 | c.2978A>G | p.D993G | ND | 0.8 | 9 |
| 7-PBMC | >95 | c.1442G>C | p.C481S | 23.3 | 23.3 | 18.2 | c.2978A>G | p.D993G | ND | 1.3 | ||
| 8 | Relapsed | >95 | c.1442G>C | p.C481S | 31.2 | 31.2 | 29.6 | c.3422T>G | p.M1141R | 4.0 | 4.0 | 15 |
| c.1441T>A | p.C481S | ND | ND | 0.3 | ||||||||
| 9 | Relapsed | 85 | c.1442G>C | p.C481S | 33.2 | 33.2 | 35.6 | None | None | NA | NA | 43 |
| 10 | Relapsed | 95 | c.1442G>C | p.C481S | 7.8 | 7.8 | 10.0 | c.2977G>T | p.D993Y | 32.7 | 32.7 | 40 |
| c.1441T>A | p.C481S | ND | ND | inconclusive | c.2977G>C | p.D993H | 2.0 | 2.0 | ||||
| c.2978A>G | p.D993G | ND | 1.3 | |||||||||
| c.3418G>A | p.D1140N | ND | 0.6 | |||||||||
| 11 | Relapsed | 95 | c.1442G>C | p.C481S | 42.7 | 42.8 | 41.6 | c.2535A>T | p.L845F | ND | 0.8 | 43 |
| c.1441T>A | p.C481S | ND | 0.8 | 2.2 | c.2977G>C | p.D993H | ND | 0.7 | ||||
| 12 | Relapsed | 94 | c.1442G>C | p.C481S | 62.5 | 62.5 | 62.7 | c.2977G>C | p.D993H | ND | 0.2 | 16 |
| 13 | Relapsed | 90 | c.1442G>C | p.C481S | 17.9 | 17.9 | 18.0 | None | None | NA | NA | 32 |
| c.1441T>A | p.C481S | ND | ND | 0.2 | ||||||||
| 14 | Relapsed | 85 | c.1442G>C | p.C481S | 5.8 | 5.8 | 6.8 | None | None | NA | NA | 41 |
| c.1441T>A | p.C481S | ND | 0.7 | 1.0 | ||||||||
| 15-PBMC | Relapsed | 70 | c.1442G>C | p.C481S | 41.9 | 41.8 | 43.8 | c.2535A>C | p.L845F | NA | 0.7 | 37 |
| c.1441T>A | p.C481S | ND | 0.5 | 0.7 | c.2535A>T | p.L845F | NA | 0.7 | ||||
| 15-BM | 38 | c.1442G>C | p.C481S | 11.5 | 11.5 | 13.2 | None | None | NA | NA | ||
| c.1441T>A | p.C481S | ND | ND | 0.1 | ||||||||
| 16 | Relapsed | 35 | c.1442G>C | p.C481S | 3.0 | 3.0 | not tested | None | None | NA | NA | 56 |
| 17 | Relapsed | unknown | c.1442G>C | p.C481S | 61.6 | 61.5 | 66.3 | None | None | NA | NA | 42 |
| c.1441T>A | p.C481S | ND | ND | 0.3 | ||||||||
| c.1442G>A | p.C481Y | ND | 0.5 | not tested | ||||||||
| 18 | Relapsed | 99 | c.1442G>C | p.C481S | 15.7 | 15.7 | 17.2 | c.2535A>T | p.L845F | NA | 0.3 | 62 |
| c.1441T>A | p.C481S | ND | 0.1 | 0.2 | ||||||||
| c.1442G>A | p.C481Y | ND | 1.4 | not tested | ||||||||
| 19 | Relapsed | unknown | c.1441T>A | p.C481S | 37.9 | 37.9 | 50.3 | None | None | NA | NA | 34 |
| c.1442G>C | p.C481S | 23.4 | 23.4 | 39.2 | ||||||||
| 20-BM | Relapsed | 66 | c.1442G>C | p.C481S | 2.3 | 2.3 | 2.2 | c.3412_3414del | p.1138_1138del | 2.7 | 2.7 | 34 |
| c.2535A>C | p.L845F | 3.4 | 3.4 | |||||||||
| c.2535A>T | p.L845F | 2.7 | 2.6 | |||||||||
| c.1442G>A | p.C481Y | ND | 0.2 | not tested | c.2977G>C | p.D993H | 1.7 | 1.7 | ||||
| c.1993C>T | p.R665W | ND | 0.8 | |||||||||
| c.2120C>T | p.S707F | ND | 0.3 | |||||||||
| c.3418G>A | p.D1140N | ND | 0.7 | |||||||||
| c.3422T>A | p.M1141K | ND | 0.7 | |||||||||
| c.3422T>G | p.M1141R | ND | 0.2 | |||||||||
| 20-PBMC | 92 | c.1442G>C | p.C481S | ND | 1.4 | 1.8 | c.3412_3414del | p.1138_1138del | 12.7 | 12.7 | ||
| c.2535A>C | p.L845F | 2.5 | 2.5 | |||||||||
| c.2535A>T | p.L845F | 2.0 | 2.0 | |||||||||
| c.2977G>C | p.D993H | 1.8 | 1.8 | |||||||||
| c.1993C>T | p.R665W | ND | 0.9 | |||||||||
| c.3418G>A | p.D1140N | ND | 0.4 | |||||||||
| c.3422T>A | p.M1141K | ND | 0.6 | |||||||||
| c.3422T>G | p.M1141R | ND | 0.6 | |||||||||
| 21 | Relapsed | unknown | c.1441T>C | p.C481R | 51.8 | 51.7 | not tested | None | None | NA | NA | 42 |
| c.1442G>C | p.C481S | 5.5 | 5.5 | 7.2 | ||||||||
| c.1441T>A | p.C481S | 1.8 | 1.8 | 2.7 | ||||||||
| c.1442G>A | p.C481Y | 2.8 | 2.8 | not tested | ||||||||
| c.1443C>A | p.C481 | 2.2 | 2.2 | not tested | ||||||||
| 22 | Relapsed | 73 | c.1442G>T | p.C481F | 33.5 | 33.5 | not tested | None | None | NA | NA | 38 |
| c.1442G>C | p.C481S | 4.8 | 4.8 | 8.6 | ||||||||
| 23 | Relapsed | 81 | c.1442G>C | p.C481S | 79.3 | 79.3 | 79.9 | None | None | NA | NA | 37 |
| c.1441T>A | p.C481S | ND | 0.4 | 2.4 | ||||||||
| 24 | Relapsed | 97 | c.1441T>A | p.C481S | 47.8 | 47.8 | 69.2 | None | None | NA | NA | 37 |
| c.1442G>C | p.C481S | 22 | 22 | 57.9 | ||||||||
| c.1442G>A | p.C481Y | 4.8 | 4.8 | not tested | ||||||||
| c.1442G>T | p.C481F | 4.6 | 4.6 | not tested | ||||||||
| c.1441T>C | p.C481R | ND | 0.8 | not tested | ||||||||
| 25 | Relapsed | unknown | None | None | NA | NA | NA | c.2120C>T | p.S707F | 21.2 | 21.2 | 42 |
| c.3412_3414del | p.1138_1138del | ND | 0.2 | |||||||||
| 26 | Relapsed | 75 | None | None | NA | NA | NA | c.3416A>G | p.E1139G | 3 | 3.2 | 10 |
| 27 | Relapsed | 74 | c.1442G>C | p.C481S | ND | 1.2 | 0.8 | None | None | NA | NA | 5 |
| 28 | Relapsed | 18 | c.1442G>C | p.C481S | ND | 0.3 | 0.1 | None | None | NA | NA | 43 |
| 29 | Relapsed | unknown | c.1441T>A | p.C481S | ND | 0.9 | 1.2 | None | None | NA | NA | 33 |
| c.1442G>C | p.C481S | ND | 1.5 | ND | ||||||||
| c.1441T>C | p.C481R | ND | 1.3 | not tested | ||||||||
| 30 | Relapsed | 75 | c.1442G>C | p.C481S | ND | 0.1 | 0.1 | None | None | NA | NA | 45 |
| 31 | Relapsed | 81 | c.1442G>C | p.C481S | ND | ND | 0.2 | None | None | NA | NA | 56 |
| 32 | Relapsed | 99 | c.1442G>C | p.C481S | ND | ND | 0.1 | None | None | NA | NA | 56 |
| 33 | Responsive | unknown | c.1442G>C | p.C481S | 19.5 | 19.5 | 18.7 | c.2977G>C | p.D993H | 2.9 | 2.9 | 32 |
| c.2535A>C | p.L845F | ND | 1.4 | |||||||||
| c.3419A>G | p.D1140G | ND | 0.4 | |||||||||
| 34 | Responsive | unknown | c.1442G>C | p.C481S | 4.4 | 4.4 | 5.4 | c.3419A>G | p.D1140G | 5.5 | 5.5 | 40 |
| c.2535A>T | p.L845F | 3.4 | 2.7 | |||||||||
| c.1442G>A | p.C481Y | ND | 0.7 | not tested | c.2535A>C | p.L845F | 2.7 | 2.1 | ||||
| c.1441T>A | p.C481S | ND | ND | 0.2 | ||||||||
| 35 | Responsive | 98 | c.1442G>C | p.C481S | 2.7 | 2.7 | 2.7 | None | None | NA | NA | 28 |
| 36 | Responsive | 88 | c.1442G>C | p.C481S | ND | ND | 0.4 | None | None | NA | NA | 18 |
| 37 | Responsive | 89 | c.1442G>C | p.C481S | ND | ND | 1.2 | None | None | NA | NA | 37 |
| 38 | Responsive | 89 | c.1442G>C | p.C481S | ND | ND | 0.7 | None | None | NA | NA | 20 |
| Patient ID . | Cohort . | % CD19+ in sample . | BTK hotspot mutations . | PLCG2 hotspot mutations . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coding DNA description . | Protein description . | Standard NGS VAF (%) . | Per base NGS VAF (%) . | ddPCR VAF (%) . | Coding DNA description . | Protein description . | Standard NGS VAF (%) . | Per base NGS VAF (%) . | Time on ibrutinib at sampling (months) . | |||
| 1 | Relapsed | 99 | c.1442G>C | p.C481S | 79.5 | 79.5 | 85.0 | c.3418G>A | p.D1140N | 3.9 | 3.9 | 46 |
| c.1441T>A | p.C481S | 2.6 | 2.6 | 11 | ||||||||
| c.1442G>A | p.C481Y | ND | 0.5 | not tested | ||||||||
| c.1441T>C | p.C481R | ND | 0.3 | not tested | c.2977G>T | p.D993Y | ND | 0.9 | ||||
| c.3422T>A | p.M1141K | ND | 0.4 | |||||||||
| 2 | Relapsed | >90 | c.1441T>A | p.C481S | 53.4 | 53.7 | 65 | c.2978A>G | p.D993G | 7.9 | 7.8 | 12 |
| c.1442G>C | p.C481S | 12.8 | 12.8 | 30.7 | c.2977G>T | p.D993Y | 4.8 | 4.8 | ||||
| 3 | Relapsed | 85 | c.1442G>C | p.C481S | 2.7 | 2.7 | 2.8 | c.3418G>A | p.D1140N | ND | 0.4 | 36 |
| c.1441T>A | p.C481S | 2.4 | 2.5 | 2.2 | c.3422T>G | p.M1141R | ND | 0.3 | ||||
| 4 | Relapsed | unknown | c.1441T>C | p.C481R | 40.3 | 40.1 | not tested | None | None | NA | NA | 17 |
| 5 | Relapsed | >90 | c.1442G>C | p.C481S | 32.0 | 32.0 | 32.7 | None | None | NA | NA | 47 |
| c.1441T>A | p.C481S | ND | ND | 0.9 | ||||||||
| 6 | Relapsed | unknown | c.1442G>C | p.C481S | 6.1 | 6.1 | 7.1 | c.3412_3414del | p.1138_1138del | 13.0 | 13.0 | 11 |
| c.1441T>A | p.C481S | ND | ND | 0.5 | c.2543T>G | p.L848R | 9.3 | 9.3 | ||||
| c.3422T>A | p.M1141K | 2.0 | 2.0 | |||||||||
| c.3418G>A | p.D1140N | ND | 1.0 | |||||||||
| c.3422T>G | p.M1141R | ND | 1.7 | |||||||||
| 7-BM | Relapsed | >95 | c.1442G>C | p.C481S | 7.1 | 7.1 | 8.0 | c.2978A>G | p.D993G | ND | 0.8 | 9 |
| 7-PBMC | >95 | c.1442G>C | p.C481S | 23.3 | 23.3 | 18.2 | c.2978A>G | p.D993G | ND | 1.3 | ||
| 8 | Relapsed | >95 | c.1442G>C | p.C481S | 31.2 | 31.2 | 29.6 | c.3422T>G | p.M1141R | 4.0 | 4.0 | 15 |
| c.1441T>A | p.C481S | ND | ND | 0.3 | ||||||||
| 9 | Relapsed | 85 | c.1442G>C | p.C481S | 33.2 | 33.2 | 35.6 | None | None | NA | NA | 43 |
| 10 | Relapsed | 95 | c.1442G>C | p.C481S | 7.8 | 7.8 | 10.0 | c.2977G>T | p.D993Y | 32.7 | 32.7 | 40 |
| c.1441T>A | p.C481S | ND | ND | inconclusive | c.2977G>C | p.D993H | 2.0 | 2.0 | ||||
| c.2978A>G | p.D993G | ND | 1.3 | |||||||||
| c.3418G>A | p.D1140N | ND | 0.6 | |||||||||
| 11 | Relapsed | 95 | c.1442G>C | p.C481S | 42.7 | 42.8 | 41.6 | c.2535A>T | p.L845F | ND | 0.8 | 43 |
| c.1441T>A | p.C481S | ND | 0.8 | 2.2 | c.2977G>C | p.D993H | ND | 0.7 | ||||
| 12 | Relapsed | 94 | c.1442G>C | p.C481S | 62.5 | 62.5 | 62.7 | c.2977G>C | p.D993H | ND | 0.2 | 16 |
| 13 | Relapsed | 90 | c.1442G>C | p.C481S | 17.9 | 17.9 | 18.0 | None | None | NA | NA | 32 |
| c.1441T>A | p.C481S | ND | ND | 0.2 | ||||||||
| 14 | Relapsed | 85 | c.1442G>C | p.C481S | 5.8 | 5.8 | 6.8 | None | None | NA | NA | 41 |
| c.1441T>A | p.C481S | ND | 0.7 | 1.0 | ||||||||
| 15-PBMC | Relapsed | 70 | c.1442G>C | p.C481S | 41.9 | 41.8 | 43.8 | c.2535A>C | p.L845F | NA | 0.7 | 37 |
| c.1441T>A | p.C481S | ND | 0.5 | 0.7 | c.2535A>T | p.L845F | NA | 0.7 | ||||
| 15-BM | 38 | c.1442G>C | p.C481S | 11.5 | 11.5 | 13.2 | None | None | NA | NA | ||
| c.1441T>A | p.C481S | ND | ND | 0.1 | ||||||||
| 16 | Relapsed | 35 | c.1442G>C | p.C481S | 3.0 | 3.0 | not tested | None | None | NA | NA | 56 |
| 17 | Relapsed | unknown | c.1442G>C | p.C481S | 61.6 | 61.5 | 66.3 | None | None | NA | NA | 42 |
| c.1441T>A | p.C481S | ND | ND | 0.3 | ||||||||
| c.1442G>A | p.C481Y | ND | 0.5 | not tested | ||||||||
| 18 | Relapsed | 99 | c.1442G>C | p.C481S | 15.7 | 15.7 | 17.2 | c.2535A>T | p.L845F | NA | 0.3 | 62 |
| c.1441T>A | p.C481S | ND | 0.1 | 0.2 | ||||||||
| c.1442G>A | p.C481Y | ND | 1.4 | not tested | ||||||||
| 19 | Relapsed | unknown | c.1441T>A | p.C481S | 37.9 | 37.9 | 50.3 | None | None | NA | NA | 34 |
| c.1442G>C | p.C481S | 23.4 | 23.4 | 39.2 | ||||||||
| 20-BM | Relapsed | 66 | c.1442G>C | p.C481S | 2.3 | 2.3 | 2.2 | c.3412_3414del | p.1138_1138del | 2.7 | 2.7 | 34 |
| c.2535A>C | p.L845F | 3.4 | 3.4 | |||||||||
| c.2535A>T | p.L845F | 2.7 | 2.6 | |||||||||
| c.1442G>A | p.C481Y | ND | 0.2 | not tested | c.2977G>C | p.D993H | 1.7 | 1.7 | ||||
| c.1993C>T | p.R665W | ND | 0.8 | |||||||||
| c.2120C>T | p.S707F | ND | 0.3 | |||||||||
| c.3418G>A | p.D1140N | ND | 0.7 | |||||||||
| c.3422T>A | p.M1141K | ND | 0.7 | |||||||||
| c.3422T>G | p.M1141R | ND | 0.2 | |||||||||
| 20-PBMC | 92 | c.1442G>C | p.C481S | ND | 1.4 | 1.8 | c.3412_3414del | p.1138_1138del | 12.7 | 12.7 | ||
| c.2535A>C | p.L845F | 2.5 | 2.5 | |||||||||
| c.2535A>T | p.L845F | 2.0 | 2.0 | |||||||||
| c.2977G>C | p.D993H | 1.8 | 1.8 | |||||||||
| c.1993C>T | p.R665W | ND | 0.9 | |||||||||
| c.3418G>A | p.D1140N | ND | 0.4 | |||||||||
| c.3422T>A | p.M1141K | ND | 0.6 | |||||||||
| c.3422T>G | p.M1141R | ND | 0.6 | |||||||||
| 21 | Relapsed | unknown | c.1441T>C | p.C481R | 51.8 | 51.7 | not tested | None | None | NA | NA | 42 |
| c.1442G>C | p.C481S | 5.5 | 5.5 | 7.2 | ||||||||
| c.1441T>A | p.C481S | 1.8 | 1.8 | 2.7 | ||||||||
| c.1442G>A | p.C481Y | 2.8 | 2.8 | not tested | ||||||||
| c.1443C>A | p.C481 | 2.2 | 2.2 | not tested | ||||||||
| 22 | Relapsed | 73 | c.1442G>T | p.C481F | 33.5 | 33.5 | not tested | None | None | NA | NA | 38 |
| c.1442G>C | p.C481S | 4.8 | 4.8 | 8.6 | ||||||||
| 23 | Relapsed | 81 | c.1442G>C | p.C481S | 79.3 | 79.3 | 79.9 | None | None | NA | NA | 37 |
| c.1441T>A | p.C481S | ND | 0.4 | 2.4 | ||||||||
| 24 | Relapsed | 97 | c.1441T>A | p.C481S | 47.8 | 47.8 | 69.2 | None | None | NA | NA | 37 |
| c.1442G>C | p.C481S | 22 | 22 | 57.9 | ||||||||
| c.1442G>A | p.C481Y | 4.8 | 4.8 | not tested | ||||||||
| c.1442G>T | p.C481F | 4.6 | 4.6 | not tested | ||||||||
| c.1441T>C | p.C481R | ND | 0.8 | not tested | ||||||||
| 25 | Relapsed | unknown | None | None | NA | NA | NA | c.2120C>T | p.S707F | 21.2 | 21.2 | 42 |
| c.3412_3414del | p.1138_1138del | ND | 0.2 | |||||||||
| 26 | Relapsed | 75 | None | None | NA | NA | NA | c.3416A>G | p.E1139G | 3 | 3.2 | 10 |
| 27 | Relapsed | 74 | c.1442G>C | p.C481S | ND | 1.2 | 0.8 | None | None | NA | NA | 5 |
| 28 | Relapsed | 18 | c.1442G>C | p.C481S | ND | 0.3 | 0.1 | None | None | NA | NA | 43 |
| 29 | Relapsed | unknown | c.1441T>A | p.C481S | ND | 0.9 | 1.2 | None | None | NA | NA | 33 |
| c.1442G>C | p.C481S | ND | 1.5 | ND | ||||||||
| c.1441T>C | p.C481R | ND | 1.3 | not tested | ||||||||
| 30 | Relapsed | 75 | c.1442G>C | p.C481S | ND | 0.1 | 0.1 | None | None | NA | NA | 45 |
| 31 | Relapsed | 81 | c.1442G>C | p.C481S | ND | ND | 0.2 | None | None | NA | NA | 56 |
| 32 | Relapsed | 99 | c.1442G>C | p.C481S | ND | ND | 0.1 | None | None | NA | NA | 56 |
| 33 | Responsive | unknown | c.1442G>C | p.C481S | 19.5 | 19.5 | 18.7 | c.2977G>C | p.D993H | 2.9 | 2.9 | 32 |
| c.2535A>C | p.L845F | ND | 1.4 | |||||||||
| c.3419A>G | p.D1140G | ND | 0.4 | |||||||||
| 34 | Responsive | unknown | c.1442G>C | p.C481S | 4.4 | 4.4 | 5.4 | c.3419A>G | p.D1140G | 5.5 | 5.5 | 40 |
| c.2535A>T | p.L845F | 3.4 | 2.7 | |||||||||
| c.1442G>A | p.C481Y | ND | 0.7 | not tested | c.2535A>C | p.L845F | 2.7 | 2.1 | ||||
| c.1441T>A | p.C481S | ND | ND | 0.2 | ||||||||
| 35 | Responsive | 98 | c.1442G>C | p.C481S | 2.7 | 2.7 | 2.7 | None | None | NA | NA | 28 |
| 36 | Responsive | 88 | c.1442G>C | p.C481S | ND | ND | 0.4 | None | None | NA | NA | 18 |
| 37 | Responsive | 89 | c.1442G>C | p.C481S | ND | ND | 1.2 | None | None | NA | NA | 37 |
| 38 | Responsive | 89 | c.1442G>C | p.C481S | ND | ND | 0.7 | None | None | NA | NA | 20 |
BTK hotspots VAFs were not sex-normalized.
ND, not detected; NA, not available.
Samples from 47 of 49 relapsed patients (95.9%) were analyzed by ddPCR targeting BTK hotspot mutations as well. This analysis confirmed all NGS-detected BTK p.C481S mutation(s), with both tests reporting similar VAFs for the majority of mutations (Table 2). Discrepancies were observed in 3 patients (#1, #2, #24), who harbored a p.C481S mutation stemming from 2 different nucleotide substitutions (c.1441T>A and c.1442G>C) with very different VAFs, thus the discordance likely being the result of cross-reactivity in the ddPCR assays because of the strong positivity of the major clone.
The ddPCR assay identified additional BTK p.C481S low-VAF mutations (range, 0.1%-2.4%) in 16 relapsed patients (Table 2). Ten of them carried also a major BTK-mutated clone identified by both standard NGS and ddPCR analysis and hence were already classed as BTK mutated; in 5 of 11 of these samples the additional BTK mutation(s) were confirmed by per base NGS reanalysis (Table 2). In the remaining 6 patients, wildtype by standard NGS analysis (1% sensitivity), the ddPCR assay identified low-VAF BTK mutations (range, 0.1%-1.2%; 5/6 <1%), which were confirmed by per base NGS reanalysis in 4 of 6 samples. In addition, in 1 of these patients (#29), 2 additional low-VAF mutations were retrieved by per base NGS analysis only (Table 2). Samples from BM and peripheral blood at the time of relapse were available for 3 patients (#7, #15 and #20). The BTK p.C481S mutation was found in both the BM and PBMC sample from all 3 cases (Table 2), with a higher VAF detected in the PBMC in case #7 and #15, and with similarly low levels in patient #20, though only detected by either per base NGS analysis or ddPCR in the PBMC sample.
A per base NGS reanalysis was performed for PLCG2 gene as well at selected hotspots, to detect mutations with VAF <1%. Twelve relapsed patients harboring BTK mutation(s) also carried hotspot PLCG2 mutation(s). In 4 of them (#3, #6, #10 and #20), carrying BTK mutations at low-VAF only (<10%), multiple PLCG2 mutations with VAFs in the range of 0.2% to 32.7% were present (Table 2). Among the 25 of 49 relapsed patients (51%) negative for BTK mutations, only 2 cases (#2 and#26) carried hotspot PLCG2 mutations (Table 2).
Taking all analyzes together, 65% of relapsed patients carried at least 1 hotspot mutation in BTK and/or PLCG2, with 12 of 30 (40%) carrying BTK-mutated clone(s) at a VAF <10%, of which 6 at a VAF ≤1.2% (Table 2). No hotspot mutations in BTK or PLCG2 were detected in any of the matched baseline samples.
Only 3 of 49 responders (#33, #34 and #35) carried the BTK p.C481S substitution at varying allelic frequencies (19.5%, 4.4% and 2.7%, respectively). Two of them (#33 and #34), progressed 6 and 15 months after sampling, respectively, and were also found to harbor PLCG2 hotspots mutations at low VAFs (Table 2). The third patient (#35) remained in response at last follow-up (10 months after sampling) and carried no detectable PLCG2 mutation.
All BTK mutations detected by NGS in the 3 responsive patients were confirmed by ddPCR at similar VAFs. In patient #34, the ddPCR assay detected an additional BTK-mutated (BTKmut) minor subclone (VAF 0.2%) not confirmed by the NGS per base reanalysis; however, another small subclone (VAF 0.7%) was detected by the NGS per base reanalysis (Table 2).
In addition, samples at time point ≥1 year for 43 of 46 responders, wildtype for BTK as assessed by standard NGS analysis, were also analyzed by ddPCR. In 3 of 43 patients (#36, #37, #38) a p.C481S mutation was found at very low VAF (0.4%-1.2%) (Table 2) and was not detected by the NGS per base reanalysis. These 3 patients have maintained a response to ibrutinib at 26, 31 and 33 months after sampling.
Taking all analyzes together, 6 of 49 responders (12%) carried a hotspot mutation in BTK/PLCG2, with 5 of 6 cases (83%) harboring BTK mutations at a VAF <10%.
Finally, 9 mutations with a VAF <10% and lying outside the known hotspot regions within BTK (n = 4) and PLCG2 (n = 5) were detected, the majority of which had not been reported previously (Table 3). Six of them were found in 5 relapsed patients, all of whom harbored a hotspot mutation in BTK/PLCG2 genes (Table 3), whereas 3 were observed in responders, with 2 of them having no detectable hotspot mutation (Table 3).
BTK and PLCG2 nonhotspot mutations in relapsed and responsive cohorts, as assessed by targeted NGS analysis
| Patient ID . | Cohort . | Time point . | Gene . | coding DNA description . | protein description . | protein domain . | NGS VAF (%) . | References . | Other BTK hotspot mutations . | Other PLCG2 hotspot mutations . |
|---|---|---|---|---|---|---|---|---|---|---|
| 18 | Relapsed | relapse | BTK | c.136C>T | p.R46C | PH | 2.6 | none | yes | no |
| 26 | Relapsed | relapse | BTK | c.946A>G | p.T316A | SH2 | 3.0 | Sharma et al 2016; Kadri et al 2017; Jones et al 2017 | no | yes |
| c.1003G>C | p.V335L | SH2 | 3.6 | none | ||||||
| 34 | Responsive | ≥1 y | BTK | c.1283C>A | p.A428D | TK | 6.0 | Wang et al 2022 | yes | yes |
| 7-PBMC | Relapsed | relapse | PLCG2 | c.601C>G | p.Q201E | EF | 2.7 | none | yes | no |
| 1 | Relapsed | relapse | PLCG2 | c.683T>A | p.F228Y | EF | 1.7 | none | yes | yes |
| 6 | Relapsed | relapse | PLCG2 | c.2543T>G | p.L848R | PH | 9.3 | Landau et al 2017 | yes | yes |
| 60 | Responsive | ≥1 y | PLCG2 | c.846G>C | p.E282D | EF | 3.8 | none | no | no |
| 83 | Responsive | ≥1 y | PLCG2 | c.3076A>T | p.A1026T | Y | 3.2 | none | no | no |
| Patient ID . | Cohort . | Time point . | Gene . | coding DNA description . | protein description . | protein domain . | NGS VAF (%) . | References . | Other BTK hotspot mutations . | Other PLCG2 hotspot mutations . |
|---|---|---|---|---|---|---|---|---|---|---|
| 18 | Relapsed | relapse | BTK | c.136C>T | p.R46C | PH | 2.6 | none | yes | no |
| 26 | Relapsed | relapse | BTK | c.946A>G | p.T316A | SH2 | 3.0 | Sharma et al 2016; Kadri et al 2017; Jones et al 2017 | no | yes |
| c.1003G>C | p.V335L | SH2 | 3.6 | none | ||||||
| 34 | Responsive | ≥1 y | BTK | c.1283C>A | p.A428D | TK | 6.0 | Wang et al 2022 | yes | yes |
| 7-PBMC | Relapsed | relapse | PLCG2 | c.601C>G | p.Q201E | EF | 2.7 | none | yes | no |
| 1 | Relapsed | relapse | PLCG2 | c.683T>A | p.F228Y | EF | 1.7 | none | yes | yes |
| 6 | Relapsed | relapse | PLCG2 | c.2543T>G | p.L848R | PH | 9.3 | Landau et al 2017 | yes | yes |
| 60 | Responsive | ≥1 y | PLCG2 | c.846G>C | p.E282D | EF | 3.8 | none | no | no |
| 83 | Responsive | ≥1 y | PLCG2 | c.3076A>T | p.A1026T | Y | 3.2 | none | no | no |
Mutational profiling of the relapsed and responsive cohort
We next investigated the frequency of mutations within the 11 additional genes included in the HaloPlex panel. A total of 415 somatic mutations were detected; no significant difference in the average number of mutations per case in the relapsed vs responsive cohort was observed (4.6 vs 3.9, respectively). The VAF range spanned from 1.4% to 100%, however, half of all variants were found at allelic frequencies <10% (203/415; 48.9%) (supplemental Table 2).
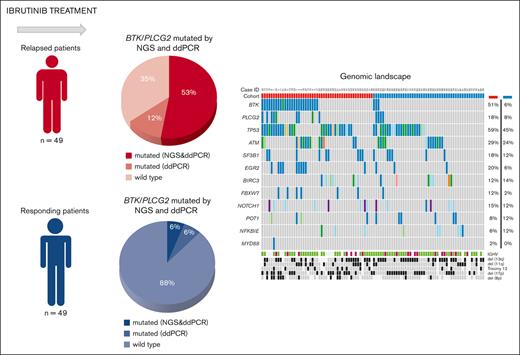
Aside from mutations in BTK and PLCG2, the most frequently mutated genes in the relapsed patients at progression were: TP53 (29/49, 59%), ATM (14/49, 29%), EGR2 (10/49, 20%), SF3B1 (9/49, 18%), NOTCH1 (8/49, 16%) and BIRC3 (6/49, 12%), and in the responsive patients at ≥1 year sampling after therapy initiation: TP53 (22/49, 45%), ATM (12/49, 24%), BIRC3 (7/49, 14%), SF3B1 (6/49, 12%) and NOTCH1 (6/49, 12%) (Figure 1). Two relapsed and 9 responsive patients (without BTK/PLCG2 mutations) did not carry mutations in any genes tested. Combining FISH and mutational data, 32 of 49 of the relapsed patients (65%) displayed TP53 aberrations compared with that of 28 of 49 of the responsive patients (57%). Along the same lines, 19 of 49 patients (39%) in the relapsed cohort carried an ATM alteration (mutation and/or deletion) vs 21 of 49 (43%) in the responsive cohort.
Oncoplot displaying the somatic variants detected in the 13 genes analyzed by a HaloPlex high sensitivity custom panel in the relapsed and responding cohorts. Illustrated are the distribution of the somatic variants, IGHV mutational status, cytogenetic profile determined by fluorescence in situ hybridization or lymphoid panel (del[8p]), and the mutation frequency of each gene in the relapsed and responding cohorts, respectively. Patient identifiers are indicated across the top row.
Oncoplot displaying the somatic variants detected in the 13 genes analyzed by a HaloPlex high sensitivity custom panel in the relapsed and responding cohorts. Illustrated are the distribution of the somatic variants, IGHV mutational status, cytogenetic profile determined by fluorescence in situ hybridization or lymphoid panel (del[8p]), and the mutation frequency of each gene in the relapsed and responding cohorts, respectively. Patient identifiers are indicated across the top row.
An asymmetric distribution of mutations in other genes was noted in BTKmut vs BTKwt relapsed patients. Specifically, EGR2 was mutated in 9 of 24 BTKmut vs 1 of 25 BTKwt patients (P < .01), whereas BIRC3 (n = 6; P < .05) and NFKBIE (n = 3, P > .05) mutations were only detected in the BTKwt group (Figure 1). In contrast, TP53 mutation burden was not significantly different between BTKmut and BTKwt relapsed cases.
In 19 relapsed patients, samples at both baseline and relapse were available. The total number of somatic mutations at baseline was 36 (average: 1.9; range, 1-4), compared with that of 60 mutations carried by the matched relapse samples (average: 3.2; range, 0-7), the difference being mainly because of the appearance of BTK/PLCG2 mutations (n = 18). In other words, for all other genes analyzed, only a few patients acquired mutations at the relapse vs the baseline time point, including 2 patients who gained EGR2 mutations at low allelic burdens (#5 and #30) (supplemental Figure 1; supplemental Figure 2A). Accordingly, BIRC3 and NFKBIE mutated patients carried mutations at both time points, except for patient #41 who was NFKBIE mutated only at baseline with a low allelic burden (supplemental Figure 2B,C).
Paired samples (at baseline and at time point ≥1 year) were available for 31 patients in the responsive cohort. The total number of somatic mutations at baseline was 70 (average: 2.3; range, 0-10), compared with that of 84 mutations detected at time point ≥1 year (average: 2.7; range, 0-11). The frequency of mutated genes in samples at baseline compared with that of at ≥1 year time point was not significantly different (supplemental Figure 3).
Additional genetic aberrations assessed by the lymphoid panel
To investigate if additional genetic aberrations may be present in relapsed vs responsive patients, we applied a capture-based lymphoid panel and analyzed the mutational status of the 239 genes not included in the HaloPlex panel and the genome-wide copy-number status in 104 gDNA samples from 72 patients (38/49 relapsed, 34/49 responsive). We detected recurrent mutations in ASXL1, BRAF, IKZF3, KRAS, MED12, MGA, RPS15, SPEN, and ZFN292; however, only BRAF, and potentially IKZF3, showed a predominance in relapsed cases (supplemental Table 3; supplemental Figure 4). Considering that occasional patients progressing on ibrutinib15,16 have displayed EIF2AK3, EP300, and KMT2D mutations and del(8p), we specifically looked for these aberrations. Only 1 relapsed patient (BTKmut by ddPCR only) carried an EP300 mutation (VAF 47.9%) at relapse, whereas 2 KMT2D mutations (VAF 28.9; 65.7%) were retrieved in 1 relapsed BTKwt patient at relapse. EIF2AK3 was wildtype in all patients (supplemental Table 3). Finally, del(8p) was detected in 13 patients: 11 relapsed, 4 of them BTKwt by HaloPlex analysis (1 mutated by ddPCR only), and 2 responsive BTKwt patients (supplemental Table 4).
TP53 clonal dynamics under ibrutinib treatment
Among 19 relapsed patients with paired samples, 7 of 19 were TP53 wildtype at both time points (1 of them BTKmut by HaloPlex analysis) and 12 of 19 carried a TP53 mutation at some point (7/12 with at least 1 BTK hotspot mutation detected by HaloPlex analysis). Among the 12 patients with TP53 mutation, 3 showed expansion (n = 2) of an existing clone or the appearance (n = 1) of a new clone at relapse; 1 showed a reduction of an existing clone; 5 carried a stable mutant clone, at both time points. One patient carried 3 different TP53 clones and, interestingly, 1 expanded, 1 reduced and 1 appeared at relapse (Figure 2A). In 2 patients the clonal dynamics could not be reliably analyzed because of missing CD19+ purity data.
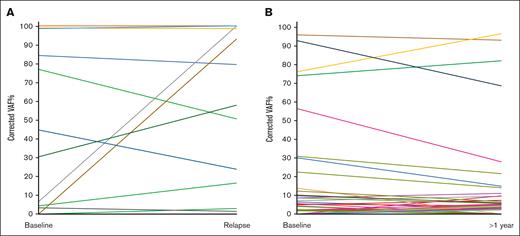
TP53 clonal dynamics in the relapsed and responsive cohorts. (A) TP53 clonal dynamics in 10 mutated patients of the relapsed cohort. Each patient is represented by a different color. The vertical axis displays the mutation VAF%, corrected according to the % of CD19+ cells in the sample. Two patients were not included in the chart because of missing CD19+ purity data. (B) TP53 clonal dynamics in 18 mutated patients of the responsive cohort. Each patient is represented by a different color. The vertical axis displays the mutation VAF%, corrected according to the % of CD19+ cells in the sample. Three patients were not included in the chart because of the missing CD19+ purity data.
TP53 clonal dynamics in the relapsed and responsive cohorts. (A) TP53 clonal dynamics in 10 mutated patients of the relapsed cohort. Each patient is represented by a different color. The vertical axis displays the mutation VAF%, corrected according to the % of CD19+ cells in the sample. Two patients were not included in the chart because of missing CD19+ purity data. (B) TP53 clonal dynamics in 18 mutated patients of the responsive cohort. Each patient is represented by a different color. The vertical axis displays the mutation VAF%, corrected according to the % of CD19+ cells in the sample. Three patients were not included in the chart because of the missing CD19+ purity data.
Among 31 responsive patients with paired samples, 10 were TP53 wildtype at both time points and 21 of 31 carried at least 1 TP53 mutation at 1 or both time points. Among the latter, 7 patients showed expansion (n = 2) of an existing clone or the appearance (n = 5) of 1 or more TP53 mutations at the time point ≥1 year (VAF<10%); 5 showed a reduction (n = 1) or the disappearance (n = 4) of existing clones; 4 carried a stable mutant clone, with either high (n = 1) or low (n = 3) VAF, at both time points (Figure 2B). The remaining 5 patients carried multiple TP53 mutations at both time points, of which 4 were mainly characterized by clonal stability or decrease, with the appearance of new clones with VAF <10% in 2 of them (Figure 2B).
Correlation of NGS data with clinical outcome in relapsed cases
Among relapsed patients, those with BTK and/or PLCG2 hotspot mutations (n = 32, assessed by any method) experienced a longer TTP than those without mutations (n = 17) (median TTP, 36 months; range, 5-56 vs 14.5 months; range, 5-66, respectively; P = .053). Clinical indications of progression on ibrutinib did not differ in the 2 subgroups with the most frequent being the presence of lymphadenopathies (22/32 in the BTK/PLCG2 mutated vs 9/17 in the BTK/PLCG2 wildtype cases), followed by anemia and/or thrombocytopenia because of BM infiltration (7/32 vs 3/18), splenomegaly (0/31 vs 3/18) and lymphocyte doubling time (3/31 vs 2/18). After a median follow-up of 43 months (8-84), overall survival in the relapsed cohort was 58 months, without statistically significant difference between the 2 subgroups.
Discussion
In this study, we performed targeted NGS in a real-world cohort of patients with CLL relapsed on or responsive to ibrutinib treatment, to gain further insights into the mechanisms implicated in the emergence of resistance.
We show that up to 65% of patients relapsing on ibrutinib carried at least 1 hotspot BTK mutation at the cysteine 481 residue, and/or ≥1 hotspot PLCG2 mutations, by integrating NGS analysis (1% sensitivity) with more sensitive techniques such as per base NGS analysis and ddPCR (0.1% sensitivity). This prevalence of BTK/PLCG2 mutations is lower than those reported in most previous studies, where the overall frequency of BTK and/or PLCG2 mutated relapsed patients (1% VAF cutoff) was up to 100%,11-13,25 hence indicating the existence of alternative mechanisms of resistance. Similar to other cohorts, we found a large proportion (40%) of relapsed BTK-mutated cases harboring hotspot mutation(s) with a VAF <10%, including several cases with VAFs bordering 1%, thus questioning how such clones substantially contribute to resistance. This is further complicated by the finding that, also in the responsive cohort, we detected BTK mutations in 6 patients (12%), of whom 3 were with low VAF (0.4%-1.2%). Although 2 of 6 progressed 6 and 15 months after sampling, the others remained in response (3/4 patients <2 years after sampling). For comparison, 6 patients in the relapsed cohort carried similar BTK clones at low VAF (0.1%-1.5%) but experienced relapse.
Reasonable assumptions could be that the minor mutant clone may exert a dominant effect on the response of the overall wildtype tumor cell population, as suggested for ibrutinib-resistant patients with either CLL26 or Waldenström Macroglobulinemia,27 though this would not explain the detection of such mutations in responding patients even after 2 years of follow-up. Alternatively, resistant and mutated cells may reside in a tissue compartment not analyzed or other mechanisms acting independently or co-operatively may exist.
To explore the latter possibility, we characterized our cohort by NGS for other genes associated with disease progression and dismal prognosis.25,28 Notably, the frequency of TP53-mutated clones in both the relapsed and responsive cohorts did not differ significantly. However, there was a striking difference between the 2 cohorts in terms of clonal size, with the mean VAF% of TP53 mutant clones being higher in the relapsed vs the responsive cohort, at both baseline (57% vs 19%) and relapse/≥1 year time point (59% vs 16%), with almost all TP53 mutations in the relapsed group having a VAF ≥10%. On the contrary, in the responsive cohort the majority of TP53 mutations was present at a VAF <10%, at both baseline and at ≥1 year time point. That said, it is interesting to note that ibrutinib treatment did not seem to favor the selection of TP53-aberrant clones even in the relapsed cohort, in which the majority of clones either remained stable or decreased in size at the time of relapse, similar to the responding cohort. This suggests little if any direct involvement of TP53 aberrations in the onset of ibrutinib resistance, though the presence of large TP53-aberrant clones may give a higher propensity toward clonal evolution because of genomic instability and the occurrence of mutations in other genes or pathways ultimately responsible for drug resistance.
In the relapsed cohort, we detected a biased distribution of other gene mutations in BTK/PLCG2 mutated vs wildtype subgroups. Interestingly, the transcription factor EGR2 was almost exclusively mutated in the BTKmut relapsed group. EGR2 is activated through ERK phosphorylation upon BcR stimulation, thus suggesting that EGR2 mutations might lead to a constitutively dysregulated BcR signaling,29 cooperating with the existing BTK/PLCG2 mutations toward ibrutinib resistance.
Conversely, BIRC3 and NFKBIE mutations in the relapsed cohort were exclusively detected in the BTKwt vs BTKmut group (also at baseline), pointing toward an aberrant activation of the canonical/noncanonical NF-κB pathway as a potential mechanism leading to earlier progression and drug escape. BIRC3 aberrations have been suggested as predictive factors for poor response to chemoimmunotherapy in patients with CLL.30 Our results potentially extend the role of this gene also in shaping resistance to novel therapies, although in the phase 3 RESONATE study progression-free survival in patients treated with ibrutinib was not affected by baseline BIRC3 mutational status.31 Notably, BIRC3 and/or NFKBIE mutations were present also in a minor proportion (14% and 12%) of the responsive patients, respectively. Longer follow-up will be needed to ascertain if the presence of these mutations may associate with future resistance to ibrutinib treatment.8,9
Additional genomic aberrations, including del(8p) and mutations in EIF2AK3, EP300, KMT2D,15,16 have been reported in smaller BTKwt patient series. By applying a capture-based panel, we confirmed an enrichment of del(8p) in relapsing cases (11 relapsing vs 2 responsive) but with only 3 of them wildtype for BTK/PLCG2. Moreover, although recurrent mutations were seen in some other known CLL driver genes, only BRAF and IKZF3 mutations showed a predominance in relapsed cases. In contrast, no clear predilection of mutations in EIF2AK3, EP300, and KMT2D was observed in relapsing vs responsive cases.
Though the existence of additional genomic aberrations explaining the resistance deserves further studies, it is also intriguing to hypothesize the occurrence of “functional resistance,” in the absence of BTK/PLCG2 mutations, because of either decreased dependence on proximal BcR signaling or to its bypass through the modulation of the functionality of other non-BcR immune pathways.32 Noteworthy, in a recent study,33 in patients with CLL progressing on idelalisib treatment, IGF1R overexpression was associated with progression in the absence of mutations that could explain resistance, highlighting nongenetic mechanisms as causes of secondary resistance.
In conclusion, although we confirm that BTK/PLCG2 mutations are present in patients with CLL relapsing on ibrutinib, more than one-third of them do not harbor such mutations even after high sensitivity analyses. We also validate enrichment of del(8p) in relapsing patients, mainly in combination with BTK mutations. Importantly, we show that additional genetic mechanisms, in particular aberrant activation of the BcR and NF-κB pathways, may cooperate in determining progression on ibrutinib in some cases. BTK/PLCG2 mutations may appear at low frequency and can be identified also in a small fraction of patients responding to ibrutinib for <2 years. As a consequence, though we demonstrate the feasibility of a targeted NGS approach to detect such mutations in real-world, its use should not be applied in routine clinical practice, as currently stated in the international recommendations, till we accumulate enough evidence on how to guide treatment decisions when a mutation in these (or other) genes are detected before clinical progression.
Acknowledgments
This project was supported by Cancer Research UK (ECRIN-M3 accelerator award C42023/A2937). This work was in part supported by an unrestricted grant by Sunesis; Associazione Italiana per la Ricerca sul Cancro--AIRC, Milano, Italy (Investigator Grant #20246 and Special Program on Metastatic Disease--5 per mille #21198); ERA NET TRANSCAN-2 Joint Transnational Call for Proposals: JTC 2014 (project #143 GCH-CLL) and JTC 2016 (project #179 NOVEL), project code (MIS) 5041673; Bando della Ricerca Finalizzata 2018, Ministero della Salute, Roma, Italy (progetto RF-2018-12368231); the Hellenic Precision Medicine Network in Oncology; project ODYSSEAS (Intelligent and Automated Systems for enabling the Design, Simulation and Development of Integrated Processes and Products) implemented under the “Action for the Strategic Development on the Research and Technological Sector,” funded by the Operational Programme “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014-2020) and cofinanced by Greece and the European Union, with grant agreement number MIS 5002462; the Swedish Cancer Society, the Swedish Research Council, the Knut and Alice Wallenberg Foundation, Karolinska Institutet, Karolinska University Hospital, and Radiumhemmets Forskningsfonder, Stockholm; Lion’s Cancer Research Foundation, Uppsala; and EU’s Horizon 2020 research and innovation program under grant 739593. The authors acknowledge Clinical Genomics Uppsala, Science for Life Laboratory, Department of Immunology, Genetics and Pathology, Uppsala University, Sweden for providing assistance with droplet digital PCR and analysis. The authors acknowledge ACRF and Peter MacCallum molecular laboratories and the CLL Global Foundation for their support.
Authorship
Contribution: S.B. coordinated sample collection, performed NGS experiments, interpreted data and wrote the manuscript; L.-A.S. performed NGS experiments, interpreted data, and wrote the manuscript; V.L. performed bioinformatics analysis and wrote the manuscript; A.C. analyzed and interpreted clinical data; T.P. and S.W. performed ddPCR experiments and analysis; H.F.-A and A.S. performed bioinformatic analysis on the lymphoid panel data; A.G. and A.L. prepared and sequenced libraries for the lymphoid panel; F.G. interpreted lymphoid panel data and wrote the manuscript; P.R. performed sample purification and DNA extraction; L.S. designed the study, coordinated clinical data collection, analyzed and interpreted clinical data and wrote the manuscript; P.G. and R.R. designed the study, interpreted data and wrote the manuscript; and all authors provided samples and clinical data, and edited and approved the manuscript for submission.
Conflict-of-interest disclosure: G.G. is on the advisory board/speaker’s bureau of Janssen, AbbVie, AstraZeneca, and BeiGene. F.F. received honoraria and/or is on the advisory board of AbbVie, Acerta/AstraZeneca, Janssen-Cilag, and BC platforms. O.J. reports consultancy for AstraZeneca and Eli Lilly, and is on the speaker’s bureau of AbbVie, AstraZeneca, and Johnson & Johnson. R.W. reports meeting sponsorship from AbbVie and Janssen; is on the advisory board of AstraZeneca, Janssen, SecuraBio, and AbbVie; and is on the speaker's bureau of AbbVie, AstraZeneca, Janssen, and BeiGene. A.O. receives research funding from BeiGene, Janssen, AstraZeneca and Gilead. P.B. received honoraria from AbbVie, Gilead, and Janssen, and received research funding from Gilead. K.S. received honoraria and/or is on the advisory board of AbbVie, Acerta/AstraZeneca, Gilead, and Janssen, and received research funding from AbbVie, Gilead, and Janssen. L.S. is on the advisory board of AbbVie, AstraZeneca, and Janssen. R.R. received honoraria and/or is an advisory board member in AbbVie, AstraZeneca, Janssen, Illumina and Roche. P.G. received honoraria and/or is on the advisory board of AbbVie, Acerta/AstraZeneca, Adaptive, ArQule/MSD, BeiGene, CelGene/Juno, Gilead, Janssen, Loxo/Lilly, and Sunesis, and received research funding from AbbVie, Gilead, Janssen, Novartis, and Sunesis. The remaining authors declare no competing financial interests.
Correspondence: Paolo Ghia, Division of Experimental Oncology, Università Vita Salute San Raffaele, Via Olgettina 58, Milan 20132, Italy; e-mail: ghia.paolo@hsr.it.
References
Author notes
∗S.B., L.-A.S., and V.L. are the joint first authors.
†L.S., R.R., and P.G. are the joint last authors.
NGS data for HaloPlex (fastq files) have been deposited to the European nucleotide archive, https://www.ebi.ac.uk/ena/ (accession ID PRJEB44894), whereas the lymphoid panel NGS data (CRAM files) are available via Figshare at https://doi.org/10.17044/scilifelab.19721998.
The online version of this article contains a data supplement.

![Oncoplot displaying the somatic variants detected in the 13 genes analyzed by a HaloPlex high sensitivity custom panel in the relapsed and responding cohorts. Illustrated are the distribution of the somatic variants, IGHV mutational status, cytogenetic profile determined by fluorescence in situ hybridization or lymphoid panel (del[8p]), and the mutation frequency of each gene in the relapsed and responding cohorts, respectively. Patient identifiers are indicated across the top row.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/7/12/10.1182_bloodadvances.2022008821/2/m_blooda_adv-2022-008821-gr1.jpeg?Expires=1765885003&Signature=uHhTvLdPAfsWfsLU652FIjayvgSjQPGCigDNhIwBZ40bnwanZwtDXyrxo6g7Rkl7qyTr0aZg7JUuVi1Q3cfJvSnh-gfKbfpSz2WSxCRUqX0kCg9L2Ppy65VC3pwnL6mxjSkCx01mjBm1K0UWCRjpUhUnaPZTTyGP2duygQA-rc9IROu5rj6RmsT4wOU9QOQ93WbyyhTFNJjhvIQg3igyl9AQPER3ICUVgqsx9rv93wwhwTIu9fbFFN3xc0Dnjev9QK~uBwq01gk5YvyXbTtm1pKwtsB9eAO03G5kMriIxdJQKqa2aUmWjeA2RlzvWBkZ3fY9kW3HuSmgRS361t~jdw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)