Key Points
The development of second primary malignancy post–auto-HSCT for myeloma is associated with inferior PFS and OS.
MM remains the primary cause of death among patients with second primary malignancy after auto-HSCT.
Abstract
The overall survival (OS) has improved significantly in multiple myeloma (MM) over the last decade with the use of proteasome inhibitor and immunomodulatory drug-based combinations, followed by high-dose melphalan and autologous hematopoietic stem cell transplantation (auto-HSCT) and subsequent maintenance therapies in eligible newly diagnosed patients. However, clinical trials using auto-HSCT followed by lenalidomide maintenance have shown an increased risk of second primary malignancies (SPM), including second hematological malignancies (SHM). We evaluated the impact of SPM and SHM on progression-free survival (PFS) and OS in patients with MM after auto-HSCT using CIBMTR registry data. Adult patients with MM who underwent first auto-HSCT in the United States with melphalan conditioning regimen from 2011 to 2018 and received maintenance therapy were included (n = 3948). At a median follow-up of 37 months, 175 (4%) patients developed SPM, including 112 (64%) solid, 36 (20%) myeloid, 24 (14%) SHM, not otherwise specified, and 3 (2%) lymphoid malignancies. Multivariate analysis demonstrated that SPM and SHM were associated with an inferior PFS (hazard ratio [HR] 2.62, P < .001 and HR 5.01, P < .001, respectively) and OS (HR 3.85, P < .001 and HR 8.13, P < .001, respectively). In patients who developed SPM and SHM, MM remained the most frequent primary cause of death (42% vs 30% and 53% vs 18%, respectively). We conclude the development of SPM and SHM leads to a poor survival in patients with MM and is an important survivorship challenge. Given the median survival for MM continues to improve, continued vigilance is needed to assess the risks of SPM and SHM with maintenance therapy post–auto-HSCT.
Introduction
Autologous hematopoietic stem cell transplantation (auto-HSCT) for eligible patients with multiple myeloma (MM) offers the possibility of long-term disease control. Auto-HSCT with melphalan-based conditioning has become the standard of care for eligible patients.1,2 High-dose chemotherapy followed by auto-HSCT is generally preceded by induction therapy with drug combinations involving a proteasome inhibitor and an immunomodulatory agent.3 This treatment approach along with the inclusion of maintenance therapies after auto-HSCT in patients with MM have reduced relapse risk and improved outcomes, now with median overall survival (OS) >10 years in certain MM populations.4,5 Although the all-cause late mortality following auto-HSCT recipients has declined over the last 3 decades, that from second primary malignancies (SPM) has not improved.6 Therefore, given the improving survival, understanding the impact of long-term complications, such as SPM, is paramount.
Although auto-HSCT is recognized as a standard of care for patients newly diagnosed with MM who are eligible for transplant owing to the improved long-term disease control and survival it provides, the exposure to high-dose chemotherapy is associated with an increased risk of SPM, with a subset of SPM categorized as second hematologic malignancies (SHM).7-9 A population-based Swedish study conducted before wide adaptation of lenalidomide maintenance revealed that increased cumulative doses of alkylating therapy with melphalan was associated with a 2.8-fold higher risk of therapy-related myeloid neoplasms (t-MN).10 A similar Center for International Blood and Marrow Transplant Research (CIBMTR) study of auto-HSCT, limited to studying the development of t-MN from 1995 to 2010, reported the risk of a subsequent t-MN was higher in males, aged ≥55 years, and those who received ≥3 lines pre-HSCT therapy.11 Prior CIBMTR analysis of new cancers after auto-HSCT in MM from 1990 to 2010, with only 11% of the study cohort receiving lenalidomide maintenance, revealed higher than expected rates of melanoma and acute myeloid leukemia (AML) compared with age-, race-, and gender-adjusted comparison subjects.12
With several randomized trials confirming the progression-free survival (PFS) and OS benefit when lenalidomide-based maintenance is provided after auto-HSCT, this treatment paradigm is now the nearly universal approach for transplant-eligible patients with MM.13-16 Although lenalidomide maintenance has improved survival in MM, post-HSCT lenalidomide maintenance has been shown to increase the risk of SPM and SHM by approximately 2.5- and 5-fold, respectively.14,17-19 Recent meta-analysis revealed the development of SPM after lenalidomide was specific to use in MM, with no observed increase of SPM after lenalidomide use in lymphoma, chronic lymphocytic leukemia, or myelodysplastic syndromes.20 As confirmed by this recent meta-analysis, SPM after lenalidomide use in MM was independent of transplant status.20,21 However, the risk of t-MN is increased in the setting of prior melphalan exposure compared with those who received lenalidomide in the absence of auto-HSCT or prior melphalan.21 A recent single center analysis of patients with MM undergoing auto-HSCT revealed that lenalidomide exposure was associated with an approximately 9-fold increase in the risk of SHM, specifically t-MN.22 Survival after the diagnosis of t-MN is approximately 1 year, representing 1 of the most aggressive malignancies known.23,24 Although allogeneic HSCT (allo-HSCT) is considered the “gold-standard” for treatment of t-MN, <10% t-MN patients received allo-HSCT.22
There are several clinical trials evaluating new maintenance therapies after auto-HSCT in MM, including maintenance daratumumab plus lenalidomide vs lenalidomide alone (NCT03901963), maintenance teclistamab combined with lenalidomide vs lenalidomide alone (NCT05243797), and iberdomide maintenance (NCT05354557), which makes further elucidation of type, timing, and outcome of SPM necessary to fill an important knowledge gap.25 We hypothesized that the development of SPM and SHM after auto-HSCT in patients with MM are associated with significantly inferior PFS and OS. The purpose of this study was to examine data from the CIBMTR registry to determine the impact of SPM on PFS and OS in patients with MM after auto-HSCT in the modern era.
Methods
Data source
The CIBMTR is a nonprofit research collaboration between the National Marrow Donor Program (NMDP)/Be The Match and the Medical College of Wisconsin. It encompasses a voluntary working group of >350 transplant centers worldwide. Participating centers are required to report all transplants and cellular therapies consecutively; compliance is monitored by onsite audits and patients are followed longitudinally. Computerized checks for discrepancies, physicians’ review of submitted data, and onsite audits of participating centers ensure data quality. It is estimated that almost all United States (US) allo-HSCTs and about 85% of auto-HSCTs are reported to the CIBMTR. Currently, the CIBMTR research database includes long-term clinical data for >600 000 patients. The CIBMTR collects transplant data on 2 levels, using a Transplant Essential Data (TED) form and a Comprehensive Report Form (CRF). The CIBMTR collects TED data on all patients. Using a regularly reviewed, weighted algorithm, the CIBMTR selects a subset of patients for a more detailed CRF data collection. TED- and CRF-level data are collected pretransplant, 100 days after transplant, 6 months after transplant, annually until year 6 after transplant, and biannually thereafter. The CIBMTR subjects data to a series of automated and manual quality checks. In addition, the CIBMTR audits each transplant center periodically. These validations and verifications produce high-quality data. If a center fails to meet data quality standards, its data is removed (embargoed) from research studies. The NMDP/Be The Match Institutional Review Board reviews the CIBMTR’s research. Patients and/or guardian(s) give informed consent for research.
Study design
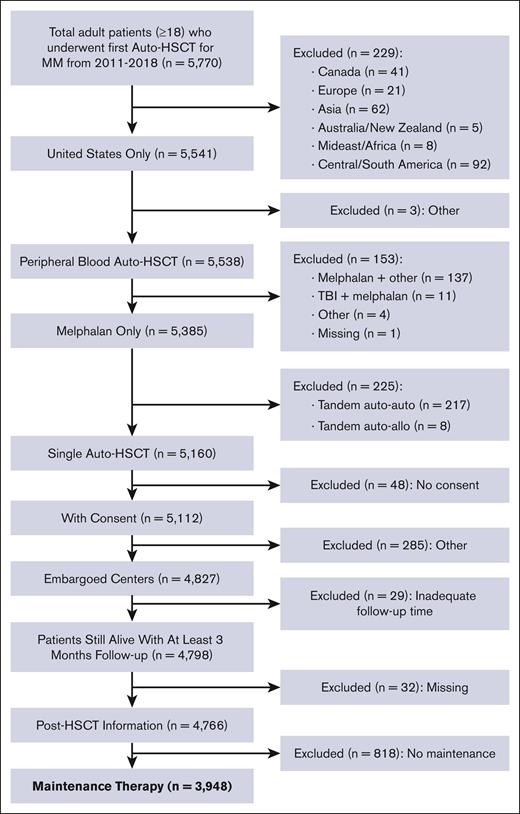
The data were collected in the CIBMTR registry database, and the study was conducted by the Plasma Cell Disorders Working Committee of the CIBMTR. Adult patients with MM who underwent first single auto-HSCT in the US with a melphalan only conditioning regimen between 2011 and 2018 and subsequently received maintenance therapy were included. Owing to the high rate of use of maintenance therapy after auto-HSCT during this study period, patients were excluded if they did not receive maintenance after auto-HSCT. Patients were also excluded if they underwent transplantation at a non-US center, received a tandem auto-HSCT, or had <3 available months of follow-up data if still alive. Nonmelanoma skin cancers were excluded as new malignancies in this analysis. Details regarding cause of death were reported to the CIBMTR from respective transplant centers.
Study outcomes
The primary objective of this study was to determine the impact of SPM and SHM on OS and PFS in patients with MM after auto-HSCT. The secondary objectives were to characterize different types of SPM after auto-HSCT and evaluate the utilization rate of allo-HSCT in patients with SHM.
Statistical analysis
Descriptive statistics were used to summarize the study population. Time to diagnosis of SPM and SHM from auto-HSCT was determined. OS was defined as the time from auto-HSCT to death from any cause, with the surviving subjects censored at the time of the last follow-up. PFS was defined as the time from auto-HSCT to MM relapse or death from any cause, with alive patients censored at the last follow-up. The cumulative incidence of SPM and SHM from auto-HSCT was determined with death as a competing risk. Kaplan-Meier method and log-rank testing for univariate comparisons was used to determine probabilities of OS and PFS. Multivariate analysis (MVA) was performed using a Cox proportional hazards regression model using both the variables as time-dependent covariates to determine the impact of SPM and SHM on PFS or OS. A stepwise model building approach was adopted and variables that attain a P-value <5% were retained in the final model. The following patient-related factors were considered in the model building: age, gender, and race. The disease-related covariates considered were Karnofsky Performance Score (KPS), hematopoietic cell transplant comorbidity index (HCT-CI), MM International Staging System (ISS) stage, cytogenetics, number of lines of therapies, and disease status before HSCT. The transplant-related covariates considered were conditioning regimen, interval from diagnosis to transplant, and year of transplant. Statistical analysis was performed using SAS (version 9.4, Cary, North Carolina, United States). All P-values shown were from 2-sided tests, and the reported confidence intervals (CIs) refer to 95% boundaries.
Results
Patient characteristics
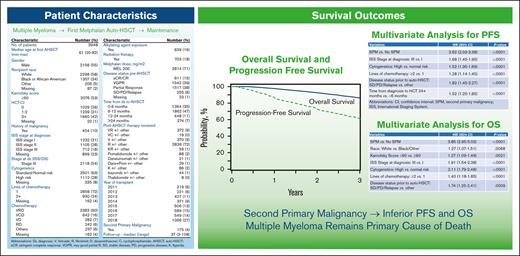
A total of 3948 adult patients with MM who underwent first auto-HSCT in the United States with a melphalan conditioning regimen between 2011 and 2018 and received post-HSCT maintenance therapy were included (Figure 1). Follow-up data was available for 100%, 93%, and 90% of patients at 1-, 2-, and 3-years, respectively. Median follow-up time was 37 months (range, 3-108). Patient characteristics are shown in Table 1. The median age at the time of the first auto-HSCT was 61 years (range, 20-82), with 44% (n = 1727) of patients receiving transplantation between the ages of 60 to 69. Recipient race was reported as White in 58% (n = 2298) and Black or African-American (AA) in 34% (n = 1 357) of patients. A total of 400 patients (10%) had prior malignancy at the time of auto-HSCT, with a majority (n = 334) reported as a history of solid tumor. High-risk MM cytogenetics were observed in 28% (n = 1112) of the population, and most (n = 2856, 72%) received just 1 line of chemotherapy before auto-HSCT. Bortezomib, lenalidomide, dexamethasone (VRD) was the most used therapy before auto-HSCT, and 639 (16%) had exposure to alkylating agents before auto-HSCT. A total of 71% (n = 2814) of patients received melphalan dosing of 200 mg/m2. Most patients underwent transplant with very good partial response (VGPR) or partial response (PR) of 39% (n = 1542) and 38% (n = 1517), respectively. Patients underwent transplant most often within 6 to 12 months (n = 1862; 47%) or 0 to 6 months (n = 1364; 35%) from diagnosis. Lenalidomide, either as a single agent or in combination, was the most commonly reported maintenance regimen after auto-HSCT (n = 2836; 72%). Notably, the most transplants performed in any 1 year were observed in 2018 (n = 1 066; 27%).
Characteristics of patients with MM undergoing first autologous stem cell transplant from 2011 to 2018 in the United States
| Characteristic . | Number (%) . |
|---|---|
| No. of patients | 3948 |
| Median age at first auto-HSCT (min-max) | 61 (20-82) |
| Age at transplant, y | |
| 18-39 | 110 (3) |
| 40-49 | 471 (12) |
| 50-59 | 1256 (32) |
| 60-69 | 1727 (44) |
| 70+ | 384 (10) |
| Gender | |
| Male | 2156 (55) |
| Female | 1792 (45) |
| Region | |
| US | 3948 (100) |
| Recipient race | |
| White | 2298 (58) |
| Black or African-American | 1357 (34) |
| Other | 206 (5) |
| Missing | 87 (2) |
| Karnofsky score | |
| ≥ 90 | 2076 (53) |
| < 90 | 1798 (46) |
| Missing | 74 (2) |
| HCT-CI | |
| 0 | 1029 (26) |
| 1 | 559 (14) |
| 2 | 680 (17) |
| 3 | 744 (19) |
| 4 | 436 (11) |
| 5 | 216 (5) |
| 6+ | 264 (7) |
| Missing | 20 (1) |
| History of solid tumor (excluding nonmelanoma skin cancers) | |
| No | 3591 (91) |
| Yes | 334 (8) |
| Missing | 23 (1) |
| History of malignancy (any prior malignancy including solid tumors) | |
| No | 3544 (90) |
| Yes | 404 (10) |
| ISS stage at diagnosis | |
| ISS stage I | 1232 (31) |
| ISS stage II | 1105 (28) |
| ISS stage III | 712 (18) |
| Missing | 899 (23) |
| Stage at diagnosis (ISS/DS) | |
| Stage III | 2118 (54) |
| Stage I-II | 1694 (43) |
| Missing | 136 (3) |
| Immunochemical subtype | |
| IgG | 2357 (60) |
| IgA | 751 (19) |
| Light chain | 764 (19) |
| Nonsecretory | 36 (1) |
| Others | 40 (1) |
| Bone marrow plasma cells at diagnosis | |
| <10% | 384 (10) |
| ≥10% | 3048 (77) |
| Missing | 516 (13) |
| Bone marrow plasma cells at transplant | |
| <10% | 2686 (68) |
| ≥10% | 488 (12) |
| Missing | 774 (20) |
| Hemoglobin at diagnosis, g/dL | |
| <10 g/dL | 1355 (34) |
| ≥ 10 g/dL | 2234 (57) |
| Missing | 359 (9) |
| Hemoglobin before transplant, g/dL | |
| <10 g/dL | 816 (21) |
| ≥ 10 g/dL | 3104 (79) |
| Missing | 28 (1) |
| LDH at diagnosis | |
| <upper limit | 1389 (35) |
| ≥upper limit | 383 (10) |
| Missing | 2176 (55) |
| Beta-2 microglobulin level at diagnosis, mg/L | |
| 0- 3.5 mg/L | 1737 (44) |
| 3.5-5.5 mg/L | 632 (16) |
| ≥5.5mg/L | 692 (18) |
| Missing | 887 (22) |
| Serum creatinine prior to transplant, mg/dL | |
| <2 mg/dL | 3713 (94) |
| ≥ 2 mg/dL | 198 (5) |
| Missing | 37 (1) |
| Cytogenetics | |
| Standard risk | 2501 (63) |
| High risk | 1112 (28) |
| t(4;14) | 122 (3) |
| t(14;16) | 32 (1) |
| t(14;20) | 4 (0) |
| del17p | 144 (4) |
| +1q | 557 (14) |
| ≥2 HR | 253 (6) |
| Missing | 335 (8) |
| Lines of chemotherapy | |
| 1 | 2856 (72) |
| 2 | 930 (24) |
| Missing | 162 (4) |
| Chemotherapy | |
| VTD | 37 (1) |
| VRD | 2383 (60) |
| VCD | 642 (16) |
| VD | 282 (7) |
| RD | 242 (6) |
| KRD | 75 (2) |
| Daratumumab (Dara) | 89 (2) |
| Other∗ | 36 (1) |
| Missing | 162 (4) |
| Alkylating agent exposure | |
| No | 3153 (80) |
| Yes | 639 (16) |
| Missing | 156 (4) |
| Radiation therapy on any line of treatment | |
| No | 3149 (80) |
| Yes | 703 (18) |
| Missing | 96 (2) |
| Melphalan dose in conditioning regimen, mg/m2 | |
| MEL 140 | 1134 (29) |
| MEL 200 | 2814 (71) |
| Disease status before transplant | |
| sCR/CR | 611 (15) |
| VGPR | 1542 (39) |
| PR | 1517 (38) |
| SD | 194 (5) |
| PD/Relapse | 61 (2) |
| Missing | 23 (1) |
| Type of transplant | |
| Single auto | 3948 (100) |
| Time from diagnosis to transplant | |
| 0-6 mo | 1364 (35) |
| 6-12 mo | 1862 (47) |
| 12-24 mo | 448 (11) |
| >24 mo | 274 (7) |
| Missing | 0 (0) |
| Initial platelet count ≥ 20 × 109/L achieved | |
| No | 6 (0) |
| Yes | 3827 (97) |
| Never dropped below | 84 (2) |
| Missing | 31 (1) |
| Post-HSCT therapy received | |
| VR +/− other | 372 (9) |
| VC +/− other | 19 (0) |
| V +/− other | 370 (9) |
| R +/− other | 2836 (72) |
| KR +/− other | 59 (1) |
| K +/− other | 86 (2) |
| Other† | 16 (0) |
| Dara+Pom +/− other | 29 (1) |
| Dara +/− other | 21 (1) |
| Pom +/− other | 88 (2) |
| Thalidomide +/− other | 8 (0) |
| Ixazomib +/− other | 44 (1) |
| Y of transplant | |
| 2011 | 219 (6) |
| 2012 | 221 (6) |
| 2013 | 427 (11) |
| 2014 | 371 (9) |
| 2015 | 506 (13) |
| 2016 | 589 (15) |
| 2017 | 549 (14) |
| 2018 | 1066 (27) |
| SPM‡ | |
| No | 3773 (96) |
| Yes | 175 (4) |
| Follow-up--median (range) | 37 (3-108) |
| Characteristic . | Number (%) . |
|---|---|
| No. of patients | 3948 |
| Median age at first auto-HSCT (min-max) | 61 (20-82) |
| Age at transplant, y | |
| 18-39 | 110 (3) |
| 40-49 | 471 (12) |
| 50-59 | 1256 (32) |
| 60-69 | 1727 (44) |
| 70+ | 384 (10) |
| Gender | |
| Male | 2156 (55) |
| Female | 1792 (45) |
| Region | |
| US | 3948 (100) |
| Recipient race | |
| White | 2298 (58) |
| Black or African-American | 1357 (34) |
| Other | 206 (5) |
| Missing | 87 (2) |
| Karnofsky score | |
| ≥ 90 | 2076 (53) |
| < 90 | 1798 (46) |
| Missing | 74 (2) |
| HCT-CI | |
| 0 | 1029 (26) |
| 1 | 559 (14) |
| 2 | 680 (17) |
| 3 | 744 (19) |
| 4 | 436 (11) |
| 5 | 216 (5) |
| 6+ | 264 (7) |
| Missing | 20 (1) |
| History of solid tumor (excluding nonmelanoma skin cancers) | |
| No | 3591 (91) |
| Yes | 334 (8) |
| Missing | 23 (1) |
| History of malignancy (any prior malignancy including solid tumors) | |
| No | 3544 (90) |
| Yes | 404 (10) |
| ISS stage at diagnosis | |
| ISS stage I | 1232 (31) |
| ISS stage II | 1105 (28) |
| ISS stage III | 712 (18) |
| Missing | 899 (23) |
| Stage at diagnosis (ISS/DS) | |
| Stage III | 2118 (54) |
| Stage I-II | 1694 (43) |
| Missing | 136 (3) |
| Immunochemical subtype | |
| IgG | 2357 (60) |
| IgA | 751 (19) |
| Light chain | 764 (19) |
| Nonsecretory | 36 (1) |
| Others | 40 (1) |
| Bone marrow plasma cells at diagnosis | |
| <10% | 384 (10) |
| ≥10% | 3048 (77) |
| Missing | 516 (13) |
| Bone marrow plasma cells at transplant | |
| <10% | 2686 (68) |
| ≥10% | 488 (12) |
| Missing | 774 (20) |
| Hemoglobin at diagnosis, g/dL | |
| <10 g/dL | 1355 (34) |
| ≥ 10 g/dL | 2234 (57) |
| Missing | 359 (9) |
| Hemoglobin before transplant, g/dL | |
| <10 g/dL | 816 (21) |
| ≥ 10 g/dL | 3104 (79) |
| Missing | 28 (1) |
| LDH at diagnosis | |
| <upper limit | 1389 (35) |
| ≥upper limit | 383 (10) |
| Missing | 2176 (55) |
| Beta-2 microglobulin level at diagnosis, mg/L | |
| 0- 3.5 mg/L | 1737 (44) |
| 3.5-5.5 mg/L | 632 (16) |
| ≥5.5mg/L | 692 (18) |
| Missing | 887 (22) |
| Serum creatinine prior to transplant, mg/dL | |
| <2 mg/dL | 3713 (94) |
| ≥ 2 mg/dL | 198 (5) |
| Missing | 37 (1) |
| Cytogenetics | |
| Standard risk | 2501 (63) |
| High risk | 1112 (28) |
| t(4;14) | 122 (3) |
| t(14;16) | 32 (1) |
| t(14;20) | 4 (0) |
| del17p | 144 (4) |
| +1q | 557 (14) |
| ≥2 HR | 253 (6) |
| Missing | 335 (8) |
| Lines of chemotherapy | |
| 1 | 2856 (72) |
| 2 | 930 (24) |
| Missing | 162 (4) |
| Chemotherapy | |
| VTD | 37 (1) |
| VRD | 2383 (60) |
| VCD | 642 (16) |
| VD | 282 (7) |
| RD | 242 (6) |
| KRD | 75 (2) |
| Daratumumab (Dara) | 89 (2) |
| Other∗ | 36 (1) |
| Missing | 162 (4) |
| Alkylating agent exposure | |
| No | 3153 (80) |
| Yes | 639 (16) |
| Missing | 156 (4) |
| Radiation therapy on any line of treatment | |
| No | 3149 (80) |
| Yes | 703 (18) |
| Missing | 96 (2) |
| Melphalan dose in conditioning regimen, mg/m2 | |
| MEL 140 | 1134 (29) |
| MEL 200 | 2814 (71) |
| Disease status before transplant | |
| sCR/CR | 611 (15) |
| VGPR | 1542 (39) |
| PR | 1517 (38) |
| SD | 194 (5) |
| PD/Relapse | 61 (2) |
| Missing | 23 (1) |
| Type of transplant | |
| Single auto | 3948 (100) |
| Time from diagnosis to transplant | |
| 0-6 mo | 1364 (35) |
| 6-12 mo | 1862 (47) |
| 12-24 mo | 448 (11) |
| >24 mo | 274 (7) |
| Missing | 0 (0) |
| Initial platelet count ≥ 20 × 109/L achieved | |
| No | 6 (0) |
| Yes | 3827 (97) |
| Never dropped below | 84 (2) |
| Missing | 31 (1) |
| Post-HSCT therapy received | |
| VR +/− other | 372 (9) |
| VC +/− other | 19 (0) |
| V +/− other | 370 (9) |
| R +/− other | 2836 (72) |
| KR +/− other | 59 (1) |
| K +/− other | 86 (2) |
| Other† | 16 (0) |
| Dara+Pom +/− other | 29 (1) |
| Dara +/− other | 21 (1) |
| Pom +/− other | 88 (2) |
| Thalidomide +/− other | 8 (0) |
| Ixazomib +/− other | 44 (1) |
| Y of transplant | |
| 2011 | 219 (6) |
| 2012 | 221 (6) |
| 2013 | 427 (11) |
| 2014 | 371 (9) |
| 2015 | 506 (13) |
| 2016 | 589 (15) |
| 2017 | 549 (14) |
| 2018 | 1066 (27) |
| SPM‡ | |
| No | 3773 (96) |
| Yes | 175 (4) |
| Follow-up--median (range) | 37 (3-108) |
V, Velcade; T, Thalidomide; R, Revlimid; D, dexamethasone; K, Kyprolis; C, cyclophosphamide; Pom, Pomalidomide; sCR, stringent complete response; VGPR, very good partial R; SD, stable disease; PD, progressive disease.
Other chemo: TD (n = 4), VDD/DVD (n = 21), VAD/similar (n = 3), K+/− other (n = 15), Pomalidomide (n = 1)
Other post-HSCT: Cellular therapy (n = 3), BMT CTN1401 (n = 2), Oprozomib (n = 1), CPD (n = 1), Nivolumab/Ipilimumab (n = 1), Panobinostat (n = 3), Vectibix (n = 1), Venetoclax (n = 2), Atezolizumab (n = 1), Dasatinib (n = 1)
Nonmelanoma skin cancers not included as new malignancies
Characteristics of new malignancies
SPMs were reported in 4% (n = 175) of observed patients, shown in Table 2. Solid tumors accounted for 64% (n = 112) of the new SPMs (supplemental Table 1), with a median time of development of solid tumor of 33 months (range, 2-96). Melanoma (n = 22, 19%) and genitourinary malignancies (n = 21, 18%) were the most commonly observed SPMs after auto-HSCT. Of the 63 patients with SHM, 36 (57%) were myeloid, 24 (38%) were classified as SHM, not otherwise specified, and 3 (5%) were lymphoid. The median interval from auto-HSCT to SPM was 33 months (range, 2-96) and SHM was 35 months (range, 3-93), respectively.
Characteristics of new malignancies
| Characteristic . | SPM . |
|---|---|
| No. of patients | 175 |
| No. of centers | 66 |
| Number of new malignancies--no. (%) | |
| 1 | 165 (94) |
| 2 | 10 (6) |
| Classification of new malignancies--no. (%) | |
| Myeloid∗ | 36 (21) |
| Lymphoid† | 3 (2) |
| SHM, not otherwise specified‡ | 24 (14) |
| Solid tumor | 112 (64) |
| Time from HCT to new malignancy--median (min-max) | 33 (2-96) |
| New malignancy: AML/MDS--no. (%) | |
| No | 139 (79) |
| Yes | 36 (21) |
| Time from HSCT to SHM--median (min-max) | 35 (3-93) |
| Time from HSCT to solid tumor--median (min-max) | 33 (2-96) |
| Characteristic . | SPM . |
|---|---|
| No. of patients | 175 |
| No. of centers | 66 |
| Number of new malignancies--no. (%) | |
| 1 | 165 (94) |
| 2 | 10 (6) |
| Classification of new malignancies--no. (%) | |
| Myeloid∗ | 36 (21) |
| Lymphoid† | 3 (2) |
| SHM, not otherwise specified‡ | 24 (14) |
| Solid tumor | 112 (64) |
| Time from HCT to new malignancy--median (min-max) | 33 (2-96) |
| New malignancy: AML/MDS--no. (%) | |
| No | 139 (79) |
| Yes | 36 (21) |
| Time from HSCT to SHM--median (min-max) | 35 (3-93) |
| Time from HSCT to solid tumor--median (min-max) | 33 (2-96) |
MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm.
Myeloid diagnoses: AML, n = 9; MDS, n = 14: AML+MDS, n = 2; AML+MDS/MPN, n = 2; MDS/MPN, n = 9.
Lymphoid diagnoses: ALL, n = 1; Hodgkin’s lymphoma, n = 1; lymphoproliferative disorder, n = 1.
SHM, not otherwise specified diagnoses: other leukemia, n = 21; other leukemia+breast cancer, n = 1; other leukemia+melanoma, n = 1; other leukemia+thyroid cancer, n = 1.
For the entire cohort, the cumulative incidences of SPM, SHM, and t-MN at 3-years were 3.3% (95% CI, 2.6%-3.9%), 1.1% (95% CI, 0.7%-1.5%), and 0.7% (95% CI, 0.4%-1%), respectively.
Reviewing the 404 patients with a prior malignancy history, 31 developed another malignancy after auto-HSCT for MM. Prior malignancy diagnoses included the following: 13 nonspecified, 7 nonmelanomatous skin cancers, 5 genitourinary cancers, 1 breast cancer, 2 leukemias, 1 bone cancer, 1 plasmacytoma, and 1 amyloidosis. Of the patients with a specified prior malignancy, the malignancy reported after transplant was different than before.
Of the 3773 patients who did not develop SPM, 616 (16%) were deceased at the last follow-up, with MM as the most common cause of death (n = 523, 85%). In contrast, of the 175 patients who developed SPM, 66 (38%) were deceased at the last follow-up, with primary disease of MM as the most common cause of death (n = 28, 42%), followed by SPM (n = 20, 30%) (supplemental Table 2). Similarly, of the 63 patients with SHM, 54% (n = 34) were deceased, with MM as the most common cause of death (n = 18, 53%), followed by SHM (n = 6, 18%).
The incidence of SPM was not significantly different when comparing patients by maintenance regimen received (supplemental Tables 3-6). There was a higher incidence of SHM in patients receiving lenalidomide-based or lenalidomide only maintenance compared with that of nonlenalidomide regimens (supplemental Table 5-6). The time to development of SPM after auto-HSCT was shorter in patients receiving nonlenalidomide-based maintenance regimens compared with those receiving lenalidomide-based maintenance regimens (supplemental Table 5-6). MM remained as the primary cause of death in all groups (supplemental Tables 3-6).
Characteristics for patients with and without SPM are shown in supplemental Table 7.
Predictors of survival after Auto-HSCT
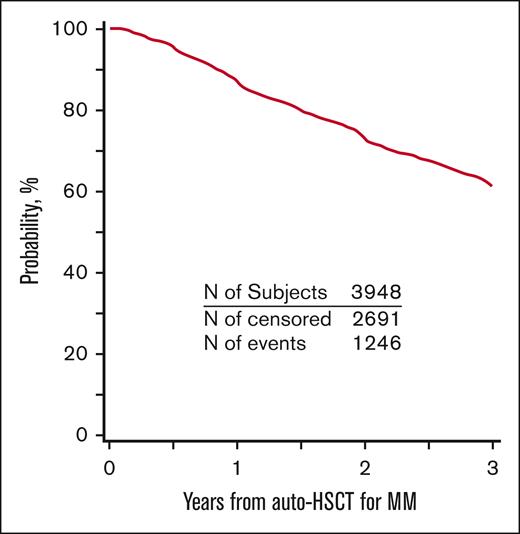
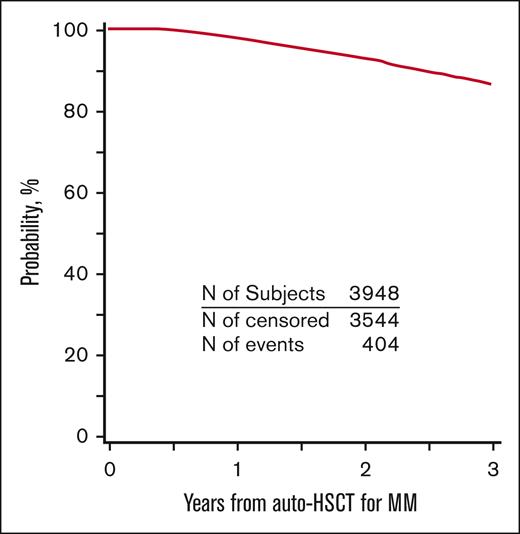
PFS and OS for the entire cohort are demonstrated in Figures 2 and 3. Patients who developed SPM had an inferior PFS (hazard ratio [HR] = 2.62; 95% CI, 2.03-3.38; P < .001) compared with patients who did not develop SPM (Table 3). Further, in the subcohort of patients whose SPM was classified as an SHM, an even lower PFS was demonstrated (HR = 5.01; 95% CI 3.41-7.37; P < .001) (supplemental Table 8). Presence of SPM was associated with an inferior OS (HR = 3.85, 95% CI 2.95-5.02; P < .001) (Table 4). Among those with SPM, the presence of SHM was associated with a >8-fold lower OS (HR = 8.13; 95%CI, 5.67-11.65; P < .001) (supplemental Table 9).
PFS for patients with MM who underwent first auto-HSCT in the United States with a melphalan conditioning regimen from 2011 to 2018 and received post-HSCT maintenance.
PFS for patients with MM who underwent first auto-HSCT in the United States with a melphalan conditioning regimen from 2011 to 2018 and received post-HSCT maintenance.
OS for patients with MM who underwent first auto-HSCT in the United States with a melphalan conditioning regimen from 2011 to 2018 and received post-HSCT maintenance.
OS for patients with MM who underwent first auto-HSCT in the United States with a melphalan conditioning regimen from 2011 to 2018 and received post-HSCT maintenance.
Multivariate analysis--impact of SPM on progression-free survival
| Covariate . | N . | Hazard ratio . | 95% Hazard ratio confidence limits . | P-value . | |
|---|---|---|---|---|---|
| Time-dependent: SPM | |||||
| No | - | 1.00 | Reference | <.0001 | |
| Yes | - | 2.62 | 2.03 | 3.38 | <.0001 |
| Baseline covariates | |||||
| Gender | |||||
| Female | 1791 | 1.00 | Reference | .0742 | |
| Male | 2155 | 1.10 | 0.99 | 1.21 | .0742 |
| Karnofsky score | |||||
| ≥90 | 2076 | 1.00 | Reference | .1922 | |
| <90 | 1796 | 1.09 | 0.99 | 1.21 | .0939 |
| Missing | 74 | 1.20 | 0.81 | 1.77 | .3677 |
| ISS Stage at diagnosis | |||||
| Stage I | 1231 | 1.00 | Reference | <.0001 | |
| Stage II | 1104 | 1.27 | 1.11 | 1.46 | .0006 |
| Stage III | 712 | 1.68 | 1.45 | 1.95 | <.0001 |
| Missing | 899 | 1.28 | 1.11 | 1.48 | .0007 |
| Cytogenetics | |||||
| Standard/Normal Risk | 2496 | 1.00 | Reference | <.0001 | |
| High risk | 1114 | 1.52 | 1.35 | 1.69 | <.0001 |
| Missing | 336 | 1.34 | 1.12 | 1.61 | .0017 |
| Lines of chemotherapy | |||||
| 1 | 2856 | 1.00 | Reference | <.0001 | |
| 2+ | 929 | 1.28 | 1.14 | 1.45 | <.0001 |
| Missing | 161 | 0.74 | 0.54 | 1.01 | .0574 |
| Disease status before transplant | |||||
| sCR/CR | 611 | 1.00 | Reference | <.0001 | |
| VGPR | 1538 | 1.22 | 1.03 | 1.44 | .0206 |
| PR | 1520 | 1.53 | 1.30 | 1.81 | <.0001 |
| SD/PD/Relapse | 255 | 1.82 | 1.45 | 2.27 | <.0001 |
| Missing | 22 | 0.95 | 0.39 | 2.32 | .9151 |
| Time from diagnosis to HCT | |||||
| 0-6 mo | 1367 | 1.00 | Reference | <.0001 | |
| 6-12 mo | 1861 | 0.93 | 0.83 | 1.04 | .2134 |
| 12-24 mo | 445 | 1.07 | 0.90 | 1.27 | .4431 |
| 24+ mo | 273 | 1.52 | 1.25 | 1.85 | <.0001 |
| Covariate . | N . | Hazard ratio . | 95% Hazard ratio confidence limits . | P-value . | |
|---|---|---|---|---|---|
| Time-dependent: SPM | |||||
| No | - | 1.00 | Reference | <.0001 | |
| Yes | - | 2.62 | 2.03 | 3.38 | <.0001 |
| Baseline covariates | |||||
| Gender | |||||
| Female | 1791 | 1.00 | Reference | .0742 | |
| Male | 2155 | 1.10 | 0.99 | 1.21 | .0742 |
| Karnofsky score | |||||
| ≥90 | 2076 | 1.00 | Reference | .1922 | |
| <90 | 1796 | 1.09 | 0.99 | 1.21 | .0939 |
| Missing | 74 | 1.20 | 0.81 | 1.77 | .3677 |
| ISS Stage at diagnosis | |||||
| Stage I | 1231 | 1.00 | Reference | <.0001 | |
| Stage II | 1104 | 1.27 | 1.11 | 1.46 | .0006 |
| Stage III | 712 | 1.68 | 1.45 | 1.95 | <.0001 |
| Missing | 899 | 1.28 | 1.11 | 1.48 | .0007 |
| Cytogenetics | |||||
| Standard/Normal Risk | 2496 | 1.00 | Reference | <.0001 | |
| High risk | 1114 | 1.52 | 1.35 | 1.69 | <.0001 |
| Missing | 336 | 1.34 | 1.12 | 1.61 | .0017 |
| Lines of chemotherapy | |||||
| 1 | 2856 | 1.00 | Reference | <.0001 | |
| 2+ | 929 | 1.28 | 1.14 | 1.45 | <.0001 |
| Missing | 161 | 0.74 | 0.54 | 1.01 | .0574 |
| Disease status before transplant | |||||
| sCR/CR | 611 | 1.00 | Reference | <.0001 | |
| VGPR | 1538 | 1.22 | 1.03 | 1.44 | .0206 |
| PR | 1520 | 1.53 | 1.30 | 1.81 | <.0001 |
| SD/PD/Relapse | 255 | 1.82 | 1.45 | 2.27 | <.0001 |
| Missing | 22 | 0.95 | 0.39 | 2.32 | .9151 |
| Time from diagnosis to HCT | |||||
| 0-6 mo | 1367 | 1.00 | Reference | <.0001 | |
| 6-12 mo | 1861 | 0.93 | 0.83 | 1.04 | .2134 |
| 12-24 mo | 445 | 1.07 | 0.90 | 1.27 | .4431 |
| 24+ mo | 273 | 1.52 | 1.25 | 1.85 | <.0001 |
Multivariate analysis--impact of SPM on OS
| Covariate . | N . | Hazard ratio . | 95% Hazard ratio confidence limits . | P-value . | |
|---|---|---|---|---|---|
| Time-dependent: SPM | |||||
| No | - | 1 | Reference | <.0001 | |
| Yes | - | 3.85 | 2.95 | 5.02 | <.0001 |
| Baseline covariates | |||||
| Gender | |||||
| Female | 1791 | 1.00 | Reference | .2683 | |
| Male | 2155 | 1.09 | 0.94 | 1.27 | .2683 |
| Race | |||||
| Black | 1356 | 1.00 | Reference | .0129 | |
| White | 2300 | 1.27 | 1.07 | 1.51 | .0068 |
| Other | 204 | 0.82 | 0.53 | 1.25 | .3492 |
| Missing | 86 | 0.98 | 0.54 | 1.75 | .9351 |
| Karnofsky score | |||||
| ≥90 | 2076 | 1.00 | Reference | .009 | |
| <90 | 1796 | 1.27 | 1.09 | 1.49 | .0021 |
| Missing | 74 | 1.16 | 0.63 | 2.12 | .6297 |
| HCT-CI | |||||
| 0 | 1030 | 1.00 | Reference | .1121 | |
| 1-2 | 1237 | 1.03 | 0.84 | 1.27 | .7629 |
| 3+ | 1659 | 1.21 | 1.00 | 1.47 | .0547 |
| Missing | 20 | 0.38 | 0.05 | 2.69 | .329 |
| ISS stage at diagnosis | |||||
| Stage I | 1231 | 1.00 | Reference | <.0001 | |
| Stage II | 1104 | 1.31 | 1.05 | 1.62 | .0152 |
| Stage III | 712 | 1.91 | 1.54 | 2.38 | <.0001 |
| Missing | 899 | 1.39 | 1.11 | 1.74 | .0046 |
| Cytogenetics | |||||
| Standard/Normal Risk | 2496 | 1.00 | Reference | <.0001 | |
| High risk | 1114 | 2.11 | 1.79 | 2.48 | <.0001 |
| Missing | 336 | 1.48 | 1.13 | 1.95 | .0048 |
| Lines of chemotherapy | |||||
| 1 | 2856 | 1.00 | Reference | .0005 | |
| 2+ | 929 | 1.40 | 1.18 | 1.65 | <.0001 |
| Missing | 161 | 1.04 | 0.66 | 1.64 | .8805 |
| Disease status before transplant | |||||
| sCR/CR | 611 | 1.00 | Reference | .0081 | |
| VGPR | 1538 | 1.14 | 0.89 | 1.46 | .3154 |
| PR | 1520 | 1.22 | 0.95 | 1.56 | .1126 |
| SD/PD/Relapse | 255 | 1.74 | 1.25 | 2.41 | .0009 |
| Missing | 22 | 2.24 | 0.82 | 6.13 | .1175 |
| Covariate . | N . | Hazard ratio . | 95% Hazard ratio confidence limits . | P-value . | |
|---|---|---|---|---|---|
| Time-dependent: SPM | |||||
| No | - | 1 | Reference | <.0001 | |
| Yes | - | 3.85 | 2.95 | 5.02 | <.0001 |
| Baseline covariates | |||||
| Gender | |||||
| Female | 1791 | 1.00 | Reference | .2683 | |
| Male | 2155 | 1.09 | 0.94 | 1.27 | .2683 |
| Race | |||||
| Black | 1356 | 1.00 | Reference | .0129 | |
| White | 2300 | 1.27 | 1.07 | 1.51 | .0068 |
| Other | 204 | 0.82 | 0.53 | 1.25 | .3492 |
| Missing | 86 | 0.98 | 0.54 | 1.75 | .9351 |
| Karnofsky score | |||||
| ≥90 | 2076 | 1.00 | Reference | .009 | |
| <90 | 1796 | 1.27 | 1.09 | 1.49 | .0021 |
| Missing | 74 | 1.16 | 0.63 | 2.12 | .6297 |
| HCT-CI | |||||
| 0 | 1030 | 1.00 | Reference | .1121 | |
| 1-2 | 1237 | 1.03 | 0.84 | 1.27 | .7629 |
| 3+ | 1659 | 1.21 | 1.00 | 1.47 | .0547 |
| Missing | 20 | 0.38 | 0.05 | 2.69 | .329 |
| ISS stage at diagnosis | |||||
| Stage I | 1231 | 1.00 | Reference | <.0001 | |
| Stage II | 1104 | 1.31 | 1.05 | 1.62 | .0152 |
| Stage III | 712 | 1.91 | 1.54 | 2.38 | <.0001 |
| Missing | 899 | 1.39 | 1.11 | 1.74 | .0046 |
| Cytogenetics | |||||
| Standard/Normal Risk | 2496 | 1.00 | Reference | <.0001 | |
| High risk | 1114 | 2.11 | 1.79 | 2.48 | <.0001 |
| Missing | 336 | 1.48 | 1.13 | 1.95 | .0048 |
| Lines of chemotherapy | |||||
| 1 | 2856 | 1.00 | Reference | .0005 | |
| 2+ | 929 | 1.40 | 1.18 | 1.65 | <.0001 |
| Missing | 161 | 1.04 | 0.66 | 1.64 | .8805 |
| Disease status before transplant | |||||
| sCR/CR | 611 | 1.00 | Reference | .0081 | |
| VGPR | 1538 | 1.14 | 0.89 | 1.46 | .3154 |
| PR | 1520 | 1.22 | 0.95 | 1.56 | .1126 |
| SD/PD/Relapse | 255 | 1.74 | 1.25 | 2.41 | .0009 |
| Missing | 22 | 2.24 | 0.82 | 6.13 | .1175 |
For SPM and SHM patients, other factors associated with an inferior PFS included male sex, low KPS, higher ISS stage at diagnosis, high-risk MM cytogenetics, ≥2 lines of therapy pre-HSCT, not achieving complete remission or stringent complete remission (CR/sCR) at the time of auto-HSCT, and undergoing auto-HSCT ≥2 years after the initial diagnosis (Table 3 and supplemental Table 8). The same factors were also associated with an inferior OS (Table 4 and supplemental Table 9). In addition, HCT-CI ≥3 was associated with an inferior PFS after SPM and SHM. MVA also revealed that White patients with SHM had an inferior OS compared with other racial groups.
Patients receiving lenalidomide single agent maintenance had lower rates of relapse or progression and improved PFS and OS compared with all other maintenance regimens (supplemental Table 10). When comparing single agent lenalidomide maintenance vs lenalidomide combinations, patients receiving single agent had lower rates of relapse or progression and improved PFS and OS (supplemental Table 11). Patients receiving lenalidomide-based or lenalidomide only maintenance had lower rates of relapse and progression and higher PFS and OS compared with those receiving nonlenalidomide-based maintenance (supplemental Tables 12 and 13). Additionally, nonrelapse mortality was higher in the nonlenalidomide-based maintenance cohort when compared with lenalidomide only or lenalidomide containing regimens.
Characteristics of patients receiving Allo-HSCT
Of the 63 patients with MM who developed SHM after auto-HSCT, only 9 (14%) underwent an allo-HSCT (4 for t-AML and 5 for therapy-related myelodysplastic syndromes [t-MDS]) (supplemental Tables 14 and 15). The patients undergoing allo-HSCT were more likely to have KPS ≥90 (100% vs 50%, P = .02) compared with those who did not. In patients receiving allo-HSCT for SHM, 1-year OS from the time of allo-HSCT was 66.7% (95% CI, 34.6%-91.9%).
Discussion
To our knowledge, this is the largest and the most recent analysis to demonstrate the unfavorable impact of post-HSCT SPM on PFS and OS in the modern era when maintenance therapies for MM are widely accepted and used. This retrospective analysis of the prospectively collected CIBMTR data revealed that patients with MM who developed SPM after auto-HSCT had >3 times worse OS and >2.5 times worse PFS than those who did not develop SPM. For those with SPM that developed a hematologic malignancy, there was an even worse PFS and OS (5- and 8-fold respectively). Prior reports demonstrated there was no increase in the risk of death from SPM.26 Additionally, a more recent California Cancer Registry analysis for patients with MM diagnosed between 1991 and 2014 reported a low attributable 10-year mortality for patients with SPM compared with myeloma-related mortality.27 Our analysis contrasts these findings and confirms that although MM remains the main cause of death for patients with SPM, survival is inferior for patients with SPM compared with those with MM who do not develop SPM. With the continued improvement in median OS for MM in recent years owing to the broad application of maintenance therapy and new drug approvals, the negative impact of SPM on patient outcomes appears to be greater in the current era of MM treatment. Thus, this analysis highlights a particularly vulnerable population with poor outcomes. The cohort size was complemented by a robust completeness of follow-up and the availability of a large array of patient- and disease-related factors, lending credence to our observations.
The International Myeloma Working Group (IMWG) consensus for SPM in MM does not recommend routine cancer screening beyond what is suggested for the general population.28 However, the IMWG does recommend enhanced monitoring and precise measurement of second cancers on clinical trials, with the suggestion to include SPM as a defined end point.28 No consensus recommendations exist to guide the management of MM in the setting of SPM or vice versa. Patients are often taken off clinical trial or excluded from future trials with an SPM diagnosis. This, along with potential discontinuation of maintenance or MM disease-directed therapy, may influence the inferior PFS and OS in this study cohort. With the survival benefit observed for lenalidomide-based maintenance compared with nonlenalidomide maintenance, yet comparable SPM incidence between groups, discontinuation of maintenance may account for the inferior survival observed for patients with SPM in this cohort. Unfortunately, discontinuation details were not available.
Unlike our analysis, previous analyses of similar populations of patients with MM did include nonmelanomatous skin cancers in SPM cohorts.29 Because the primary risk factors for superficial skin cancers are sun-exposure and older age, these were not included as SPMs in this analysis. Melanoma was included, as prior CIBMTR analyses examining the incidence of SPM from 1990 to 2010 revealed there was an increased risk of hematologic malignancies and melanoma in patients with MM after upfront auto-HSCT compared with the general population.12 Although the intent of this analysis was not to assess risk compared with the population, melanoma was confirmed as the most commonly diagnosed solid tumor SPM, followed by genitourinary malignancies.
Thirty-four percent of patients in this cohort were Black or AA, which is much higher than the recently reported VA Corporate Data Warehouse study cohort from 1999 to 2018, in which it was identified that AA patients did not have a higher incidence of SPM overall.30 Future planned analyses examining cumulative incidence of SPM for this cohort after additional follow-up will explore whether this population of patients experience a higher incidence of SPM. In this analysis, when evaluating OS and PFS after SPM or SHM by race, it was demonstrated that reported race of Black or AA did not yield inferior OS.
Even in those who developed SPM/SHM, the most common primary cause of death remained MM, which was even more apparent among the SHM subgroup. Just as other reports have demonstrated, MM remains the greatest driver of mortality.27,28 This is of interest, as SHM, especially t-MN, are aggressive malignancies with poor survival. Therefore, this analysis brings to the forefront the challenge of simultaneously managing MM and SPM/SHM and that the prioritization of treatment for SPM and MM should be done on a case-by-case basis.
The above discussion strengthens the argument that strategies targeted toward treatment of both SPM and MM are needed, with 1 such modality being allo-HSCT.31 However, only 9 of 63 patients with SHM underwent allo-HSCT in this cohort. Because this was a retrospective registry analysis, the reasoning for proceeding to or foregoing allo-HSCT in these patients is unclear. However, these findings are congruent with a recent single-institution study that showed that <10% of patients underwent allo-HSCT for t-MN, highlighting the possible underuse of a potentially curative modality.22 Because few patients went on to allo-HSCT in this cohort, conclusions regarding survival after allo-HSCT cannot be made. A recent CIBMTR analysis of patients with t-MNs receiving allo-HSCT between 2000 and 2014 revealed inferior survival in the 17% of patients who had received prior auto-HSCT. Notably, a majority of patients who underwent prior auto-HSCT received reduced intensity allo-HSCT conditioning, which was also associated with inferior survival.32 Further investigation to determine the risk or benefit of allo-HSCT in this population is needed because allo-HSCT provides the only potential curative modality for patients with SHM.
Although much of the attention has been focused on the development of t-MN, therapy-related acute lymphoblastic leukemia (t-ALL) has also been described. Previous studies have shown that t-ALL after MM had a significantly lower white blood count at diagnosis, less likelihood of BCR/ABL1, a higher frequency of adverse cytogenetics, but better survival compared with non-MM t-ALL.33,34
When comparing patients based on maintenance regimen, there were no observed significant differences in SPM incidence. However, differences in relapse or progression, PFS and OS were revealed. It does not appear that SPM was a primary contributor to the differences in relapse or survival when comparing patients based on the maintenance regimen. There was an increased incidence of SHM in patients receiving lenalidomide only or lenalidomide-based maintenance compared with those receiving nonlenalidomide regimens. This analysis suggests that lenalidomide maintenance improved survival outcomes without demonstrating a higher likelihood of SPM compared with those receiving nonlenalidomide regimens. Longer follow-up is required to further evaluate lenalidomide as a risk factor for the development of SPM because the median time to SPM per prior studies and in this cohort was nearly equivalent to the median follow-up in this analysis.35 Further details regarding duration of lenalidomide-based maintenance would be of value for risk-attribution. With the data available from the CIBMTR, this analysis aligns with prior evidence supporting the use of lenalidomide-based maintenance strategies, as the survival benefit is evident despite the development of SPM.
Several limitations of this retrospective registry analysis should be considered. First, accurate reporting of second malignancies was incumbent upon the transplant centers, which may influence the reported incidence of SPM/SHM. Ten percent of patients had a malignancy before auto-HSCT, and recurrence of this would not be appropriately classified as SPM. Notably, patients with prior malignancy that developed another malignancy after auto-HSCT represented <1% of the entire study cohort, with a majority having a nonspecified prior malignancy or nonmelanomatous skin cancer. Thus, these patients were not excluded from this analysis. Second, data elements that may impact the incidence of or outcomes after SPM, such as the duration of maintenance therapy, post-HSCT treatments, or therapies for SPM were not available. Prior reports have demonstrated that with longer duration of follow-up after diagnosis of MM, the risk of developing SPM rises.26 The following was the twofold rationale to study the 2011-2018 cohort: (1) this era represents a wider adaptation of the maintenance approach, and (2) to minimize overlap with a prior CIBMTR study that included patients from 1995 to 2010.11 Even with a shorter follow-up period, the benefit of maintenance therapy on OS and PFS for patients, including in those with SPM, was evident. Third, the incidence of SPM/SHM was lower than that in clinical trials of lenalidomide maintenance.14,36 Of note, although the duration of maintenance therapy in these trials was variable, the mean duration was generally between 2.5 and 3.5 years, and the risk of MM disease progression was greater than the risk of developing SPM.13,15,36 Within our cohort, 27% of patients underwent auto-HSCT in 2018, representing the largest number of patients who underwent transplant in any 1 year. Given that the median time to develop SPM and SHM was 33 and 35 months respectively, longer follow-up will be required to provide an assessment of the true incidence of SPM or SHM in these patients.
In summary, compared with those without a second malignancy, the development of SPM and SHM after auto-HSCT in MM resulted in inferior PFS and OS. Furthermore, relapsed MM remained the primary cause of death, even in those with SPM/SHM. Thus, our analysis supports the use of the current paradigm of induction, auto-HSCT, and maintenance therapy, despite their potential influence on SPM. Although, owing to the inferior outcomes revealed in this analysis, it is crucial to accurately identify, and possibly mitigate, factors which contribute to or increase the risk of SPM and SHM. The NIH Hematopoietic Cell Transplantation Late Effects Initiative provided consensus recommendations for subsequent neoplasms after HSCT, which included conducting large-scale and long-term systematic follow-up post-HSCT to better understand the risks that SPM pose to patients.37 This analysis aligns with that recommendation and further reinforces the need for tailored preventive screening and therapeutic guidelines for managing SPM. Additionally, given the rapidly changing landscape of MM therapies, capturing a real-word assessment of the true incidence of SPM or SHM is necessary to determine the profundity of these iatrogenic risk factors. Further studies are ongoing to provide an update to the cumulative incidence of SPM or SHM and to identify risk factors for the development of SPM.
Acknowledgments
M.V.S. was supported by the Mayo Clinic MM SPORE Career Enhancement Award and the Leukemia Research Foundation New Investigator Award. The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases (NIAID); HHSH250201700006C from the Health Resources and Services Administration (HRSA); and N00014-20-1-2832 and N00014-21-1-2954 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc; Adaptimmune; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Allogene; Allovir, Inc; Amgen, Inc; Angiocrine; Anthem; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics; BeiGene; bluebird bio, inc; Bristol Myers Squibb (BMS); CareDx Inc; CRISPR; CSL Behring; CytoSen Therapeutics, Inc; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida-Cell, Ltd; Gilead; GlaxoSmithKline (GSK); HistoGenetics; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc; Kadmon, a Sanofi Company; Karius; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt Pharmaceuticals; medac GmbH; Medexus Pharma; Merck & Co; Mesoblast; Millennium, the Takeda Oncology Co; Miltenyi Biotec, Inc; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc; Ossium Health, Inc; Pfizer, Inc; Pharmacyclics, LLC, an AbbVie company; Pluristem; PPD Development, LP; Sanofi; Sobi, Inc; StemCyte; Takeda Pharmaceuticals; Talaris Therapeutics; Terumo Blood and Cell Technologies; TG Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc; Xenikos BV. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the US Government.
Authorship
Contribution: B.K.R., L.G., G.G., M.V.S., and S.U. conceived and designed the analysis; A.D., N.E., R.F., B.K.R., L.G., G.G., M.V.S., and S.U. performed and assisted analysis; B.K.R. wrote the original draft of the paper; B.K.R., A.D., N.E., L.G., G.G., M.D.L., S.H., M.K., N.M., R.B., A.S., G.C.H., H.M., M.B.A., M.B., L.L., S.P., H.M., Y.N., C.S., S.M.B., S.A.H., B.D., M.A., D.V., C.L., A.P., U.G., K.C.M., L.A.H., A.A., M.S., P.M., T.N., L.D.A., B.W., G.K., M.Q., N.S., S.K.K., S.U., and M.V.S. reviewed and edited the paper; A.D. and S.U. supervised.
Conflict-of-interest disclosure: B.K.R. reports advisory board participation for Astellas and Pfizer. M.V.S. reports grants or contracts in the form of institutional research funding from BMS, Astellas, and MRKR Therapeutics. A.D. reports compensation for a clinical trial as site PI for AbbVie, Caelum, TeneoBio, Takeda, Sanofi, Janssen, and Prothena; ad board for BMS; consulting for Prothena; Independent Review Committee participation for Janssen; Payments: A.D. is a member of an independent review committee for a phase 3 clinical trial. In this ongoing role, A.D. performs blinded review of disease response. A.D. is compensated for every hour of consulting work, and this has amounted to more than $5000 compensation in a year. G.K. reports advisory board participation and has received honoraria from BMS, Sanofi, Cellectar and Arcellx. R.B. reports consulting fees for Clearview Healthcare Partners, Guidepoint Global, Genentech/Roche, Janssen Oncology, Sanofi Pasteur, and SparkCures; and payment or honoraria from Clinical Care Options, Curio Science, and i3 Health. A.S. reports royalties or licenses from In8Bio Inc; and consulting fees from Kite Pharma, Magenta Therapeutics, Incyte Pharma, and CareDx. H.M. reports advisory board participation for Janssen, GSK, BMS, Takeda, Pfizer, Amgen, AbbVie, Sanofi, and FORUS; and research funding from Janssen. M.B. reports research support for unrelated project paid to institute from Novartis. H.S.M. reports advisory board participation for CRISPR Therapeutics and Senti Biosciences. C.S. reports consulting fees from Jazz Pharmaceuticals; payment or honoraria from Iowa Oncology Society; and participation on a data safety monitoring board or advisory board for GSK. S.A.H. reports consulting fees from Janssen. D.H.V. reports speakers bureau participation for Amgen, Takeda, BMS, GSK, Karyopharm, Janssen, and Sanofi; participation on a data safety monitoring board or advisory board for BMS; International Response Committee for Eloquent-1 trial (completed); and stock or stock options for AbbVie, Abbott, Biogen, BMS, Gilead, Ionis, Johnson & Johnson, and Lilly Sorrento. C.H.L. reports payment for chairing meeting for BMS and advisory board participation for Janssen, Antengene, and BMS. L.A.H. reports study funding to institution from Seattle Genetics, Sanofi, Millennium-Takeda, BMS, Merck, Janssen, and iTeos Therapeutics; payment from UpToDate; data safety monitoring board or advisory board participation for iTeas Therapeutics (no payment); and leadership or fiduciary role in other board, society, committee, or advocacy group participation (no payment) for NCCN Guidelines MM, Waldenström macroglobulinemia, and Primary Systemic Amyloidosis. M.S. reports speakers bureau participation for BMS and GSK. T.N. reports research support (unrelated to the current study) to the institution from Novartis (clinical trial) and Karyopharm (drug supply only). L.D.A. Jr reports research funding from GSK, Janssen, BMS, Celgene, Amgen, AbbVie, and Regeneron; consulting fees from GSK, Janssen, BMS, Celgene, Karyopharm, Beigene, Amgen, and AbbVie; and data safety monitoring board or advisory board participation for Prothena. M.Q. reports payment from Amgen Inc, Bioline Rx Ld., Janssen Scientific, Angiocrine Bioscience, and NexImmune Inc. N.S. reports as voluntary clinical faculty at the University of California, San Francisco, in the Hematology-Bone Marrow Transplant Clinic. S.K.K. reports research funding for clinical trials to the institution from AbbVie, Amgen, BMS, Carsgen, Janssen, AstraZeneca, Novartis, Roche-Genentech, Takeda, Tenebio, and Molecular Templates; consulting/advisory board participation (with no personal payments) from AbbVie, Amgen, BMS, Janssen, Roche-Genentech, Takeda, AstraZeneca, Bluebird Bio, Epizyme, Secura Biotherapeutics, Monterosa Therapeutics, and Trillium; and consulting/advisory board participation (with personal payment) from Oncopeptides, Beigene, Antengene, and GLH Pharma. S.Z.U. reports grants or contracts from Amgen, Array BioPharma, BMS, Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDx, and Takeda; and consulting fees from AbbVie, Amgen, BMS, Celgene, EdoPharma, Genentech, Gilead, GSK, Janssen, Oncopeptides, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, and TeneoBio; and payment or honoraria from Amgen, Beigene, BMS, Janssen, and Sanofi.
Correspondence: Brittany K. Ragon, 1021 Morehead Medical Drive, Charlotte, NC, 28204; e-mail: Brittany.Ragon@atriumhealth.org; Mithun Vinod Shah, 200 1st Street SW, Rochester, MN, 55905; e-mail: Shah.Mithun@mayo.edu; and Saad Z. Usmani, 530 East 74th Street, New York, NY, 10065; e-mail: usmanis@mskcc.org.
References
Author notes
∗B.K.R. and M.V.S. are the co-first authors and equal contributors.
The final analysis data set will be posted to the CIBMTR website at: https://www.cibmtr.org/ReferenceCenter/PubList/PubDsDownload/Pages/default.aspx. CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases deidentified data sets that comply with all relevant global regulations regarding privacy and confidentiality.
The full-text version of this article contains a data supplement.