Key Points
Most patients with refractory B-ALL could achieve remission with a single infusion of CAR T cells.
Survival probability is excellent for patients who relapsed >6 months posttransplant and poor for patients who relapsed before 6 months.
Abstract
Patients with precursor B-cell acute lymphoblastic leukemia (pB-ALL) who have relapsed after allogeneic hematopoietic stem cell transplantation (allo-HSCT), have relapsed more than once, or are resistant upfront have a dismal prognosis. CD19-targeted chimeric antigen receptor (CAR) T cells have evolved as potent immune therapies. Tisagenlecleucel (Tisa-cel) is a commercially available autologous CD19-directed CAR T-cell product. We performed a retrospective study inviting all CAR T-cell centers in Germany to participate. Eighty-one patients with pB-ALL were included. Twenty-eight days after CAR T-cell infusion, 71 patients (87.7%) were in complete response, and 8 (9.9%) were in nonremission. At 2 years, the probabilities of event-free survival (pEFS), relapse-free survival (pRFS), and overall survival (pOS) were 45.3%, 51.7%, and 53.2%, respectively. pEFS was not different in patients without (n = 16, 55.0%) vs with prior allo-HSCT (n = 65, 43.4%). In patients treated after allo-HSCT, the time to relapse after allo-HSCT was a strong predictor of outcome. Patients relapsing within 6 months of allo-HSCT had a disappointing pEFS of 18.4% (pOS = 16.0%); the pEFS for those relapsing later was 55.5% (pOS = 74.8%). Our study provides real-world experience in pediatric, adolescent, and young adult patients with ALL treated with Tisa-cel, where most patients were treated after having relapsed after allo-HSCT. A total of 45.3% were rescued with a single dose of Tisa-cel. Our novel finding that patients with ALL after allo-HSCT had by far a better pEFS if relapse occurred beyond 6 months might be helpful in clinical decision-making and motivates studies to uncover the reasons.
Introduction
During the past decades, substantial progress has been achieved in the treatment of children, adolescents, and young adults with acute lymphoblastic leukemia (ALL). With multiagent chemotherapy protocols, up to 90% of pediatric and 70% of young adult patients with ALL can survive their disease.1 However, patients not responding to induction chemotherapy or relapsing early after initial therapy still have a very poor outcome.2,3
Most of these patients can only be rescued by reinduction treatment followed by allogeneic hematopoietic stem cell transplantation (allo-HSCT).4 Using standardized transplant approaches with total body irradiation–based conditioning regimens, up to 80% of these patients survive long term.5 Relapse after allo-HSCT, however, has a very poor prognosis, with a probability of event-free survival (pEFS) of only 15%.6 Even if another remission and a second allo-HSCT can be achieved, overall survival could not be achieved in >30% of all patients.7
These high-risk patients, who are difficult to cure with conventional therapies as outlined above, are the current target group for a novel cellular immunotherapy, namely, CD19-directed chimeric antigen receptor (CAR) T cells. This therapy was tested, and its efficacy was confirmed in larger studies for patients with precursor B-cell ALL (pB-ALL) who had relapsed subsequent to allo-HSCT, had relapsed twice, or were resistant to upfront therapy.8,9
Tisagenlecleucel (Tisa-cel) is a commercially available CD19-directed autologous CAR T-cell product. The CAR construct contains the costimulatory element 4-1BB to promote in vivo persistence. Thus, this therapy is in principle competent to serve as a standalone therapy with a curative intention. Based on the results of the ELIANA trial,10 Tisa-cel was approved by the US Food and Drug Administration and European Medicines Agency for pediatric/young adult pB-ALL in 2017 and 2018, respectively.
In clinical trials, stringent inclusion criteria narrow the risk profiles of trial participants. Treatment in clinical trials provides close guidance to physicians and patients during the trial. In contrast, postapproval reality leads to the treatment of patients with higher and more variable risk profiles, distributed over many centers.
We performed a retrospective study, asking all CAR T-cell centers in Germany approved for treatment with Tisa-cel (Kymriah) to collect treatment data reflecting postapproval reality to verify outcome data from clinical trials and possibly identify patients who might benefit most from this novel, complex, and expensive treatment.
Patients and methods
Data collection and participating centers
All pediatric centers performing allo-HSCT and CAR T-cell therapy are organized via the Pediatric Registry for Stem Cell Transplantation and Cell Therapy, and all adult centers are listed in the German Registry for Stem Cell Transplantation. Both registries report treatment procedures to the European Society for Blood and Marrow Transplantation (EBMT) registry and are part of the Joint Accreditation Committee HSCT-Europe & EBMT accreditation. Currently, 26 centers are approved to perform CAR T-cell therapies using Tisa-cel in Germany. The centers were trained and qualified by the manufacturer, Novartis, for application and toxicity management. Furthermore, as per the federal directive, all centers were obliged to adhere to the defined quality assurance measures based on the requirements of the Federal Joint Committee.11 All centers were invited to participate in this retrospective study; 18 of them agreed and reported patients. The collection of data required written informed consent to report pseudonymized data to the aforementioned registries on a standard Pediatric Registry for Stem Cell Transplantation and Cell Therapy/German Registry for Stem Cell Transplantation/EBMT template, following the European Data Protection Law.
Study design
The study was performed retrospectively between 1 September 2018 and 1 January 2022, and allowed us to include all consenting patients with pB-ALL who received CAR T-cell treatment with Tisa-cel according to approval by the European Medicines Agency (ie, age ≤25 years, primary refractory disease, ≥2 relapses, or relapse after allo-HSCT).
Centers were contacted to provide patient, treatment, and follow-up information using data documentation fields. The collected data underwent quality control and query validation and resolution by the study center in Frankfurt. The study was performed in accordance with the Declaration of Helsinki with the approval of the Ethics Committee of the University of Frankfurt am Main (number 2021-376).
Statistical analysis
Numerical data are presented as the median with range. Categorical data are shown as numbers and percentages. Group comparisons for numeric and count data were performed with the Wilcoxon rank-sum test and χ2 test, respectively. The hematological response was assessed by microscopy. Complete response (CR) was defined as <5% leukemia blasts in the bone marrow (BM). More than 5% of the blasts in the BM were defined as nonremission (NR).
Median follow-up was calculated using the inverse Kaplan-Meier approach.
Survival analyses were performed using the Kaplan-Meier method. For probability of overall survival (pOS), death from any cause was considered an event, and for pEFS, any of the following events was regarded, whichever occurred first: death from any cause, nonresponse to CAR T-cell therapy (NR), relapse, and secondary malignancy. The probability of relapse-free survival (pRFS), cumulative incidences of relapse (CIR), nonrelapse mortality (NRM), and probability of duration of B-cell aplasia (pDBA) were evaluated for all patients with CR after CAR T-cell therapy who were not lost to follow-up before day 28 after CAR T-cell therapy. For pRFS, the time from CR to any of the following events was evaluated: death from any cause, relapse, or secondary malignancy. CIR and NRM were estimated considering relapse, NRM, secondary malignancy, and subsequent HSCT as competing risks. For estimation of duration of B-cell aplasia, a loss of B-cell aplasia or a CD19+ relapse were considered events.
Censoring was applied on the date of the last follow-up. For estimations of pEFS and pRFS, patients were also censored on the date of a subsequent HSCT (n = 1); for estimation of pDBA, patients were censored on the day of second bag infusion, consecutive HSCT, CD19− relapse, or the day of the last recorded negative B-cell measurement. Two-year estimates are provided for every survival outcome.
The log-rank test was used for comparisons of survival and cumulative incidences. P < .05 was considered significant. Data analysis was performed with the statistical software R 4.1.1.
Results
Patient characteristics
Eighteen centers included all pediatric and young adult patients with pB-ALL (n = 81) who had received Tisa-cel between August 2018 and January 2022. This allows for a minimal follow-up of 5 month for surviving patients. The median age was 11.5 years (range, 1.0-25.0); 53 (65.4%) were males, and 28 (34.6%) were females. The median body weight was 41.6 kg (range, 8.0-135). Sixty-five patients (80.2%) relapsed after HSCT, and 15 (18.5%) suffered a second relapse without HSCT. Only 1 had primary refractory disease. Cytogenetics were classified as favorable (eg, ETV6/RUNX1 or hyperdiploid) in 11 patients (13.6%), intermediate risk in 41 patients (50.6%), and high risk in 29 patients (35.8%; eg, KMT2A rearranged or t(9;22); the details are summarized in Table 1.
Patient characteristics
| . | All patients (N = 81) . | With HSCT (N = 65) . | Without HSCT (N = 16) . | P value . |
|---|---|---|---|---|
| Age, y | .302 | |||
| Median | 11.5 | 10.0 | 14.5 | |
| Range | 1.0-25.0 | 1.0-25.0 | 1.2-25.0 | |
| Sex, n (%) | .755 | |||
| Female | 28 (34.6) | 23 (35.4) | 5 (31.2) | |
| Male | 53 (65.4) | 42 (64.6) | 11 (68.8) | |
| Body weight, kg | .299 | |||
| Median | 41.6 | 38.0 | 45.5 | |
| Range | 8.0-135.0 | 8.0-135.0 | 9.7-84.0 | |
| Preliminary treatment, n (%) | ||||
| Primary refractory | 1 (1.2) | 0 (0.0) | 1 (6.2) | |
| Relapsed refactory | 15 (18.5) | 0 (0.0) | 15 (93.8) | |
| HSCT | 65 (80.2) | 65 (100.0) | NA | |
| Leukemia cytogenetics, n (%) | .277 | |||
| Favorable | 11 (13.6) | 7 (10.8) | 4 (25.0) | |
| ETV6/RUNX1 | 8 (9.8) | 5 (7.7) | 3 (18.7) | |
| Other∗ | 3 (3.7) | 2 (3.1) | 1 (6.2) | |
| Intermediate risk | 41 (50.6) | 33 (50.8) | 8 (50.0) | |
| No specification | 33 (40.7) | 27 (41.5) | 6 (37.5) | |
| Other† | 8 (9.9) | 6 (9.2) | 2 (12.5) | |
| High risk | 29 (35.8) | 25 (38.5) | 4 (25.0) | |
| KMT2A rearrangement | 10 (12.3) | 8 (12.3) | 2 (12.5) | |
| BCR/ABL1 | 8 (9.9) | 8 (12.3) | 0 (0.0) | |
| Other‡ | 11 (13.6) | 9 (13.8) | 2 (12.5) | |
| Leukemia site involvement, n (%) | .892 | |||
| Isolated BM | 41 (50.6) | 34 (52.3) | 7 (43.8) | |
| Isolated CNS | 7 (8.6) | 5 (7.7) | 2 (12.5) | |
| BM + CNS | 13 (16.0) | 10 (15.4) | 3 (18.8) | |
| Other | 20 (24.7) | 16 (24.6) | 4 (25.0) | |
| Time from HSCT to relapse, mo, n (%) | ||||
| <6 | 22 (33.8) | 22 (33.8) | NA | |
| ≥6 | 43 (66.2) | 43 (66.2) | NA | |
| Bridging therapy, n (%) | .116 | |||
| Blinatumomab | 3 (3.7) | 2 (3.1) | 1 (6.2) | |
| Inotuzumab | 4 (4.9) | 2 (3.1) | 2 (12.5) | |
| Low-dose chemotherapy | 72 (88.9) | 60 (92.3) | 12 (75.0) | |
| Intensive chemotherapy | 1 (1.2) | 0 (0.0) | 1 (6.2) | |
| None | 1 (1.2) | 1 (1.5) | 0 (0.0) | |
| Remission status at LDC, n (%) | .196 | |||
| <5% blasts | 44 (54.3) | 33 (50.8) | 11 (68.8) | |
| ≥5% blasts | 37 (45.7) | 32 (49.2) | 5 (31.2) | |
| Cyclophosphamide dose for LDC, mg/m2, n (%) | .173 | |||
| No data | 3 | 3 | 0 | |
| <1000 | 26 (33.3) | 22 (35.5) | 4 (25.0) | |
| 1000-1499 | 28 (35.9) | 24 (38.7) | 4 (25.0) | |
| ≥1500 | 24 (30.8) | 16 (25.8) | 8 (50.0) | |
| Fludarabine dose for LDC, mg/m2, n (%) | .963 | |||
| No data | 3 | 3 | 0 | |
| <120 | 24 (30.8) | 19 (30.6) | 5 (31.2) | |
| ≥120 | 54 (69.2) | 43 (69.4) | 11 (68.8) |
| . | All patients (N = 81) . | With HSCT (N = 65) . | Without HSCT (N = 16) . | P value . |
|---|---|---|---|---|
| Age, y | .302 | |||
| Median | 11.5 | 10.0 | 14.5 | |
| Range | 1.0-25.0 | 1.0-25.0 | 1.2-25.0 | |
| Sex, n (%) | .755 | |||
| Female | 28 (34.6) | 23 (35.4) | 5 (31.2) | |
| Male | 53 (65.4) | 42 (64.6) | 11 (68.8) | |
| Body weight, kg | .299 | |||
| Median | 41.6 | 38.0 | 45.5 | |
| Range | 8.0-135.0 | 8.0-135.0 | 9.7-84.0 | |
| Preliminary treatment, n (%) | ||||
| Primary refractory | 1 (1.2) | 0 (0.0) | 1 (6.2) | |
| Relapsed refactory | 15 (18.5) | 0 (0.0) | 15 (93.8) | |
| HSCT | 65 (80.2) | 65 (100.0) | NA | |
| Leukemia cytogenetics, n (%) | .277 | |||
| Favorable | 11 (13.6) | 7 (10.8) | 4 (25.0) | |
| ETV6/RUNX1 | 8 (9.8) | 5 (7.7) | 3 (18.7) | |
| Other∗ | 3 (3.7) | 2 (3.1) | 1 (6.2) | |
| Intermediate risk | 41 (50.6) | 33 (50.8) | 8 (50.0) | |
| No specification | 33 (40.7) | 27 (41.5) | 6 (37.5) | |
| Other† | 8 (9.9) | 6 (9.2) | 2 (12.5) | |
| High risk | 29 (35.8) | 25 (38.5) | 4 (25.0) | |
| KMT2A rearrangement | 10 (12.3) | 8 (12.3) | 2 (12.5) | |
| BCR/ABL1 | 8 (9.9) | 8 (12.3) | 0 (0.0) | |
| Other‡ | 11 (13.6) | 9 (13.8) | 2 (12.5) | |
| Leukemia site involvement, n (%) | .892 | |||
| Isolated BM | 41 (50.6) | 34 (52.3) | 7 (43.8) | |
| Isolated CNS | 7 (8.6) | 5 (7.7) | 2 (12.5) | |
| BM + CNS | 13 (16.0) | 10 (15.4) | 3 (18.8) | |
| Other | 20 (24.7) | 16 (24.6) | 4 (25.0) | |
| Time from HSCT to relapse, mo, n (%) | ||||
| <6 | 22 (33.8) | 22 (33.8) | NA | |
| ≥6 | 43 (66.2) | 43 (66.2) | NA | |
| Bridging therapy, n (%) | .116 | |||
| Blinatumomab | 3 (3.7) | 2 (3.1) | 1 (6.2) | |
| Inotuzumab | 4 (4.9) | 2 (3.1) | 2 (12.5) | |
| Low-dose chemotherapy | 72 (88.9) | 60 (92.3) | 12 (75.0) | |
| Intensive chemotherapy | 1 (1.2) | 0 (0.0) | 1 (6.2) | |
| None | 1 (1.2) | 1 (1.5) | 0 (0.0) | |
| Remission status at LDC, n (%) | .196 | |||
| <5% blasts | 44 (54.3) | 33 (50.8) | 11 (68.8) | |
| ≥5% blasts | 37 (45.7) | 32 (49.2) | 5 (31.2) | |
| Cyclophosphamide dose for LDC, mg/m2, n (%) | .173 | |||
| No data | 3 | 3 | 0 | |
| <1000 | 26 (33.3) | 22 (35.5) | 4 (25.0) | |
| 1000-1499 | 28 (35.9) | 24 (38.7) | 4 (25.0) | |
| ≥1500 | 24 (30.8) | 16 (25.8) | 8 (50.0) | |
| Fludarabine dose for LDC, mg/m2, n (%) | .963 | |||
| No data | 3 | 3 | 0 | |
| <120 | 24 (30.8) | 19 (30.6) | 5 (31.2) | |
| ≥120 | 54 (69.2) | 43 (69.4) | 11 (68.8) |
NA, not applicable.
Two patients with hyperdiploidy and 1 patient with CALB3.
Two patients with CRLF2 mutation, 2 patients with IKZF1plus, 1 patient with del13q14, 1 patient with cMYC rearrangement, 1 patient with t(12;22), and 1 patient with PAX5-ZNF521-fusion transcript t(9;18).
Two patients with iAMP21, 2 patients with IKFZ deletion, 2 patients with BCR-ABL1–like, 1 patient with complex aberrant karyotype, 1 patient with MEF2D-BCL9, 1 patient with TCF3/HLF, 1 patient with JAK2-MPRIP t(9;17), and 1 patient with TP53 mutation.
Forty-one patients (50.6%) had isolated BM disease, and 7 patients (8.6%) had isolated central nervous system (CNS) involvement. Thirteen patients (16.0%) had combined BM and CNS involvement, and in 20 patients (24.7%), other manifestations, such as the testis, skin, kidney, or various combinations thereof, were affected. For bridging therapy, 3 patients (3.7%) received blinatumomab, 4 (4.9%) received inotuzumab, and 72 (88.9%) received low-dose chemotherapy. One patient was bridged with more intensive chemotherapy, and 1 patient with extramedullary disease did not require any bridging therapy. At the time lymphodepleting chemotherapy (LDC) was started, 44 patients (54.3%) had <5% leukemia blasts (morphological remission), and 37 patients (45.7%) had ≥5% leukemia blasts (nonremission). The LDC consisted of cyclophosphamide and fludarabine in all patients; however, the intensity of LDC was individually adapted according to the physicians’ choice.
Twenty-six patients (33.3%) received <1000 mg/m2, 28 patients (35.9%) received between 1000 and 1499 mg/m2, and 24 patients received ≥1500 mg/m2 cyclophosphamide. Fludarabine was given to 24 patients (30.8%) with <120 mg/m2 and to 54 patients (69.2%) with ≥120 mg/m2. In 3 patients, no data on LDC were reported. Table 1 provides details of the patient data in the comparison of patients with and without prior allo-HSCT.
Toxicity
CRS and immune cell–associated neurological syndrome (ICANS)
Cytokine release syndrome (CRS) was observed in 55 patients (67.9%) (grade I-II, n = 50 [61.7%]; grade III-IV, n = 4 [6.2%];) and grade V, n = 2 [2.5%]). This was less frequent than in the ELIANA trial, in which 58 patients (77.3%) had developed any (grades I-IV) of the majority (n = 35, 46.7%), grade III, or IV (P < .001). There was no difference in the risk of CRS between patients who received Tisa-cel for relapse after HSCT, 42 (64.6%) compared with 13 (81.2%) without prior allo-HSCT (P = .20). A tendency of more CRS was observed in patients in nonremission: 29 of 37 patients (78.4%) compared with 26 of 44 patients (59.1%) in remission at the start of LDC (P = .064). Patients with a higher disease burden (≥5% blasts) than patients with <5% blasts at LDC had a higher incidence of CRS grade ≥3 (P < .001).
ICANS was also less frequent than in the ELIANA trial. Altogether, 6 patients (7.4%) developed ICANS (grade I, n = 2; grade III-IV, n = 4), compared with 30 (40%) in the ELIANA trial (P < .001); details are given in Table 2.
Adverse events after CAR T-cell infusion
| . | All patients (N = 81) . | With HSCT (N = 65) . | Without HSCT (N = 16) . | P value . | <5% blasts (N = 44) . | ≥5% blasts (N = 37) . | P value . | ELIANA (N = 75) . | P value . |
|---|---|---|---|---|---|---|---|---|---|
| CRS (1-5), n (%) | 55 (67.9) | 42 (64.6) | 13 (81.2) | .202 | 26 (59.1) | 29 (78.4) | .064 | 58 (77.3) | .188 |
| CRS (≥3), n (%) | 5 (6.2) | 4 (6.2) | 1 (6.2) | .989 | 0 (0.0) | 5 (13.5) | .012 | 35 (46.7) | <.001 |
| CRS (5), n (%) | 2 (2.5) | 2 (3.1) | 0 (0.0) | .477 | 0 (0.0) | 2 (5.4) | .118 | 0 (0.0) | .171 |
| ICANS (1-5), n (%) | 6 (7.4) | 5 (7.7) | 1 (6.2) | .844 | 2 (4.5) | 4 (10.8) | .283 | 30 (40.0) | <.001 |
| ICANS (≥3), n (%) | 4 (4.9) | 3 (4.6) | 1 (6.2) | .787 | 1 (2.3) | 3 (8.1) | .227 | 10 (13.3) | .067 |
| ICANS (5), n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) | NA |
| . | All patients (N = 81) . | With HSCT (N = 65) . | Without HSCT (N = 16) . | P value . | <5% blasts (N = 44) . | ≥5% blasts (N = 37) . | P value . | ELIANA (N = 75) . | P value . |
|---|---|---|---|---|---|---|---|---|---|
| CRS (1-5), n (%) | 55 (67.9) | 42 (64.6) | 13 (81.2) | .202 | 26 (59.1) | 29 (78.4) | .064 | 58 (77.3) | .188 |
| CRS (≥3), n (%) | 5 (6.2) | 4 (6.2) | 1 (6.2) | .989 | 0 (0.0) | 5 (13.5) | .012 | 35 (46.7) | <.001 |
| CRS (5), n (%) | 2 (2.5) | 2 (3.1) | 0 (0.0) | .477 | 0 (0.0) | 2 (5.4) | .118 | 0 (0.0) | .171 |
| ICANS (1-5), n (%) | 6 (7.4) | 5 (7.7) | 1 (6.2) | .844 | 2 (4.5) | 4 (10.8) | .283 | 30 (40.0) | <.001 |
| ICANS (≥3), n (%) | 4 (4.9) | 3 (4.6) | 1 (6.2) | .787 | 1 (2.3) | 3 (8.1) | .227 | 10 (13.3) | .067 |
| ICANS (5), n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) | NA |
NRM and cause of death
Four patients succumbed to nonrelapse events (NRM). Of these cases, 2 patients (20 and 11.5 years) developed fulminant, severe CRS (grade V), and macrophage activation that could not be controlled; the patients passed away 1.2 or 0.8 months after CAR T-cell infusion. Two more patients died of treatment-related mortality (TRM), which resulted from infection or multiorgan toxicity at 4.6 or 1.2 months after CAR T-cell infusion, respectively. One patient with a prior allo-HSCT followed by CAR T-cell treatment developed secondary myelodysplastic syndrome. There was no vector integration observed (supplemental Table 1).
Response
By day 28, 71 patients (87.8%) were in CR, 8 patients (9.9%) did not respond (NR), and 1 patient each (1.2%) had died because of NRM and TRM as described above. One young adult patient was consolidated with allogeneic SCT after having achieved CR at day +28. Of the 71 patients who achieved CR at day +28, minimal residual disease (MRD) data were available in 69 patients (97.2%). In all 69 patients, MRD detection was negative by TCR/IG-rearrangement real-time polymerase chain reaction with a minimum level of detection of residual leukemia cells to be 10−4 performed according to the standards of the European Study Group on MRD detection in ALL.12
The median follow-up was 20.8 months (range, 0.6-44.7). At the last follow-up, of the 71 patients who were in CR at day +28, 42 patients (59%) remained in remission (only 1 patient received an allo-HSCT while being in remission; the other 42 patients received no further treatment), 26 patients (37%) had relapsed, and 1 patient (1.2%) each had died because of NRM, died because of TRM, or developed secondary myelodysplastic syndrome (supplemental Table 2).
All 7 patients with isolated CNS relapse achieved CR at day +28. Two patients relapsed with CD19+ blasts at 1.94 and 11.57 months. At the last follow-up (median, 21; range, 14.5-27.5), 5 of 7 patients (71%) were alive in CR at the last follow-up.
Survival
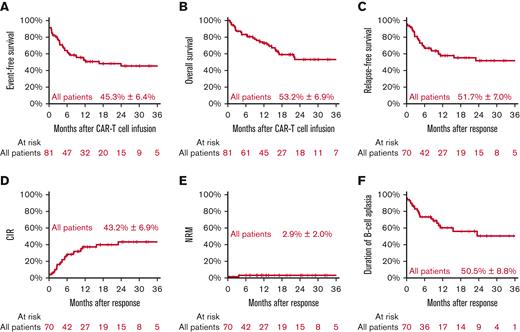
Two years after CAR T-cell infusion, pEFS was 45.3% (±6.4%) and pOS was 53.2% (±6.9%). Seventy-one of 81 patients (87.8%) responded at day +28 and achieved morphological remission. Of these 71 patients, 1 patient from abroad was lost to follow-up. Of the remaining 70 of 71 responding patients, the 2-year pRFS was 51.7% (±7.0%), the CIR was 43.2% (±6.9%), the cumulative incidence of NRM was 2.9% (±2.0%), and the pDBA was 50.5% (±8.8%) (Figure 1). The cumulative incidences of CD19+ and CD19− relapses were 29.3% (±6.4%) and 13.9% (±4.3%), respectively (supplemental Figure 1).
All patients. Event-free survival (A), overall survival (B) with estimates for 24 months after CAR T-cell infusion, relapse-free survival (C), CIR (D), NRM (E), and duration of B-cell aplasia with estimates for 24 months after response.
All patients. Event-free survival (A), overall survival (B) with estimates for 24 months after CAR T-cell infusion, relapse-free survival (C), CIR (D), NRM (E), and duration of B-cell aplasia with estimates for 24 months after response.
The analysis of potential risk factors such as age, cytogenetics, sites of leukemia involvement, or intensity of LDC showed that only disease burden at LDC itself was noticeably associated with pEFS. Patients with high-level disease (≥5%) had a pEFS of 29.6% (±10.9%), compared with 55.4% (±8.1%) in patients with low-level disease (<5% blasts) (P = .049) (Table 3; supplemental Figure 2). Patients with isolated CNS relapse showed pEFS, pOS, pRFS, and pDBA rates of 57.1%, 71.4%, 57.1%, and 50.0%, respectively. There was no difference in patients who had isolated BM relapse, combined BM relapse, or involvement at other sites (supplemental Figure 3).
Risk factor analysis in all patients: estimates at 24 months
| Variable . | Group . | N∗ . | pEFS, % . | P value . | OS, % . | P value . | pRFS, % . | P value . | pDBA, % . | P value . | CIR, % . | P value . | CIR CD19+, % . | P value . | CIR CD19−, % . | P value . | NRM, % . | P value . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | 1-6 | 19/17 | 37.6 | .438 | 32.9 | .503 | 42.0 | .189 | 42.9 | .126 | 51.7 | .278 | 45.4 | .109 | 6.3 | .568 | 0.0 | .614 |
| 7-12 | 26/25 | 50.7 | 56.4 | 52.7 | 31.3 | 43.1 | 22.2 | 20.9 | 4.2 | |||||||||
| 13-17 | 16/13 | 46.4 | 66.7 | 57.1 | 61.2 | 42.9 | 26.7 | 16.2 | 0.0 | |||||||||
| 18-25 | 20/15 | 46.8 | 62.2 | 58.7 | 62.7 | 32.1 | 25.5 | 6.7 | 6.7 | |||||||||
| Patient category | Pediatric | 61/55 | 45.4 | .912 | 50.6 | .883 | 50.3 | .548 | 44.4 | .482 | 45.9 | .305 | 30.2 | .617 | 15.7 | .439 | 1.9 | .306 |
| Adults | 20/15 | 46.8 | 62.2 | 58.7 | 62.7 | 32.1 | 25.5 | 6.7 | 6.7 | |||||||||
| Leukemia cytogenetics | Favorable | 11/10 | 45.5 | .405 | 60.6 | .190 | 50.0 | .808 | 31.1 | .407 | 40.0 | .998 | 30.0 | .279 | 10.0 | .116 | 10.0 | .276 |
| Intermediate risk | 41/33 | 39.9 | 42.3 | 49.5 | 61.4 | 43.9 | 19.8 | 24.1 | 3.4 | |||||||||
| High risk | 29//27 | 52.2 | 65.2 | 54.1 | 47.1 | 44.4 | 40.7 | 3.7 | 0.0 | |||||||||
| Leukemia site involvement | Isolated BM | 41/33 | 44.5 | .930 | 54.2 | .913 | 55.3 | .895 | 58.3 | .506 | 38.6 | .584 | 23.4 | .447 | 15.3 | .380 | 3.0 | .273 |
| Isolated CNS | 7/7 | 57.1 | 71.4 | 57.1 | 50.0 | 28.6 | 28.6 | 0.0 | 14.3 | |||||||||
| BM + CNS | 13/12 | 46.3 | 44.4 | 46.3 | 48.6 | 53.7 | 26.9 | 26.9 | 0.0 | |||||||||
| Other | 20/18 | 46.9 | 45.2 | 52.1 | 38.5 | 45.6 | 39.2 | 6.4 | 0.0 | |||||||||
| HSCT before CAR T-cell therapy | No | 16/13 | 55.0 | .614 | 55.2 | .851 | 67.7 | .294 | 76.9 | .356 | 32.3 | .501 | 16.2 | .392 | 16.2 | .868 | 0.0 | .490 |
| Yes | 65/57 | 43.4 | 53.2 | 48.6 | 45.0 | 45.1 | 31.9 | 13.1 | 3.6 | |||||||||
| Remission status at LDC | <5% blasts | 44/42 | 55.4 | .049 | 59.1 | .105 | 58.1 | .374 | 50.5 | .798 | 41.9 | .863 | 26.6 | .851 | 15.3 | .692 | 0.0 | .083 |
| ≥5% blasts | 37/28 | 29.6 | 46.3 | 37.9 | 52.7 | 49.0 | 37.4 | 11.6 | 7.3 | |||||||||
| Cyclophosphamide dose for LDC (mg/m2) | <1000 | 26/21 | 41.2 | .719 | 47.3 | .781 | 51.0 | .553 | 35.3 | .713 | 49.0 | .488 | 49.0 | .405 | 0.0 | .062 | 0.0 | .427 |
| 1000-1499 | 28/25 | 36.8 | 50.3 | 41.2 | 42.2 | 52.3 | 27.2 | 25.1 | 4.2 | |||||||||
| ≥1500 | 24/21 | 54.9 | 59.6 | 59.9 | 72.9 | 35.4 | 20.4 | 15.0 | 0.0 | |||||||||
| Fludarabine dose for LDC (mg/m2) | <120 | 24/21 | 49.7 | .988 | 51.1 | .739 | 56.8 | .988 | 27.2 | .843 | 36.7 | .637 | 31.6 | .565 | 5.1 | .164 | 4.8 | .149 |
| ≥120 | 54/46 | 43.6 | 54.9 | 50.1 | 55.0 | 47.6 | 29.0 | 18.7 | 0.0 |
| Variable . | Group . | N∗ . | pEFS, % . | P value . | OS, % . | P value . | pRFS, % . | P value . | pDBA, % . | P value . | CIR, % . | P value . | CIR CD19+, % . | P value . | CIR CD19−, % . | P value . | NRM, % . | P value . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | 1-6 | 19/17 | 37.6 | .438 | 32.9 | .503 | 42.0 | .189 | 42.9 | .126 | 51.7 | .278 | 45.4 | .109 | 6.3 | .568 | 0.0 | .614 |
| 7-12 | 26/25 | 50.7 | 56.4 | 52.7 | 31.3 | 43.1 | 22.2 | 20.9 | 4.2 | |||||||||
| 13-17 | 16/13 | 46.4 | 66.7 | 57.1 | 61.2 | 42.9 | 26.7 | 16.2 | 0.0 | |||||||||
| 18-25 | 20/15 | 46.8 | 62.2 | 58.7 | 62.7 | 32.1 | 25.5 | 6.7 | 6.7 | |||||||||
| Patient category | Pediatric | 61/55 | 45.4 | .912 | 50.6 | .883 | 50.3 | .548 | 44.4 | .482 | 45.9 | .305 | 30.2 | .617 | 15.7 | .439 | 1.9 | .306 |
| Adults | 20/15 | 46.8 | 62.2 | 58.7 | 62.7 | 32.1 | 25.5 | 6.7 | 6.7 | |||||||||
| Leukemia cytogenetics | Favorable | 11/10 | 45.5 | .405 | 60.6 | .190 | 50.0 | .808 | 31.1 | .407 | 40.0 | .998 | 30.0 | .279 | 10.0 | .116 | 10.0 | .276 |
| Intermediate risk | 41/33 | 39.9 | 42.3 | 49.5 | 61.4 | 43.9 | 19.8 | 24.1 | 3.4 | |||||||||
| High risk | 29//27 | 52.2 | 65.2 | 54.1 | 47.1 | 44.4 | 40.7 | 3.7 | 0.0 | |||||||||
| Leukemia site involvement | Isolated BM | 41/33 | 44.5 | .930 | 54.2 | .913 | 55.3 | .895 | 58.3 | .506 | 38.6 | .584 | 23.4 | .447 | 15.3 | .380 | 3.0 | .273 |
| Isolated CNS | 7/7 | 57.1 | 71.4 | 57.1 | 50.0 | 28.6 | 28.6 | 0.0 | 14.3 | |||||||||
| BM + CNS | 13/12 | 46.3 | 44.4 | 46.3 | 48.6 | 53.7 | 26.9 | 26.9 | 0.0 | |||||||||
| Other | 20/18 | 46.9 | 45.2 | 52.1 | 38.5 | 45.6 | 39.2 | 6.4 | 0.0 | |||||||||
| HSCT before CAR T-cell therapy | No | 16/13 | 55.0 | .614 | 55.2 | .851 | 67.7 | .294 | 76.9 | .356 | 32.3 | .501 | 16.2 | .392 | 16.2 | .868 | 0.0 | .490 |
| Yes | 65/57 | 43.4 | 53.2 | 48.6 | 45.0 | 45.1 | 31.9 | 13.1 | 3.6 | |||||||||
| Remission status at LDC | <5% blasts | 44/42 | 55.4 | .049 | 59.1 | .105 | 58.1 | .374 | 50.5 | .798 | 41.9 | .863 | 26.6 | .851 | 15.3 | .692 | 0.0 | .083 |
| ≥5% blasts | 37/28 | 29.6 | 46.3 | 37.9 | 52.7 | 49.0 | 37.4 | 11.6 | 7.3 | |||||||||
| Cyclophosphamide dose for LDC (mg/m2) | <1000 | 26/21 | 41.2 | .719 | 47.3 | .781 | 51.0 | .553 | 35.3 | .713 | 49.0 | .488 | 49.0 | .405 | 0.0 | .062 | 0.0 | .427 |
| 1000-1499 | 28/25 | 36.8 | 50.3 | 41.2 | 42.2 | 52.3 | 27.2 | 25.1 | 4.2 | |||||||||
| ≥1500 | 24/21 | 54.9 | 59.6 | 59.9 | 72.9 | 35.4 | 20.4 | 15.0 | 0.0 | |||||||||
| Fludarabine dose for LDC (mg/m2) | <120 | 24/21 | 49.7 | .988 | 51.1 | .739 | 56.8 | .988 | 27.2 | .843 | 36.7 | .637 | 31.6 | .565 | 5.1 | .164 | 4.8 | .149 |
| ≥120 | 54/46 | 43.6 | 54.9 | 50.1 | 55.0 | 47.6 | 29.0 | 18.7 | 0.0 |
Number of patients for estimation of pEFS and pOS/number of patients for estimation of pRFS, pDBA, CIR, and NRM.
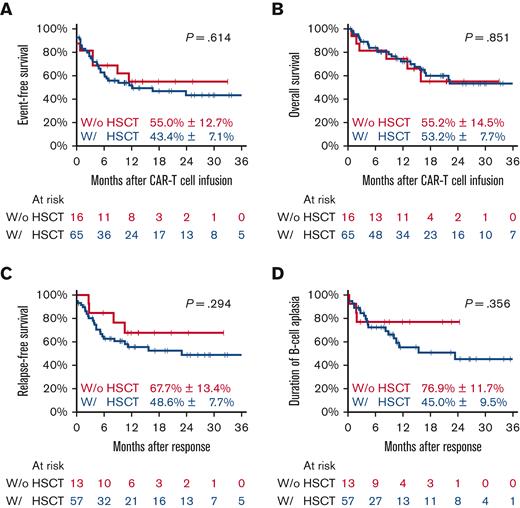
Comparing patients who had or had not received allo-HSCT, we observed pEFS of 43.4% (±7.1) and 55.0% (±12.7) (P = .614) and pOS of 53.2% (±7.7) and 55.2% (±14.5%) (P = .851), respectively. For CAR T-cell responders, the 2-year pRFS in patients with prior HSCT was 48.6% (±7.7) compared with 67.7% (±13.4) in those without prior HSCT (P = .851), and the 2-year pDBA was 45.0% (±9.5%) and 76.9% (±11.7%), respectively (P = .356) (Figure 2).
All patients according to previous allo-HSCT. Event-free survival (A), overall survival (B) with estimates for 24 months after CAR T-cell infusion, relapse-free survival (C), and duration of B-cell aplasia (D) with estimates for 24 months after response. w/, with’ w/o, without.
All patients according to previous allo-HSCT. Event-free survival (A), overall survival (B) with estimates for 24 months after CAR T-cell infusion, relapse-free survival (C), and duration of B-cell aplasia (D) with estimates for 24 months after response. w/, with’ w/o, without.
Patients with previous allo-HSCT
As mentioned above, 65 of 81 (80.2%) were patients who had received a prior allo-HSCT for high-risk or relapsed disease and subsequently relapsed before receiving CAR T-cell therapy as a standalone treatment. As such, a high proportion of patients who received allo-HSCT is unique in published real-world experience, and this group is analyzed in more detail in the following paragraph.
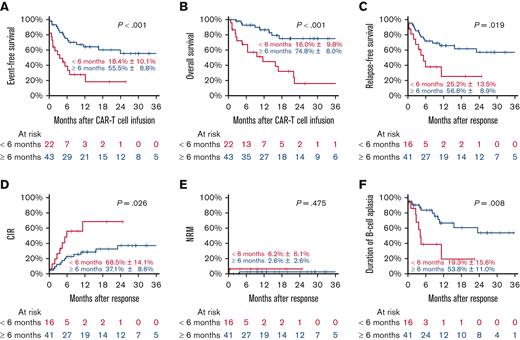
Patients relapsing within 6 months after allo-HSCT had a median follow-up of 28.8 months (range, 1.5-38.5) and a pEFS of 18.4% (±10.1%), compared with those relapsing beyond 6 months after allo-HSCT at 55.5% (±8.8%) (P < .001) (median follow-up, 20.6 months; range, 0.6-44.7). This corresponded to a pOS of 16.0% (±9.8%) and 74.8% (±8.0%) (P < .001) and a pRFS of 25.2% (±13.5%) and 56.8% (±8.9%) (P = .019) for patients relapsing within 6 months and beyond 6 months, respectively. The main reason for treatment failure was relapse, as the CIR for patients who had relapsed within 6 months of allo-HSCT was 68.5% (±14.1%), compared with 37.1% (±8.6%) for those who had relapsed beyond 6 months (P = .026) (Figure 3). The time from allo-HSCT to relapse was the sole predictor for outcome.
Patients with previous allo-HSCT according to time from HSCT to relapse. Event-free survival (A), overall survival (B) with estimates for 24 months after CAR T-cell infusion, relapse-free survival (C), CIR (D), NRM (E), and duration of B-cell aplasia (F) with estimates for 24 months after response.
Patients with previous allo-HSCT according to time from HSCT to relapse. Event-free survival (A), overall survival (B) with estimates for 24 months after CAR T-cell infusion, relapse-free survival (C), CIR (D), NRM (E), and duration of B-cell aplasia (F) with estimates for 24 months after response.
Patients who relapsed within 6 months after allo-HSCT tended to develop CD19+ relapse (CIR, 46.6%) compared with patients who relapsed beyond 6 months (CIR, 26.9%) (P = .093) (Table 4). We also observed that patients who relapsed within 6 months from allo-HSCT showed a pDBA of 19.3% at 2 years, in contrast to patients who relapsed >6 months from allo-HSCT, who had a pDBA of 53.8% at 2 years (P = .008) (Table 4).
Risk factors
| Variable . | Group . | N∗ . | pEFS, % . | P value . | pOS, % . | P value . | pRFS, % . | P value . | pDBA, % . | P value . | CIR, % . | P value . | CIR CD19+, % . | P value . | CIR CD19−, % . | P value . | NRM, % . | P value . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | 1-6 | 17/15 | 38.2 | .309 | 32.9 | .347 | 43.3 | .130 | 41.1 | .127 | 49.4 | .163 | 42.2 | .172 | 7.2 | .231 | 0.0 | .611 |
| 7-12 | 22/21 | 40.6 | 52.2 | 42.5 | 25.6 | 52.5 | 27.4 | 25.1 | 5.0 | |||||||||
| 13-17 | 11/9 | 48.5 | 72.7 | 59.3 | 60.0 | 40.7 | 29.6 | 11.1 | 0.0 | |||||||||
| 18-25 | 15/12 | 49.4 | 73.5 | 57.0 | 63.0 | 31.5 | 31.5 | 0.0 | 8.3 | |||||||||
| Patient category | Pediatric | 50/45 | 42.1 | .616 | 48.6 | .301 | 46.8 | .438 | 38.4 | .277 | 48.5 | .212 | 31.9 | .810 | 16.7 | .148 | 2.3 | .293 |
| Adults | 15/12 | 49.4 | 73.5 | 57.0 | 63.0 | 31.5 | 31.5 | 0.0 | 8.3 | |||||||||
| Leukemia cytogenetics | Favorable | 7/7 | 57.1 | .387 | 68.6 | .234 | 57.1 | .853 | 31.2 | .348 | 28.6 | .734 | 28.6 | .389 | 0.0 | .080 | 14.3 | .207 |
| Intermediate risk | 33/27 | 35.7 | 42.0 | 43.7 | 58.4 | 48.1 | 23.2 | 24.9 | 4.2 | |||||||||
| High risk | 25/23 | 46.9 | 63.8 | 48.9 | 38.0 | 49.1 | 44.7 | 4.3 | 0.0 | |||||||||
| Leukemia site involvement | Isolated BM | 34/29 | 42.3 | .952 | 54.0 | .707 | 49.6 | .953 | 53.1 | .485 | 43.5 | .938 | 26.2 | .650 | 17.2 | .738 | 3.4 | .217 |
| Isolated CNS | 5/5 | 40.0 | 60.0 | 40.0 | 25.0 | 40.0 | 40.0 | 0.0 | 20.0 | |||||||||
| BM + CNS | 10/9 | 50.8 | 57.1 | 50.8 | 43.2 | 49.2 | 36.5 | 12.7 | 0.0 | |||||||||
| Other | 16/14 | 46.7 | 35.6 | 53.3 | 40.5 | 44.0 | 35.2 | 8.7 | 0.0 | |||||||||
| Time from HSCT to relapse | <6 mo | 22/16 | 18.4 | <.001 | 16.0 | <.001 | 25.2 | .019 | 19.3 | .008 | 68.5 | .026 | 46.6 | .093 | 21.9 | .314 | 6.2 | .475 |
| ≥6 mo | 43/41 | 55.5 | 74.8 | 56.8 | 53.8 | 37.1 | 26.9 | 10.2 | 2.6 | |||||||||
| Remission status at LDC | <5% blasts | 33/31 | 54.1 | .138 | 58.6 | .289 | 57.5 | .317 | 44.9 | .824 | 42.5 | .981 | 28.8 | .860 | 13.7 | .875 | 0.0 | .123 |
| ≥5% blasts | 32/26 | 28.8 | 47.0 | 34.2 | 48.2 | 51.5 | 38.9 | 12.6 | 7.9 | |||||||||
| Cyclophosphamide dose for LDC (mg/m2) | <1000 | 22/18 | 37.7 | .685 | 45.8 | .703 | 46.1 | .610 | 29.4 | .337 | 53.9 | .426 | 53.9 | .211 | 0.0 | .116 | 0.0 | .480 |
| 1000-1499 | 24/22 | 33.6 | 51.2 | 36.6 | 34.9 | 55.8 | 31.9 | 23.9 | 4.8 | |||||||||
| ≥1500 | 16/14 | 59.5 | 62.4 | 63.5 | 79.6 | 29.4 | 15.1 | 14.3 | 0.0 | |||||||||
| Fludarabine dose for LDC (mg/m2) | <120 | 19/16 | 39.9 | .807 | 46.2 | .280 | 47.4 | .949 | 22.3 | .703 | 43.5 | .940 | 36.6 | .538 | 6.9 | .346 | 6.2 | .135 |
| ≥120 | 43/38 | 44.0 | 56.7 | 48.5 | 50.4 | 48.7 | 32.0 | 16.7 | 0.0 |
| Variable . | Group . | N∗ . | pEFS, % . | P value . | pOS, % . | P value . | pRFS, % . | P value . | pDBA, % . | P value . | CIR, % . | P value . | CIR CD19+, % . | P value . | CIR CD19−, % . | P value . | NRM, % . | P value . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | 1-6 | 17/15 | 38.2 | .309 | 32.9 | .347 | 43.3 | .130 | 41.1 | .127 | 49.4 | .163 | 42.2 | .172 | 7.2 | .231 | 0.0 | .611 |
| 7-12 | 22/21 | 40.6 | 52.2 | 42.5 | 25.6 | 52.5 | 27.4 | 25.1 | 5.0 | |||||||||
| 13-17 | 11/9 | 48.5 | 72.7 | 59.3 | 60.0 | 40.7 | 29.6 | 11.1 | 0.0 | |||||||||
| 18-25 | 15/12 | 49.4 | 73.5 | 57.0 | 63.0 | 31.5 | 31.5 | 0.0 | 8.3 | |||||||||
| Patient category | Pediatric | 50/45 | 42.1 | .616 | 48.6 | .301 | 46.8 | .438 | 38.4 | .277 | 48.5 | .212 | 31.9 | .810 | 16.7 | .148 | 2.3 | .293 |
| Adults | 15/12 | 49.4 | 73.5 | 57.0 | 63.0 | 31.5 | 31.5 | 0.0 | 8.3 | |||||||||
| Leukemia cytogenetics | Favorable | 7/7 | 57.1 | .387 | 68.6 | .234 | 57.1 | .853 | 31.2 | .348 | 28.6 | .734 | 28.6 | .389 | 0.0 | .080 | 14.3 | .207 |
| Intermediate risk | 33/27 | 35.7 | 42.0 | 43.7 | 58.4 | 48.1 | 23.2 | 24.9 | 4.2 | |||||||||
| High risk | 25/23 | 46.9 | 63.8 | 48.9 | 38.0 | 49.1 | 44.7 | 4.3 | 0.0 | |||||||||
| Leukemia site involvement | Isolated BM | 34/29 | 42.3 | .952 | 54.0 | .707 | 49.6 | .953 | 53.1 | .485 | 43.5 | .938 | 26.2 | .650 | 17.2 | .738 | 3.4 | .217 |
| Isolated CNS | 5/5 | 40.0 | 60.0 | 40.0 | 25.0 | 40.0 | 40.0 | 0.0 | 20.0 | |||||||||
| BM + CNS | 10/9 | 50.8 | 57.1 | 50.8 | 43.2 | 49.2 | 36.5 | 12.7 | 0.0 | |||||||||
| Other | 16/14 | 46.7 | 35.6 | 53.3 | 40.5 | 44.0 | 35.2 | 8.7 | 0.0 | |||||||||
| Time from HSCT to relapse | <6 mo | 22/16 | 18.4 | <.001 | 16.0 | <.001 | 25.2 | .019 | 19.3 | .008 | 68.5 | .026 | 46.6 | .093 | 21.9 | .314 | 6.2 | .475 |
| ≥6 mo | 43/41 | 55.5 | 74.8 | 56.8 | 53.8 | 37.1 | 26.9 | 10.2 | 2.6 | |||||||||
| Remission status at LDC | <5% blasts | 33/31 | 54.1 | .138 | 58.6 | .289 | 57.5 | .317 | 44.9 | .824 | 42.5 | .981 | 28.8 | .860 | 13.7 | .875 | 0.0 | .123 |
| ≥5% blasts | 32/26 | 28.8 | 47.0 | 34.2 | 48.2 | 51.5 | 38.9 | 12.6 | 7.9 | |||||||||
| Cyclophosphamide dose for LDC (mg/m2) | <1000 | 22/18 | 37.7 | .685 | 45.8 | .703 | 46.1 | .610 | 29.4 | .337 | 53.9 | .426 | 53.9 | .211 | 0.0 | .116 | 0.0 | .480 |
| 1000-1499 | 24/22 | 33.6 | 51.2 | 36.6 | 34.9 | 55.8 | 31.9 | 23.9 | 4.8 | |||||||||
| ≥1500 | 16/14 | 59.5 | 62.4 | 63.5 | 79.6 | 29.4 | 15.1 | 14.3 | 0.0 | |||||||||
| Fludarabine dose for LDC (mg/m2) | <120 | 19/16 | 39.9 | .807 | 46.2 | .280 | 47.4 | .949 | 22.3 | .703 | 43.5 | .940 | 36.6 | .538 | 6.9 | .346 | 6.2 | .135 |
| ≥120 | 43/38 | 44.0 | 56.7 | 48.5 | 50.4 | 48.7 | 32.0 | 16.7 | 0.0 |
Risk factor analysis in patients with prior HSCT: estimates at 24 months.
Number of patients for estimation of pEFS and pOS/number of patients for estimation of pRFS, pDBA, CIR, and NRM.
In contrast, age, leukemia cytogenetics, relapse site, and remission state at the start of LDC or LDC dosing were not noticeably associated with any of the long-term outcomes (Table 4).
Discussion
This analysis shows the first real-world experience in pediatric, adolescent, and young adult patients with pB-ALL in Germany treated with the CD19-CAR T-cell product Tisa-cel within the approved label. Here, 45.3% of 81 patients with reported pB-ALL with poor prognosis were rescued with a single dose of Tisa-cel, with a pOS at 2 years of 53.2%. These are very encouraging results. It is of special interest that only 1 patient was consolidated with allo-HSCT after having achieved remission with CAR T cells, in contrast to other reports where large proportions of patients who responded received allo-HSCT for consolidation.13,14
In clinical trials, stringent inclusion criteria narrow the risk profiles of trial participants. Treatment in clinical trials provides close guidance to physicians and patients during the trial. Consequently, postapproval reality leads to the treatment of patients with higher and more variable risk profiles, less stringent criteria for organ dysfunction, and many other factors that are also less restrictive in the real-world setting.13,15 In addition, the patient population can be different from that in the published licensing trials. In the ELIANA trial, for example, children below the age of 3 years, high-risk patients with isolated CNS manifestations, and patients with other organ manifestations without BM involvement could not be included. In addition, during the licensing trial, strict guidance was offered to the participating centers, and as in the real-world scenario, many centers are approved to treat patients after appropriate training.10,11
Given the particular risk profile of the patients treated in the study presented, the overall response rate at day +28 of 87.6% was as high as in the ELIANA trial.10 Although 2 patients (2.5%) died related to CAR T-cell infusion, the overall rate of CRS and ICANS was significantly lower than that in the ELIANA trial, and higher-grade (grade >3) toxicities were not more frequent. This might be because the disease load of our patients was lower than that of patients treated in the ELIANA trial.
CRS, but not ICANS, was more frequent in patients with overt leukemia at the time of LDC (Table 2). Our data confirm the findings of the French real-world experience in 51 patients, of whom 1 (2%) died by CRS/ICANS, also showing a lower rate of CRS and ICANS than in the ELIANA trial. Jacoby et al reported a retrospective study including 55 patients with CNS involvement, of whom 41 patients received Tisa-cel, 12 patients received a CD28-based CAR, and 2 patients received other constructs.16-18 CRS (grades 1-4) and ICANS (grades 1-5) occurred in 65% and 38%, respectively, which were more frequent than in our work. The fact that CD28-based CAR T cells are more prone to inducing ICANS might at least in part explain the differences observed.16-18 In the absence of other obvious reasons, we hypothesize that increasing experience and earlier interventions with tocilizumab and steroids compared with the trial phases might account for this.13,19,20
The fact that 2 patients were unable to be rescued from fulminant inflammatory complications of CAR T cells despite expert management still emphasizes the considerable risks associated with this type of therapy, especially in the context of bulky disease. Overall, the rate of TRM after CAR T-cell therapy compares favorably with conventional rescue strategies, including second and further transplants.7,21
Patients with CNS involvement at diagnosis or relapse were no less likely to benefit from CAR T-cell therapy in that pEFS and pOS were very similar; the same is true for other sites of extramedullary relapse. Our data are in line with the findings of the Pediatric Real World CAR Consortium from US centers, which showed that 40 patients with CNS involvement had no higher incidence of CRS and ICANS than patients without CNS involvement. This study demonstrated that CNS involvement was also not associated with impaired survival.22
Most recently, several studies have addressed the question of the optimal bridging to CAR T-cell therapy.23,24 The question of whether previous CD19-directed immunotherapy with blinatumomab might contribute to treatment failure has gained particular importance.19
To this end, Meyers and colleagues performed a retrospective multicenter study in 420 children and young adults treated with different CD19-directed CAR T-cell constructs between 2012 and 2019, including 77 of the 420 patients who had a history of previous treatment with blinatumomab. The primary objective of that study was to evaluate the 6-month pRFS and pEFS stratified according to prior blinatumomab use. That study revealed that nonresponse to initial treatment with blinatumomab rather than exposure to prior blinatumomab was predictive of an inferior outcome.25 Because of the low number of patients who received blinatumomab in our study during the course of their antileukemia treatment, our results do not allow a clear statement here.
Other reports17,22,26,27 found that the intensity of LDC and/or the exposure to fludarabine area under the curve to be a prognostic factor for CIR, pEFS, and pOS. In our report, we did not find a correlation between intensity of LDC and outcome. This might be caused by the excellent response to CAR T cells of the patients who relapsed late after allo-HSCT, where the dominant predictive factor was time from HSCT to relapse.
This may also be owing to the retrospective nature of the study and the individual and heterogeneous choice of dosage not being consistent based on general recommendations.
Several additional studies clearly showed that remission status before CAR T-cell infusion strongly affects prognosis; as our data confirm, the prognosis of patients receiving CAR T cells while in morphological remission is better with a decreased risk for relapse, a higher pEFS, and a better pOS.20,28 Most likely, these findings reflect the importance of the biology of leukemia as opposed to defining disease load itself as a prognostic factor.20 When analyzing the whole cohort of our patients, a high disease load at the start of LDC was correlated with an increased CIR, followed by reduced pEFS and pOS. Interestingly, this factor lost significance in patients who were treated after prior allo-HSCT.
The fact that most of our cohort (80%) received CAR T cells in the context of post-HSCT relapse makes this analysis different from all other reported studies or “real-world studies,” where post-HSCT relapses constitute smaller cohorts.13,20,25,29
Therefore, special attention was given to this fraction in our study. The prognosis of patients who relapse after allo-HSCT is generally very poor. Kuhlen and colleagues6 analyzed the outcomes of 242 children who relapsed after a first allo-HSCT enrolled in the Berlin-Frankfurt-Munster (BFM) ALL-SCT-BFM 20034 trial and ALL-SCT-BFM international 200721 studies. The 3-year pEFS was 15%, and the pOS was 20% compared with 43.4% and 53.2% at 2 years in this study, where patients were treated with a single dose of Tisa-cel.6 Until the availability of CAR T-cell therapy, the only curative treatment option for patients relapsing after allo-HSCT was a second procedure with either the same or a different donor.30 However, even after further allo-HSCT, the reported outcome is poor. Poon et al reported 31 patients who underwent a further transplant and reported 1- and 3-year pOS rates of 23% and 11%, respectively. In a large retrospective study performed by Yaniv on behalf of the Pediatric Diseases Working Party of the EBMT, 214 patients with a second transplant for ALL revealed leukemia-free survival and pOS at 2 years of 30% and 25%, respectively.21 This was associated with a TRM rate of 22%. As mentioned above, treatment with CAR T cells, as presented in our study, offers a chance for pEFS and pOS of 43.4% and 53.2%, respectively. This was associated with an impressively low NRM of 3.6%. This might well justify this approach to become the treatment of first choice in patients suffering a relapse after allo-HSCT. Remarkably, in the smaller population of patients with refractory or >1 relapsed disease receiving salvage therapy with CAR T cells without a previous transplant, the high probability of pOS of 55.2% encourages evaluation of CAR T cells even as an alternative to HSCT to avoid debilitating late effects from organ toxicities of total body irradiation and allogeneic immune reactions.
With a 2-year pOS of 74.8%, the outcome of CAR T-cell therapy for patients with post-HSCT pB-ALL relapses beyond 6 months after allo-HSCT could be regarded as already quite satisfactory. However, the survival probabilities of patients with an early relapse <6 months from allo-HSCT survival are poor. Interestingly, patients with early relapses have a significantly higher probability of B-cell recurrence than patients who relapse >6 months after allo-HSCT. These findings may reflect the lower persistence and effectivity of CAR T cells generated early after allo-HSCT. It is well known that the restoration of potent immunity after allo-HSCT is a matter of time. Often, complete restoration of polyclonal and naïve T cells might take up to 1 year.31-33 We speculate that patients with later relapses might have a more polyclonal T-cell repertoire with more naïve cells, allowing a more rapid and potent expansion and proliferation of transduced T cells both during manufacturing and later. These interactions are currently being investigated in a prospective study. It is of great importance for clinical decision-making to understand whether and if patients with early relapse after allo-HSCT may benefit from current CAR T-cell products. These patients may need alternative CAR T cells, either with different compositions or generated directly from their original stem cell donors.
However, based on our data presented, the biology of leukemia could also be a relevant prognostic factor in patients who relapse early after allo-HSCT. These questions are currently being pursued in a prospective trial.
In conclusion, in this first real-world report of CAR T-cell treatment in patients with ALL from Germany, excellent results were reported, showing that patients with refractory or relapsed disease, including patients who relapsed after allo-HSCT, could be rescued with a single infusion of CAR T cells. Among the patients with a previous transplant, patients who relapsed ≥6 months after HSCT in particular have an excellent chance to survive their disease with only a single Tisa-cel infusion and no further consolidation. Although treatment-related deaths did not occur, the toxicity profile with regard to CRS and ICANS was lower than in the initial licensing trials, and the TRM stayed far lower than expected after a second allograft procedure. Prospective studies should reveal how durable response could be improved in patients who relapsed during the first 6 months after HSCT.
Acknowledgment
No third-party funding was available for this retrospective study.
Authorship
Contribution: P.B., T.F., and A.J. were responsible for the study conception and design and data collection writing of the manuscript; P.B., A.J., and M.H. were responsible for data queries and quality checks and evaluations; patients were treated by all authors except M.H.; and all authors contributed to the conception of the study, data acquisition, writing, and approval of the final manuscript.
Conflict-of-interest disclosure: P.B. declares research grants from Neovii, Riemser, Medac, and Bristol Myers Squibb (BMS) (to institution); membership in advisory boards for Novartis, Celgene, Amgen, Medac, and Servier (personal and to institution); received speaker fees from Miltenyi, Jazz, Riemser, Novartis, and Amgen (to institution); and declares a patent and royalties from Medac. C.R. declares honoraria for lectures, consultancy, and advisory board participation by Amgen, BMS, Celgene, Novartis, Jansen, and Pfizer. F.A.A. declares honoraria from Novartis, Gilead, BMS, Janssen, Takeda, Medac, and Mallinckrodt and research support from Mallinckrodt. C.D.B. declares honoraria for lectures, consultancy, and advisory board participation by Amgen, BMS, Novartis, Gilead, Jazz, and Pfizer. H.B. acknowledges research support from Bayer, Chugai, Erydel, Miltenyi, Polyphor, Sandoz-Hexal (a Novartis company), Stage (a Celgene company), Terumo BCT, and Uniqure; honoraria and speaker fees from Chugai, Fresenius, Genzyme, Kiadis, Medac, Miltenyi, Novartis, Sandoz-Hexal, and Terumo BCT; consultancy and membership in advisory boards for Boehringer-Ingelheim, Celgene (a BMS company), Genzyme, Medac, Novartis, Sandoz-Hexal, Stage, and Terumo BCT; royalties from Medac for the MSC product not discussed in this manuscript; and stock ownership in Healthineers. G.C. and/or the study group have received research support from SHIRE, JazzPharma, Servier, SigmaTau, Amgen, and Novartis and has received honoraria or travel support from Servier, Novartis, and JazzPharma. H.E. declares honoraria from BMS/Celgene, Janssen, Amgen, Takeda, Sanofi, GSK, and Novartis; consultancy and advisory board participation with Amgen, BMS/Celgene, Janssen, Takeda, Sanofi, GSK, and Novartis; research funding by BMS/Celgene, Janssen, Amgen, GSK, and Sanofi; and travel support by BMS/Celgene, Janssen, Amgen, Takeda, Sanofi, GSK, and Novartis. U.H. declares honoraria and consultancies from BMS/Celgene, Kite/Gilead, and Novartis. C.K. declares advisory board participation from AbbVie, Amgen, BMS, EusaPharm, GSK, Janssen, Kite/Gilead, Medigene, Novartis, Roche, Sanofi, Takeda, Pfizer, and Incyte. A.K. declares honoraria for consultancy or advisory board participation from BMS and Novartis. R.M. has received consulting fees from Novartis Pharma. F.M. declares a research grant from AstraZeneca and advisory boards and honoraria from AbbVie, AstraZeneca, BMS, Janssen, Kite/Gilead, and Novartis. I.M. declares research grants from Medac (to institution) and has received fees for participation in the advisory board for Novartis, Neovii, Medac, and Servier (personal and to institution). O.P. has received honoraria or travel support from Gilead, Jazz, MSD, Novartis, Pfizer, and Therakos; has received research support from Incyte and Priothera; and is a member of advisory boards to Equillium Bio, Jazz, Gilead, Novartis, MSD, Omeros, Priothera, Shionogi, and SOBI. P.-G.S. declares advisory board participation for Novartis and bluebird bio. A.v.S. declares honoraria for lectures, consultancy, or advisory board participation from Jazz Pharmaceuticals, Amgen, Novartis, Clinigen, and Pfizer. A.J. declares speaker fees from Novartis and advisory board participation with bluebird bio and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Peter Bader, Division for Stem Cell Transplantation, Immunology, and Intensive Care Medicine, Department for Children and Adolescents, University Hospital, Goethe University, Theodor Stern Kai 7, D-60590 Frankfurt, Germany; e-mail: peter.bader@kgu.de.
References
Author notes
∗T.F. and A.J. contributed equally to this study.
Data are available on request from the corresponding author, Peter Bader (peter.bader@kgu.de).
The full-text version of this article contains a data supplement.