Key Points
Pola-DA-EPCH-R has an acceptable safety profile that is similar to that of previously published results of the standard DA-EPOCH-R regimen.
Substituting vincristine with Pola did not appear to affect the ability to escalate chemotherapy dosing beyond dose level 1.
Abstract
The POLARIX trial demonstrated the superiority of polatuzumab vedotin (Pola) over vincristine in the rituximab-cyclophosphamide-doxorubicin-vincristine-prednisone (R-CHOP) regimen for large B-cell lymphomas, but it is unknown whether Pola can be safely incorporated into intensified regimens (eg, dose-adjusted [DA]–EPOCH-R [etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab]) typically used for the highest risk histologies. This was a single-center, open-label, prospective clinical trial of 6 cycles of Pola-DA-EPCH-R (vincristine omitted) in aggressive large B-cell lymphomas. The primary end point was to estimate the safety of Pola-DA-EPCH-R as measured by the rate of dose-limiting toxicities (DLTs) in the first 2 cycles with prespecified suspension rules. Secondary and exploratory end points included efficacy and correlation with circulating tumor DNA (ctDNA) levels. We enrolled 18 patients on study, and with only 3 DLTs observed, the study met its primary end point for safety. There were 5 serious adverse events, including grade 3 febrile neutropenia (3, 17%), grade 3 colonic perforation in the setting of diverticulitis, and grade 5 sepsis/typhlitis. Among 17 evaluable patients, the best overall response rate was 100%, and the complete response rate was 76%. With a median follow-up of 12.9 months, 12-month event-free survival was 72%, and 12-month overall survival was 94%. No patient with undetectable ctDNA at the end of treatment has relapsed to date. Using Pola to replace vincristine in the DA-EPOCH-R regimen met its primary safety end point. These data support the further evaluation and use of this approach in histologies where the potential benefit of both an intensified regimen and Pola may be desired. This trial was registered at www.clinicaltrials.gov as #NCT04231877.
Introduction
R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) has been the standard regimen for untreated large B-cell lymphomas for nearly 2 decades.1-3 Unfortunately, this regimen fails in ∼40% of patients. Efforts to improve on R-CHOP, including the addition of targeted agents (lenalidomide,4 bortezomib,5 and ibrutinib6) and alternate CD20 monoclonal antibodies (obinutuzumab),7 have not clearly shown benefit in phase 3 randomized settings.
Polatuzumab vedotin (Pola) is an anti-CD79b monoclonal antibody conjugated to the microtubule toxin monomethyl auristatin E. Pola has shown activity as monotherapy and in combination for relapsed and refractory B-cell lymphomas, including diffuse large B-cell lymphoma (DLBCL).8,9 The POLARIX study was a randomized, double-blind, placebo-controlled international phase 3 study that compared R-CHOP with Pola-R-CHP.10 This study recently met its primary end point for progression-free survival at 2 years, favoring the Pola-R-CHOP arm (hazard ratio, 0.73 by Cox regression; 95% confidence interval [CI], 0.57-0.95; P = .02). However, the relevance of these data to high-risk entities such as high-grade B-cell lymphoma (HGBCL) with or without MYC/BCL-2 translocation11 and primary mediastinal B-cell lymphoma (PMBCL)12 that are more commonly treated with an intensified chemotherapy backbone as dose-adjusted (DA)–EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) are unknown.
To address this question, we hypothesized that substituting Pola for vincristine as part of the DA-EPOCH-R regimen would be feasible, safe, and effective for untreated patients with large B-cell lymphomas. We conducted a prospective trial evaluating Pola-DA-EPCH-R (Pola with etoposide, prednisone, cyclophosphamide, doxorubicin, and rituximab), and herein, we report the results of the primary analysis (www.clinicaltrials.gov, #NCT04231877).
Methods
Trial conduct
This was a single-center, open-label, investigator-initiated clinical trial of Pola-DA-EPCH-R in aggressive large B-cell lymphomas. The protocol was approved by the institutional review board at our institution. The trial was conducted in accordance with the good clinical practice guidelines of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use and the principles of the Declaration of Helsinki.13 All the patients provided written, informed consent. This was an investigator-initiated study, with research support provided by Genentech. Our institutional data safety monitoring committee reviews safety data on a regular basis. The first draft of the manuscript was written by the first author. All the authors reviewed the data and contributed to the preparation of the final version of the manuscript. The authors vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol and statistical analysis plan.
Patients
Eligible patients were at least 18 years of age, had no prior treatment for lymphoma (however, prephase corticosteroids were allowed if urgently needed for symptom control), measurable disease of at least 1 cm in extranodal sites and 1.5 cm in nodal sites, an Eastern Cooperative Oncology Group performance status of 0 to 2, and adequate hematologic and organ function. Patients with a history of HIV, hepatitis B, or hepatitis C were permitted, provided that the viral load was undetectable at the time of enrolment.
Eligible patients would have been treated with DA-EPOCH-R as a standard-of-care regimen for their large B-cell lymphoma, which could have included the following: HGBCL with MYC and BCL2 and/or BCL6 translocations; HGBCL not otherwise specified (NOS); DLBCL NOS; PMBCL; T-cell/histiocyte-rich large B-cell lymphoma; Epstein-Barr virus-associated DLBCL; anaplastic lymphoma kinase (ALK) positive large B-cell lymphoma; and B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and classical Hodgkin lymphoma. Details of trial methods, including eligibility criteria, are provided in the protocol in the supplemental Appendix.
Treatment and assessments
The experimental medication Pola was given at the dose of 1.8 mg/kg on day 1 of each of the 6 21-day cycles concurrently with DA-EPCH-R as outlined in previous studies but without the use of vincristine12 and summarized in supplemental Table 1. Dose adjustment of cyclophosphamide, etoposide, and doxorubicin followed the standard published algorithm based on neutrophil and platelet nadir. Pola was not escalated with the other drugs but could be reduced for toxicity and could not be reescalated if patients were treated below the starting dose level due to toxicity, as summarized in supplemental Table 2. All patients received granulocyte colony-stimulating factor support. Viral, pneumocystis, and fungal prophylaxis were strongly recommended per institutional standard. Bacterial prophylaxis when the absolute neutrophil count was <500 was also strongly recommended. All patients had a baseline positron emission tomography (PET)/computed tomography (CT), an option for an interim scan after cycle 2, and an end of treatment (EOT) PET 4 to 6 weeks after completion of study therapy. Treatment responses were collected per Lugano criteria14 and adverse events per Common Terminology Criteria for Adverse Events version 5.0 through EOT visit. Additional follow-up beyond the EOT visit was not specified by protocol and was up to the institutional standard of care.
End points and statistical considerations
The primary objective of the study was to estimate the safety and tolerability of combining Pola-DA-EPCH-R (vincristine omitted) using Common Terminology Criteria for Adverse Events version 5.0 through the EOT. The safety analysis was performed on all patients who received at least 1 dose of any of the study medications. The primary analysis was performed after all enrolled patients had completed study therapy. We defined the primary end point as excluding the lower level of a 1-sided 80% CI when dose-limiting toxicity (DLT) exceeds 20%. A DLT is defined in the supplement and was in general defined as a grade 3 or higher nonhematologic toxicity that lasted >72 hours or a toxicity-based delay of >14 days within the first cycle. Based on this, we planned to treat 9 patients at this dose and then evaluate with stopping rules for toxicity (supplement). If no safety signals were seen, we then planned to enroll 18 total patients in this study, and if ≤5 DLTs were observed, the regimen would be deemed sufficiently safe.
Secondary and exploratory objectives included gaining a preliminary assessment of the potential efficacy of this regimen using Lugano criteria.14 This was defined as the percentage of patients that achieve a complete remission (CR) after study treatment, event-free survival (EFS; with an event defined as death, progression, or initiation of new antilymphoma therapy), and overall survival (OS). A simple binary proportion was used to estimate CR, and the Kaplan-Meier method was used to estimate EFS and OS. An exploratory objective analyzed the proportion of patients treated at each dose level of EPCH by cycle of administration as well as correlation with circulating tumor DNA (ctDNA) levels15,16 and response by PET/CT on interim and EOT scans; these exploratory analyses were done using appropriate regression methods.
PhasED-seq for tracking ctDNA and assessing MRD
Phased variant enrichment and detection sequencing (PhasED-seq) was performed by Foresight Diagnostics Inc, as previously described,15 with exceptions noted here. PhasED-seq is a highly sensitive, tumor-informed approach for assessing minimal residual disease (MRD) by tracking somatic phased variants. Whole blood was collected in K2 EDTA tubes and processed within 6 hours or in Streck blood collection tubes and processed within 72 hours. The tubes were centrifuged twice at 1600g for 10 minutes at room temperature. After centrifugation, plasma was stored at −80°C in 1.8 mL aliquots until cell-free DNA (cfDNA) isolation. Plasma-depleted whole blood was stored at −80°C until DNA isolation from leukocytes. cfDNA was extracted from 3 to 10 mL of plasma (median, 6 mL) using the QIAamp Circulating Nucleic Acid Kit (Qiagen), according to the manufacturer’s instructions. After isolation, cfDNA was quantified using the Qubit dsDNA High Sensitivity Kit (Thermo Fisher Scientific) and the High Sensitivity Next Generation Sequencing Fragment Analyzer Kit (Agilent). Genomic DNA (gDNA) from matched plasma-depleted whole blood was extracted using the DNeasy Blood and Tissue Kit (Qiagen), quantified using the Qubit dsDNA High Sensitivity Kit, and fragmented to a target size of 170 base pairs using the Covaris S2 sonicator. Fragmented gDNA was purified using the QIAquick PCR Purification Kit (Qiagen). For cfDNA, a median of 80 ng (range, 30-80 ng) was input into library preparation. For gDNA from leukocytes, 80 ng of fragmented gDNA was input into library preparation. After sequencing on NovaSeq 6000 instruments (Illumina), data preprocessing, alignment, identification of phased variants, and detection of MRD were performed as previously described.15
Results
Patients
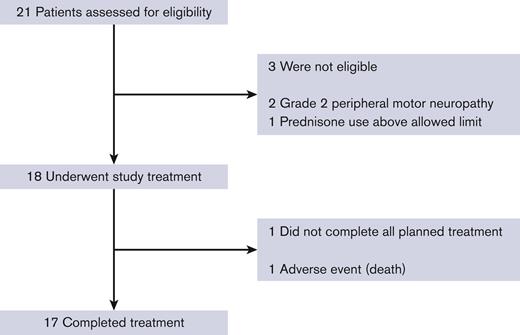
Between 28 October 2020 and 20 November 2021, 18 patients enrolled on the study (Figure 1). Three patients consented and were deemed screening failures because of grade 2 peripheral neuropathy (2) and prior use of high-dose corticosteroids (1). Enrolment ended when the accrual goal of 18 patients was met.
Patient characteristics are presented in Table 1. The median age was 64 years (range, 41-74), with most patients being stage IV (72%) at diagnosis. The median diagnosis to treatment interval was 24 days (range, 10-37 days). Six patients (33%) had HGBCL with MYC and BCL2 and/or BCL6 rearrangements, 4 (22%) had PMBCL, and the rest had DLBCL NOS (44%). Most patients had an elevated lactate dehydrogenase (78%), an International Prognostic Index of 3 or higher (72%), or a MYC rearrangement (53%). Other adverse prognostic features included prephase prednisone use (50%), bulk (39%), and Ki-67 of at least 90% (33%). Among those with DLBCL NOS, high-risk features included composite lymphoma (38%), an International Prognostic Index of 3 or 4 (100%), MYC rearrangement (38%), B symptoms (63%), and elevated lactate dehydrogenase (75%).
Patient characteristics
| Characteristic . | N = 18 . |
|---|---|
| Age, median (range), y | 64 (41-74) |
| Age category, n (%) | |
| <60 y | 7 (39) |
| ≥60 y | 11 (61) |
| Gender, n (%) | |
| Female | 8 (44) |
| Male | 10 (56) |
| Stage at diagnosis, n (%) | |
| I | 1 (6) |
| II | 4 (22) |
| III | 0 (0) |
| IV | 13 (72) |
| Baseline disease characteristics, n (%) | |
| Histology | |
| HGBCL with MYC and BCL2 and/or BCL6 rearrangement (double/triple-hit) | 6 (33) |
| DLBCL NOS | 8 (44) |
| PMBCL | 4 (22) |
| Cell of origin | |
| Germinal center B-cell–like | 13 (93) |
| Activated B-cell–like | 1 (7) |
| B symptoms | 7 (39) |
| Bulky sites (>7.5 cm) | 11 (61) |
| Extranodal disease | 14 (78) |
| Double expressor (if known) | 7 (47) |
| Elevated lactate dehydrogenase | 14 (78) |
| Eastern Cooperative Oncology Group performance status | |
| 0-1 | 18 (100) |
| 2 | 0 (0) |
| MYC rearrangement (n = 17) | 9 (53) |
| International prognostic index | |
| 0-2 | 5 (28) |
| 3-5 | 13 (72) |
| Ki-67 ≥90% (n = 15) | 5 (33) |
| Prephase prednisone use | 9 (50) |
| Diagnosis to treatment interval, median (range), d | 24 (10-37) |
| Maximum dose level achieved, n (%) | |
| 1 | 2 (11) |
| 2 | 7 (39) |
| 3 | 4 (22) |
| 4 | 3 (17) |
| 5 | 2 (11) |
| Pola dose reduction required, n (%) | 1 (6) |
| Characteristic . | N = 18 . |
|---|---|
| Age, median (range), y | 64 (41-74) |
| Age category, n (%) | |
| <60 y | 7 (39) |
| ≥60 y | 11 (61) |
| Gender, n (%) | |
| Female | 8 (44) |
| Male | 10 (56) |
| Stage at diagnosis, n (%) | |
| I | 1 (6) |
| II | 4 (22) |
| III | 0 (0) |
| IV | 13 (72) |
| Baseline disease characteristics, n (%) | |
| Histology | |
| HGBCL with MYC and BCL2 and/or BCL6 rearrangement (double/triple-hit) | 6 (33) |
| DLBCL NOS | 8 (44) |
| PMBCL | 4 (22) |
| Cell of origin | |
| Germinal center B-cell–like | 13 (93) |
| Activated B-cell–like | 1 (7) |
| B symptoms | 7 (39) |
| Bulky sites (>7.5 cm) | 11 (61) |
| Extranodal disease | 14 (78) |
| Double expressor (if known) | 7 (47) |
| Elevated lactate dehydrogenase | 14 (78) |
| Eastern Cooperative Oncology Group performance status | |
| 0-1 | 18 (100) |
| 2 | 0 (0) |
| MYC rearrangement (n = 17) | 9 (53) |
| International prognostic index | |
| 0-2 | 5 (28) |
| 3-5 | 13 (72) |
| Ki-67 ≥90% (n = 15) | 5 (33) |
| Prephase prednisone use | 9 (50) |
| Diagnosis to treatment interval, median (range), d | 24 (10-37) |
| Maximum dose level achieved, n (%) | |
| 1 | 2 (11) |
| 2 | 7 (39) |
| 3 | 4 (22) |
| 4 | 3 (17) |
| 5 | 2 (11) |
| Pola dose reduction required, n (%) | 1 (6) |
Treatment exposure and safety
Eighteen patients received at least 1 cycle of treatment and were evaluable for safety. The most common adverse events are listed in Table 2, with a complete list in the supplemental Table 3. Seventeen patients (94%) completed all study treatment. There were 5 serious adverse events observed, including 1 death from grade 5 sepsis/typhlitis (1, 6%), grade 3 febrile neutropenia (3, 17%), and grade 3 colonic perforation in the setting of diverticulitis (1, 6%). Three DLTs were observed (2 asymptomatic incidentally detected pulmonary emboli and the previously mentioned patient death from sepsis/typhlitis), which were below the prespecified threshold, meeting the study’s primary end point. Grade 3 or 4 adverse events observed in >1 patient include neutropenia (17, 94%), thrombocytopenia (10, 56%), anemia (7, 39%), oral mucositis (4, 22%), thromboembolic events (4, 22%), febrile neutropenia (3, 17%), hyperglycemia (3, 17%), abdominal pain (2, 11%), and hypokalemia (2, 11%) (Table 2). Grade 1 peripheral sensory neuropathy was common (8, 44%), but no grade 2+ neuropathy was observed. A total of 6 patients (33%) experienced a thromboembolic event. Two of these were grade 2 deep vein thrombosis (DVT), 1 of which was in a patient with prior DVT history, and another was asymptomatic and incidentally found on a restaging scan. Three patients were diagnosed with grade 3 pulmonary emboli, all of which were asymptomatic and found incidentally on restaging scans. One of these patients had a baseline history of a T10 compression fracture due to underlying lymphoma, which limited mobility compared with the prediagnosis baseline. In addition, 1 patient was diagnosed with a right atrial thrombus, which was found incidentally during a restaging scan, and this patient was found to have previously undiagnosed atrial flutter.
Adverse events
| Adverse event . | Any grade . | % . | Grade 1 . | % . | Grade 2 . | % . | Grade 3-5 . | % . | Grade 3 . | % . | Grade 4 . | % . | Grade 5 . | % . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anemia | 18 | 100 | 1 | 6 | 10 | 56 | 7 | 39 | 7 | 39 | 0 | 0 | 0 | 0 |
| Neutropenia | 17 | 94 | 0 | 0 | 0 | 0 | 17 | 94 | 0 | 0 | 17 | 94 | 0 | 0 |
| Thrombocytopenia | 17 | 94 | 4 | 22 | 3 | 17 | 10 | 56 | 6 | 33 | 4 | 22 | 0 | 0 |
| Constipation | 15 | 83 | 9 | 50 | 6 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mucositis oral | 14 | 78 | 6 | 33 | 4 | 22 | 4 | 22 | 4 | 22 | 0 | 0 | 0 | 0 |
| Elevated ALT | 13 | 72 | 10 | 56 | 2 | 11 | 1 | 6 | 0 | 0 | 1 | 6 | 0 | 0 |
| Nausea | 12 | 67 | 9 | 50 | 2 | 11 | 1 | 6 | 1 | 6 | 0 | 0 | 0 | 0 |
| Fatigue | 11 | 61 | 5 | 28 | 6 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 8 | 44 | 2 | 11 | 5 | 28 | 1 | 6 | 1 | 6 | 0 | 0 | 0 | 0 |
| Elevated AST | 8 | 44 | 7 | 39 | 0 | 0 | 1 | 6 | 0 | 0 | 1 | 6 | 0 | 0 |
| Peripheral sensory neuropathy | 8 | 44 | 8 | 44 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypokalemia | 7 | 39 | 0 | 0 | 5 | 28 | 2 | 11 | 2 | 11 | 0 | 0 | 0 | 0 |
| Edema limbs | 7 | 39 | 7 | 39 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thromboembolic event | 6 | 33 | 0 | 0 | 2 | 11 | 4 | 22 | 4 | 22 | 0 | 0 | 0 | 0 |
| Sore throat | 6 | 33 | 6 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Headache | 6 | 33 | 5 | 28 | 1 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pain in extremity | 6 | 33 | 5 | 28 | 1 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Urinary frequency | 6 | 33 | 6 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Abdominal pain | 5 | 28 | 3 | 17 | 0 | 0 | 2 | 11 | 2 | 11 | 0 | 0 | 0 | 0 |
| Back pain | 5 | 28 | 5 | 28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dysgeusia | 5 | 28 | 5 | 28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypophosphatemia | 5 | 28 | 0 | 0 | 5 | 28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fever | 5 | 28 | 4 | 22 | 1 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Insomnia | 5 | 28 | 4 | 22 | 1 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sinus bradycardia | 5 | 28 | 4 | 22 | 1 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyperglycemia | 4 | 22 | 0 | 0 | 1 | 6 | 3 | 17 | 3 | 17 | 0 | 0 | 0 | 0 |
| Hypertension | 4 | 22 | 2 | 11 | 1 | 6 | 1 | 6 | 1 | 6 | 0 | 0 | 0 | 0 |
| Anorexia | 4 | 22 | 3 | 17 | 1 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dyspepsia | 4 | 22 | 2 | 11 | 2 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nail changes | 4 | 22 | 4 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Febrile neutropenia | 3 | 17 | 0 | 0 | 0 | 0 | 3 | 17 | 3 | 17 | 0 | 0 | 0 | 0 |
| Colonic perforation | 1 | 6 | 0 | 0 | 0 | 0 | 1 | 6 | 1 | 6 | 0 | 0 | 0 | 0 |
| Hypomagnesemia | 1 | 6 | 0 | 0 | 0 | 0 | 1 | 6 | 1 | 6 | 0 | 0 | 0 | 0 |
| Supraventricular tachycardia | 1 | 6 | 0 | 0 | 0 | 0 | 1 | 6 | 1 | 6 | 0 | 0 | 0 | 0 |
| Sepsis | 1 | 6 | 0 | 0 | 0 | 0 | 1 | 6 | 0 | 0 | 0 | 0 | 1 | 6 |
| Typhlitis | 1 | 6 | 0 | 0 | 0 | 0 | 1 | 6 | 0 | 0 | 0 | 0 | 1 | 6 |
| Elevated total bilirubin | 1 | 6 | 0 | 0 | 0 | 0 | 1 | 6 | 0 | 0 | 1 | 6 | 0 | 0 |
| Adverse event . | Any grade . | % . | Grade 1 . | % . | Grade 2 . | % . | Grade 3-5 . | % . | Grade 3 . | % . | Grade 4 . | % . | Grade 5 . | % . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anemia | 18 | 100 | 1 | 6 | 10 | 56 | 7 | 39 | 7 | 39 | 0 | 0 | 0 | 0 |
| Neutropenia | 17 | 94 | 0 | 0 | 0 | 0 | 17 | 94 | 0 | 0 | 17 | 94 | 0 | 0 |
| Thrombocytopenia | 17 | 94 | 4 | 22 | 3 | 17 | 10 | 56 | 6 | 33 | 4 | 22 | 0 | 0 |
| Constipation | 15 | 83 | 9 | 50 | 6 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mucositis oral | 14 | 78 | 6 | 33 | 4 | 22 | 4 | 22 | 4 | 22 | 0 | 0 | 0 | 0 |
| Elevated ALT | 13 | 72 | 10 | 56 | 2 | 11 | 1 | 6 | 0 | 0 | 1 | 6 | 0 | 0 |
| Nausea | 12 | 67 | 9 | 50 | 2 | 11 | 1 | 6 | 1 | 6 | 0 | 0 | 0 | 0 |
| Fatigue | 11 | 61 | 5 | 28 | 6 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 8 | 44 | 2 | 11 | 5 | 28 | 1 | 6 | 1 | 6 | 0 | 0 | 0 | 0 |
| Elevated AST | 8 | 44 | 7 | 39 | 0 | 0 | 1 | 6 | 0 | 0 | 1 | 6 | 0 | 0 |
| Peripheral sensory neuropathy | 8 | 44 | 8 | 44 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypokalemia | 7 | 39 | 0 | 0 | 5 | 28 | 2 | 11 | 2 | 11 | 0 | 0 | 0 | 0 |
| Edema limbs | 7 | 39 | 7 | 39 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thromboembolic event | 6 | 33 | 0 | 0 | 2 | 11 | 4 | 22 | 4 | 22 | 0 | 0 | 0 | 0 |
| Sore throat | 6 | 33 | 6 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Headache | 6 | 33 | 5 | 28 | 1 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pain in extremity | 6 | 33 | 5 | 28 | 1 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Urinary frequency | 6 | 33 | 6 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Abdominal pain | 5 | 28 | 3 | 17 | 0 | 0 | 2 | 11 | 2 | 11 | 0 | 0 | 0 | 0 |
| Back pain | 5 | 28 | 5 | 28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dysgeusia | 5 | 28 | 5 | 28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypophosphatemia | 5 | 28 | 0 | 0 | 5 | 28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fever | 5 | 28 | 4 | 22 | 1 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Insomnia | 5 | 28 | 4 | 22 | 1 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sinus bradycardia | 5 | 28 | 4 | 22 | 1 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyperglycemia | 4 | 22 | 0 | 0 | 1 | 6 | 3 | 17 | 3 | 17 | 0 | 0 | 0 | 0 |
| Hypertension | 4 | 22 | 2 | 11 | 1 | 6 | 1 | 6 | 1 | 6 | 0 | 0 | 0 | 0 |
| Anorexia | 4 | 22 | 3 | 17 | 1 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dyspepsia | 4 | 22 | 2 | 11 | 2 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nail changes | 4 | 22 | 4 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Febrile neutropenia | 3 | 17 | 0 | 0 | 0 | 0 | 3 | 17 | 3 | 17 | 0 | 0 | 0 | 0 |
| Colonic perforation | 1 | 6 | 0 | 0 | 0 | 0 | 1 | 6 | 1 | 6 | 0 | 0 | 0 | 0 |
| Hypomagnesemia | 1 | 6 | 0 | 0 | 0 | 0 | 1 | 6 | 1 | 6 | 0 | 0 | 0 | 0 |
| Supraventricular tachycardia | 1 | 6 | 0 | 0 | 0 | 0 | 1 | 6 | 1 | 6 | 0 | 0 | 0 | 0 |
| Sepsis | 1 | 6 | 0 | 0 | 0 | 0 | 1 | 6 | 0 | 0 | 0 | 0 | 1 | 6 |
| Typhlitis | 1 | 6 | 0 | 0 | 0 | 0 | 1 | 6 | 0 | 0 | 0 | 0 | 1 | 6 |
| Elevated total bilirubin | 1 | 6 | 0 | 0 | 0 | 0 | 1 | 6 | 0 | 0 | 1 | 6 | 0 | 0 |
All grade 3-5 adverse events are listed and grade 1-2 adverse events occurring in >20% of patients are listed here. The grade 5 sepsis and typhlitis occurred in the same patient. All adverse events are listed in the supplement.
ALT, alanine transaminase; AST, aspartate transferase; CTCAE, Common Terminology Criteria for Adverse Events; N, no; Y, yes.
There was 1 grade 2 COVID-19 infection seen in patients being treated on the study. The patient was minimally symptomatic, received monoclonal antibody therapy (casirivimab and imdevimab), and did not require supplemental oxygen or experience hospitalization related to COVID-19. This patient did not experience a treatment modification or delay related to the infection.
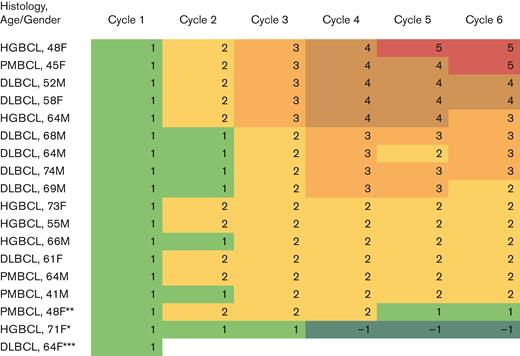
Pola dosing was reduced to 1.4 mg/kg in only 1 patient because of grade 4 thrombocytopenia after treatment at dose level 1. Among those with at least 2 cycles of treatment, 94% were able to increase chemotherapy to at least dose level 2, and 53% to dose level 3 (Figure 2). The only treatment delay was seen in the patient with a grade 3 perforation of a colonic diverticula, which required a treatment delay of 12 days before completing all expected study therapy.
Heat map displaying each patient by histology, age, and gender with each column displaying the chemotherapy dose level achieved by cycle. ∗ indicates that patient had a platelet nadir <25 000 cells per μL requiring dose reduction to DL1. ∗∗ indicates that patient had a grade 3 colonic perforation in the setting of diverticulitis requiring interruption of chemotherapy for surgery. The subsequent cycle was delayed 12 days and dose level decreased to DL1 as discretion of investigator while maintaining the standard Pola dose. ∗∗∗ indicates that patient died of sepsis during cycle 1. F, female; M, male.
Heat map displaying each patient by histology, age, and gender with each column displaying the chemotherapy dose level achieved by cycle. ∗ indicates that patient had a platelet nadir <25 000 cells per μL requiring dose reduction to DL1. ∗∗ indicates that patient had a grade 3 colonic perforation in the setting of diverticulitis requiring interruption of chemotherapy for surgery. The subsequent cycle was delayed 12 days and dose level decreased to DL1 as discretion of investigator while maintaining the standard Pola dose. ∗∗∗ indicates that patient died of sepsis during cycle 1. F, female; M, male.
Efficacy
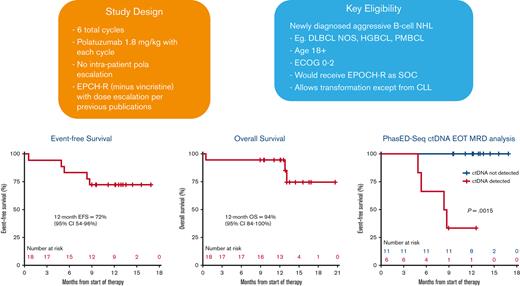
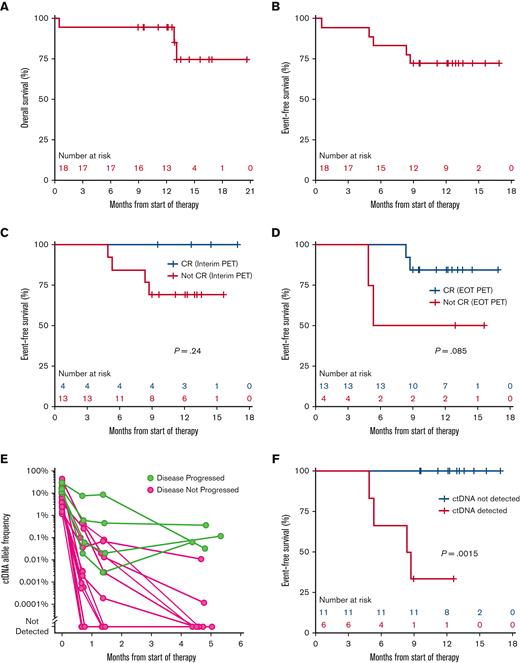
Seventeen patients had at least 1 response assessment and were evaluable for efficacy. The best overall response rate was 100%, including 76% in complete response. Overall, at the end of study treatment, responses included 13 CRs (76%), 3 partial responses (18%), and 1 progression event (6%) in a patient with a PR based on PET/CT after cycle 2. A reduction in tumor burden was seen in all patients (supplemental Figure 1). At the time of data cutoff, patients had a median follow-up of 12.9 months, 12-month EFS was 72% (95% CI, 54-96) and 12-month OS was 94% (95% CI, 84-100) (Figure 3A-B). Progression was more likely in those in PR at interim PET and EOT PET than in those who achieved a CR at those time points though this was not statistically significant (Figure 3C-D). Of the 4 patients experiencing disease progression, all (100%) received additional antilymphoma therapy, including 2 (50%) receiving chimeric antigen receptor T-cell therapy.
(A) EFS for the overall study population for the start of therapy. (B) OS for overall study population from the start of therapy. (C) EFS stratified by interim PET results. (D) EFS stratified by EOT PET results. (E) Spider plot of ctDNA allele frequency from start of therapy through EOT stratified by patients with or without known progressive disease. (F) EFS stratified by detection of ctDNA at the EOT.
(A) EFS for the overall study population for the start of therapy. (B) OS for overall study population from the start of therapy. (C) EFS stratified by interim PET results. (D) EFS stratified by EOT PET results. (E) Spider plot of ctDNA allele frequency from start of therapy through EOT stratified by patients with or without known progressive disease. (F) EFS stratified by detection of ctDNA at the EOT.
ctDNA detection by PhasED-seq predicts clinical outcomes
As an exploratory correlative for the therapeutic efficacy of this regimen, we used PhasED-seq15 to examine serial ctDNA measurements at baseline, after cycle 1, after cycle 2, and EOT. For patients that have been event-free, ctDNA levels rapidly dropped after cycle 1, and continued to drop after cycle 2 (Figure 3E). Of note, at the EOT, the ctDNA levels of patients remaining event-free became fully separated from the ctDNA levels in patients experiencing events (Figure 3E). Accordingly, clearance of ctDNA at the end of therapy significantly predicted EFS (P = .0015). To date, no patients with undetectable ctDNA at end of therapy have subsequently relapsed (Figure 3F), whereas 2 patients who achieved CR by EOT PET and were ctDNA-positive at the end of therapy have relapsed (compare Figure 3D-F). We also separately assessed serial plasma samples for early molecular response16 (at least 2 log reduction in ctDNA after 1 cycle) and major molecular response16 (at least 2.5 log reduction in ctDNA after 2 cycles). Performing a landmark analysis from the time of ctDNA assessment, improvement in EFS is seen in patients achieving an early molecular response (P = .05), major molecular response (P = .15), or clearance of ctDNA at EOT (P = .0015) (Figure 4A-C).
Discussion
This is the first study to examine the safety of substituting Pola for vincristine with an intensive infusional chemotherapy regimen for untreated patients with aggressive large B-cell lymphomas. This study met its primary end point for safety and had a toxicity profile that was similar to what was seen with DA-EPOCH-R.17 As in Pola-R-CHP, we similarly noted increased diarrhea over what would be expected with EPOCH-R, but with far less peripheral sensory neuropathy, including none over grade 1. Importantly, the potentially higher than expected rate of grade 3 to 4 thrombocytopenia compared with EPOCH-R did not appear to impair escalation of the chemotherapy dose level in most patients. All but 1 patient who received >1 cycle of treatment was able to escalate to dose level 2, similar to those in other studies with EPOCH-R.17
The safety data from our trial address 1 of the key limitations of the POLARIX study, namely the appropriateness of a CHOP-like chemotherapy foundation for large B-cell lymphomas, where intensified approaches are commonly employed. In the POLARIX study, 6.8% of patients had double- or triple-hit large B-cell lymphoma, which may have affected the similar equivalence seen in germinal center B-cell subtype lymphomas as a whole.10 However, any comparisons between the 2 arms are underpowered for any meaningful conclusions. Although patients with double/triple-hit lymphoma did numerically worse with Pola-R-CHOP vs R-CHOP,10 the sample size was small and potentially biased, given that expert guidelines in many parts of the world recommend an intensified frontline regimen over R-CHOP for these patients.
Until now, improvements to the EPOCH-R regimen have proven to be challenging. Recently, the addition of venetoclax was associated with increased cytopenias, treatment delays, and death when compared with DA-EPOCH-R.18,19 Although we observed 1 patient death due to sepsis and 1 treatment delay of 12 days due to colonic perforation in the setting of diverticulitis, significant toxicities, dose reductions, or delays that would potentially impair the effectiveness of this regimen were not seen. Prior studies of Pola-R-CHP and DA-EPOCH-R would suggest a treatment-related mortality rate of 2% to 3%.10,17 With our limited sample size and 1 patient death, this is numerically higher in this regimen (6%). We also note that 6 patients experienced a thromboembolic event, which included DVT (2), pulmonary embolism (3), and atrial thrombus (1). All of these were incidentally found on restaging scans, except for 1 DVT in a patient with a prior history of DVT. Of note, our institution has recently begun using artificial intelligence–assisted radiology reads to help detect pulmonary emboli, which may have contributed to the increase in asymptomatic venous thromboembolism. However, we are unable to ascertain the role of artificial intelligence in detecting the events that occurred in the individual patients treated in our study. We also noted higher rates of grade 3+ oral mucositis with our regimen compared with historical data (22% vs 8%).17 With the above notable exceptions, the safety profile of Pola-DA-EPCH-R appears comparable to previously published reports of EPOCH-R17 and much improved over the previous effort to combine a novel agent (venetoclax18) with an intensive upfront regimen. However, since the completion of our initial 18-patient study, the protocol has been expanded to allow for 50 total patients to better characterize the safety and efficacy of this regimen.
Because the primary end point of our trial was to rapidly assess the safety of Pola-DA-EPCH-R, we allowed a mix of histologies where an intensified regimen could be considered. The intent of this trial was not to estimate efficacy, though the high CR rate and favorable progression-free survival remain encouraging. Our study does not attempt to address any comparative questions between intensified and standard regimens.
To better understand the kinetics and relevance of tumor clearance on outcomes, our study also examined the potential of highly sensitive ctDNA monitoring relative to standard-of-care fluorodeoxyglucose (18) PET. We found that patients who had undetectable ctDNA at EOT were at significantly lower risk of recurrence, whereas those with residual ctDNA at EOT nearly all recurred. In contrast, interim and EOT PET were less effective at predicting disease recurrence. Although these will need to be confirmed with additional follow-up as well as larger data sets, the very high positive and negative predictive values of ctDNA MRD using PhasED-seq at EOT in this study with Pola-DA-EPCH-R are fully consistent with those described for R-CHOP by Kurtz et al.15 These results provide provocative clues about how ctDNA MRD could reflect clinical responses and foreshadow changes in surrogate end points of therapeutic efficacy. It may also allow for studies to examine abbreviated chemotherapy regimens in those with early ctDNA clearance, as well as early intervention for patients with detectable ctDNA at EOT with second-line treatments, such as CD19-directed chimeric antigen receptor T-cell therapy.20
In conclusion, the use of Pola at 1.8 mg/kg to replace vincristine in the DA-EPOCH-R regimen appears safe and well tolerated and has met its primary end point for safety. These safety data support the further evaluation and potential use of this approach in histologies, where the potential benefit of both an intensified regimen and Pola may be desired.
Authorship
Contribution: R.C.L., C.P., C.S.U., E.H.W.III, S.D.S., M. Shadman, S.O., M. Shelby, S.K., and K.V. performed the research; D.M.K., A.A.A., J.J.C., G.J.H., and A.S. contributed vital new reagents or analytical tools; R.C.L., K.M., S.L., and H.R. collected data; R.C.L., D.M.K., A.A.A., J.J.C., G.J.H., A.S., J.M.V., and A.K.G. analyzed and interpreted data; J.M.V., T.G., J.J.C., G.J.H., and A.S. performed statistical analysis; and R.C.L. and A.K.G. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: R.C.L. received research funding from TG Therapeutics, Incyte, Bayer, Cyteir, Genentech, Seagen, and Rapt and provided consultancy to the Cancer Study Group and Seagen. C.S.U. provided consultancy to Genentech. S.D.S. received research funding from ADC Therapeutics, AstraZeneca, Ayala (spouse), Bayer, BeiGene, Bristol Myers Squibb (spouse), De Novo Biopharma, Enterome, Genentech, Ignyta (spouse), Incyte Corporation, Kymera Therapeutics, Merck & Co, MorphoSys, Nanjing Pharmaceuticals Co Ltd, Portola Pharmaceuticals, and Viracta Therapeutics and provided consultancy or is on the advisory boards for ADC Therapeutics, AstraZeneca, BeiGene, Epizyme, Karyopharm, Kite Pharma, Incyte, Numab Therapeutics AG, AbbVie, and Coherus BioSciences (spouse). M. Shadman provides consultancy to and is a member of advisory boards, and steering committees or data safety monitoring committees for AbbVie, Genentech, AstraZeneca, Sound Biologics, Pharmacyclics, BeiGene, Bristol Myers Squibb, MorphoSys/Incyte, TG Therapeutics, Innate Pharma, Kite Pharma, Adaptive Biotechnologies, Epizyme, Eli Lilly, Adaptimmune, Mustang Bio, Regeneron, Merck, Fate Therapeutics, MEI Pharma, and Atara Biotherapeutics and received research funding from Mustang Bio, Celgene, Bristol Myers Squibb, Pharmacyclics, Gilead, Genentech, AbbVie, TG Therapeutics, BeiGene, AstraZeneca, Sunesis, Atara Biotherapeutics, Genmab, MorphoSys/Incyte, and Vincerx. D.M.K. reports PhasED-seq patent; founder, consultancy, and equity for Foresight Diagnostics; and consultancy for Genentech and Roche. A.A.A. reports ownership interest in CiberMed, FortySeven Inc, and Foresight Diagnostics; patent filings related to cancer biomarkers; research funding from Bristol Myers Squibb and Celgene; and paid consultancies from Genentech, Karyopharm, Roche, Chugai, Gilead, and Celgene. J.J.C. reports PhasED-seq patent and founder, employment, and equity with Foresight Diagnostics. G.J.H. reports employment and equity with Foresight Diagnostics and equity with Freenome. A.S. reports employment and equity with Foresight Diagnostics. A.K.G. received research funding from Merck & Co, I-Mab Biopharma, IGM Bio, Takeda, Gilead, AstraZeneca, Agios, Janssen, Bristol Myers Squibb, Seagen, Teva, Genmab; consultancy and honoraria from Incyte, Kite, MorphoSys/Incyte, ADCT, Acrotech, Merck & Co, Karyopharm, Servier, BeiGene, Cellectar, Janssen, Seagen, Epizyme, I-Mab Biopharma, Gilead, Genentech, Lilly, Caribou, and Fresenius-Kabi; and equity ownership in Compliment Corporation. The remaining authors declare no competing financial interests.
Correspondence: Ajay K. Gopal, Fred Hutchinson Cancer Center, 825 Eastlake Ave E, LG-650, Seattle, WA 98109; e-mail: agopal@uw.edu.
References
Author notes
Protocol and inquiries for data requests for collaborative purposes are available on request from the corresponding author, Ajay K. Gopal (agopal@uw.edu).
The full-text version of this article contains a data supplement.