Key Points
Enasidenib, a selective inhibitor of mutant IDH2 enzyme, is an effective treatment option in patients with IDH2mut MDS.
Durable responses occurred with enasidenib after prior HMA therapy and in combination with azacitidine in treatment-naïve patients.
Abstract
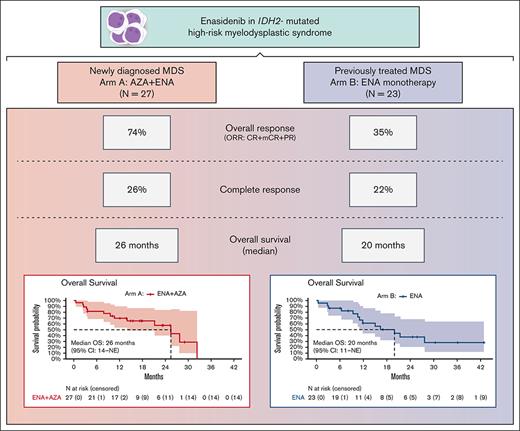
The isocitrate dehydrogenase enzyme 2 (IDH2) gene is mutated in ∼5% of patients with myelodysplastic syndrome (MDS). Enasidenib is an oral, selective, mutant IDH2 inhibitor approved for IDH2-mutated (mIDH2) relapsed/refractory acute myeloid leukemia. We designed a 2-arm multicenter study to evaluate safety and efficacy of (A) the combination of enasidenib with azacitidine for newly diagnosed mIDH2 MDS, and (B) enasidenib monotherapy for mIDH2 MDS after prior hypomethylating agent (HMA) therapy. Fifty patients with mIDH2 MDS enrolled: 27 in arm A and 23 in arm B. Median age of patients was 73 years. The most common adverse events were neutropenia (40%), nausea (36%), constipation (32%), and fatigue (26%). Hyperbilirubinemia from off-target UGT1A1 inhibition occurred in 14% of patients (8%; grades 3 and 4), and IDH-inhibitor–associated differentiation syndrome (IDH-DS) in 8 patients (16%). In the combination arm, the overall response rate (ORR: complete remission [CR] + marrow CR [mCR] + partial remission) was 74%, including 70% composite CR (CRc: CR + mCR). Median time to best response was 1 month (range, 1-4), and a median of 4 cycles was received (1-32). The median overall survival (OS) was 26 months (range, 14 to not reached). In the enasidenib monotherapy cohort after HMA failure, ORR and CRc were both 35% (n = 8), with 22% CR (n = 5). Median time to first response was 27 days, and time to best response was 4.6 months (2.7-7.6 months). A median of 7 cycles was received (range, 1-29), and the median OS was 20 months (range, 11 to not reached). Enasidenib is an effective treatment option for mIDH2 MDS, both in combination with azacitidine for treatment-naïve high-risk MDS, and as a single agent after prior HMA therapy. This trial is registered at www.clinicaltrials.gov as #NCT03383575.
Introduction
Myelodysplastic syndromes (MDSs) represent a diverse spectrum of myeloid stem cell neoplasms characterized by ineffective hematopoiesis, leading to peripheral cytopenias, cytopenia-related complications, and an associated risk of progression to acute myeloid leukemia (AML).1 MDS pathogenesis is driven by recurrent mutations and structural chromosomal alterations, which affects prognostication and outcomes and guides treatment decisions.2,3 Current standard-of-care treatment for genomically defined MDS subsets is restricted to low-risk patients, which includes lenalidomide for deletion 5q and the recent approval of luspatercept for isolated anemia and ring sideroblasts, which is frequently associated with mutations in SF3B1.4
Mutations in the genes encoding isocitrate dehydrogenase (IDH) enzymes 1 and 2 (ie, IDH1 and IDH2) occur in ∼5% of patients with MDS, with the majority affecting the IDH2 R140 residue.5 In an analysis of 1042 patients with MDS at a single institution, 60 patients (6%) had IDH1 or IDH2 mutations detected at presentation; IDH1 was detected in 1.6% and IDH2 was detected in 4.1% of the cohort.6 The presence of an IDH mutation also affects the MDS phenotype; IDH-mutated MDS is associated with a lower absolute neutrophil count, higher platelet count, and higher bone marrow blast percentage at diagnosis, and it occurs most often in the setting of diploid karyotype (60%), trisomy 8 (10%), or other intermediate-risk (23%) cytogenetic abnormalities.7
Enasidenib is an oral, selective, targeted mutant IDH2 inhibitor, approved by the Food and Drug Administration in the United States for the treatment of IDH2-mutated (mIDH2) relapsed or refractory (R/R) AML. In R/R AML, enasidenib is associated with an overall response rate (ORR) of 41%, including 30% complete remission or complete remission with incomplete hematologic recovery rate.8,9 In addition, 42% of patients achieved hematologic improvement of erythroid, neutrophil, and/or platelet counts as defined per International Working Group 2006 MDS criteria.10 Furthermore, 17 patients with mIDH2 MDS were enrolled in the original phase 1 AG221-C-001 study with 9 of 17 patients (53%) responding, including 6 of 13 patients (46%) previously treated with a hypomethylating agent (HMA), and a median overall survival (OS) of 16.9 months.11
To further investigate the role of enasidenib in mIDH2 MDS, we designed a 2-arm clinical trial to evaluate (A) the combination of enasidenib with azacitidine for newly diagnosed patients with mIDH2 MDS and (B) enasidenib monotherapy for patients with R/R mIDH2 MDS following previous HMA therapy.
Methods
This multicenter, investigator-initiated, 2-arm, phase 2 clinical trial (NCT03383575) enrolled patients at 4 institutions within the MDS Clinical Research Consortium (MD Anderson Cancer Center, Weill Cornell, Johns Hopkins, and Cleveland Clinic). The study was designed to assess the safety, tolerability, and efficacy of enasidenib, in combination with azacitidine for higher-risk HMA-naïve mIDH2 MDS (arm A) or as single-agent therapy for patients with R/R mIDH2 MDS following previous HMA therapy (arm B).
The study was approved by the institutional review boards at participating centers and was performed in accordance with the Declaration of Helsinki. Informed consent was obtained from all patients before study participation.
Patients
Patients aged ≥12 years, with an Eastern Cooperative Oncology Group performance status of 0 to 2 and adequate cardiac, renal, and liver function were eligible. Patients were required to have a confirmed diagnosis of mIDH2 myeloid neoplasm including MDS, chronic myelomonocytic leukemia, myelodysplastic syndrome/myeloproliferative neoplasm-unclassifiable (MDS/MPN-U), and refractory anemia with excess blasts in transformation (20%-30% blasts and multilineage dysplasia [WHO 2016]),12,13 with the presence of an IDH2 R140 or R172 mutation. IDH2 mutational status was evaluated locally at each participating institution via next-generation sequencing (NGS).
Study design
The dual primary objectives of the study were to determine the safety and efficacy of enasidenib monotherapy and such therapy in combination with azacitidine for patients with mIDH2 MDS. Secondary objectives included the evaluation of potential markers of antitumor activity and/or resistance, including IDH2 variant allelic frequency (VAF) and the presence of co-occurring mutations, and time-to-event analyses of OS and duration of response (DOR).
Enasidenib 100 mg was administered orally once daily in continuous 28-day cycles. In arm A, patients received enasidenib in combination with azacitidine, 75 mg/m2 per day intravenously or subcutaneously, on days 1 to 7 per treatment cycle. Institutional standards for administration were used. Cycle lengths for arm A were determined by the start of each azacitidine cycle. In June 2020, a protocol modification reduced the duration of enasidenib to days 1 to 14 per cycle for the first 3 cycles. Five patients enrolled after this amendment and received this reduced duration of enasidenib.
Dose interruptions or reductions were allowed in the setting of treatment-related toxicity and followed dosing guidelines from the package inserts of enasidenib and/or azacitidine.
Study assessments
Bone marrow evaluations were performed at screening and at the completion of cycles 2, 4, 6, and every 3 cycles thereafter. Responses were assessed based on modified International Working Group criteria for MDS, including complete remission (CR), partial remission (PR), marrow CR (mCR), and stable disease.10 A stringent definition for CR response was used (ie, bone marrow blasts < 5%, hemoglobin ≥ 11 g/dL, platelets ≥ 100 × 10⁹/L, and neutrophils ≥ 1.0 × 10⁹/L). Hematologic improvement response criteria were also assessed and included erythroid, platelet, and/or neutrophil responses lasting at least 8 weeks.
Adverse events (AEs) were graded according to the Common Terminology Criteria for AEs (NCI CTCAE v 4.0) and were routinely assessed on study and for 28 days following discontinuation.
For each applicable subject, DOR, EFS, and OS were calculated. DOR was defined as the time from the date of initial response (PR or better) to the date of first documented disease progression/relapse or death, whichever occurred first. EFS was defined as the time from the date of treatment initiation to the date of documented treatment failure, relapse from response, or death from any cause, whichever occurred first. OS was defined as the time interval from the date of treatment initiation to death from any cause. In the absence of death, OS was censored at last known follow-up.
Statistical analysis
For the arm A combination, Simon’s optimal 2-stage design was used with a planned enrollment of 50 patients and an interim futility analysis after the first 19 patients, with a target ORR of >50% considered desirable. If ≥7 of the first 19 patients responded, accrual would continue to the second stage. At the completion of enrollment, if ≥21 patients responded, combination therapy would be considered efficacious and worthy of further investigation. For arm B monotherapy, Simon’s optimal 2-stage design was used again, with a planned enrollment of 55 patients and an interim futility analysis after the first 23 patients, with a target ORR of >20% considered desirable. If ≥3 of the first 23 patients responded, accrual would continue to the second stage. At the completion of arm B enrollment, if ≥8 patients responded, enasidenib would be considered efficacious and worthy of further investigation.
No early futility or toxicity stopping criteria were met for either arm. Because of slow patient accrual related to the COVID-19 pandemic, the study supporter mandated early closure of the study in May 2021 after a total of 50 patients had enrolled (arm A: n = 27; arm B: n = 23).
Patient characteristics were summarized using median (range) for continuous variables and frequency (percentage) for categorical variables. The ORR and composite CR rate were estimated, along with the exact 95% confidence interval (CI). The probabilities of OS and DOR were estimated using the Kaplan-Meier method. Cox regression analyses were performed to assess the association between patient characteristics and OS. Hazard ratios (HRs) and the associated 95% CIs were estimated based on the fitted Cox model. P values < .05 were deemed statistically significant. All analyses were conducted using R (R Core Team, 2021).
Results
Patient characteristics
From January 2018 to May 2021, a total of 50 patients enrolled: 27 in arm A and 23 in arm B (supplemental Figure 1). At the time of data cutoff (1 September 2021), median follow-up was 25 months (range, 0.4-43 months). Baseline clinicopathologic characteristics are provided in Table 1. Median age was 73 years in both arms. Five patients (10%) had a diagnosis of chronic myelomonocytic leukemia.
Demographics
| Characteristic∗ . | Overall N = 50 . | Arm A: AZA + ENA n = 27 . | Arm B: ENA n = 23 . |
|---|---|---|---|
| Age, median (range) | 73 (46-83) | 73 (49-83) | 73 (46-82) |
| Gender, n (%) | |||
| Female | 19 (38) | 12 (44) | 7 (30) |
| Male | 31 (62) | 15 (56) | 16 (70) |
| Subtype, n (%) | |||
| RAEB-T (AML-MRC) | 3 (6.0) | 1 (3.7) | 2 (8.7) |
| CMML | 5 (10) | 2 (7.4) | 3 (13) |
| MDS-EB1 | 10 (20) | 7 (26) | 3 (13) |
| MDS-EB2 | 17 (34) | 10 (37) | 7 (30) |
| MDS-MLD | 12 (24) | 4 (15) | 8 (35) |
| MDS/MPN-U | 1 (2.0) | 1 (3.7) | — |
| MPN/MDS | 2 (4.0) | 2 (7.4) | — |
| R-IPSS, n (%) | |||
| Intermediate | 14 (28) | 6 (22) | 8 (35) |
| Low | 6 (12) | 3 (11) | 3 (13) |
| High | 19 (38) | 12 (44) | 7 (30) |
| Very high | 11 (22) | 6 (22) | 5 (22) |
| Cytogenetic risk, n (%) | |||
| Good | 23 (46) | 13 (48) | 10 (43) |
| Intermediate | 17 (34%) | 8 (30) | 9 (39) |
| Poor | 8 (16) | 5 (19) | 3 (13) |
| Very good | 2 (4.0) | 1 (3.7) | 1 (4.3) |
| IDH2 isoform, n (%) | |||
| R140 | 47 (94) | 25 (93) | 22 (96) |
| R172 | 3 (6.0) | 2 (7.4) | 1 (4.3) |
| IDH2 variant n (%) | |||
| R140L | 2 (4.0) | 1 (3.7) | 1 (4.3) |
| R140Q | 43 (86) | 23 (85) | 20 (87) |
| R140W | 2 (4.0) | 1 (3.7) | 1 (4.3) |
| R172K | 3 (6.0) | 2 (7.4) | 1 (4.3) |
| Transfusion dependent, n (%) | 15 (30) | 4 (15) | 11 (48) |
| RBC dependent | 11 (22) | 2 (7.4) | 9 (39) |
| Platelet dependent | 1 (2.0) | 1 (3.7) | — |
| RBC and platelet dependent | 3 (6.0) | 1 (3.7) | 2 (8.7) |
| Number of prior therapies, median (range) | — | — | 1 (1-4) |
| Cycles of HMA received, median (range) | — | — | 8 (6-36) |
| Baseline hematologic parameters, median (range) | |||
| Absolute neutrophil count | 0.80 (0.01-21.41) | 0.78 (0.01-21.41) | 1.24 (0.10-6.35) |
| Hemoglobin | 9.10 (7.40-14.00) | 9.60 (7.40-14.00) | 8.60 (7.60-11.80) |
| Platelet | 117 (3-436) | 116 (19-368) | 123 (3-436) |
| Characteristic∗ . | Overall N = 50 . | Arm A: AZA + ENA n = 27 . | Arm B: ENA n = 23 . |
|---|---|---|---|
| Age, median (range) | 73 (46-83) | 73 (49-83) | 73 (46-82) |
| Gender, n (%) | |||
| Female | 19 (38) | 12 (44) | 7 (30) |
| Male | 31 (62) | 15 (56) | 16 (70) |
| Subtype, n (%) | |||
| RAEB-T (AML-MRC) | 3 (6.0) | 1 (3.7) | 2 (8.7) |
| CMML | 5 (10) | 2 (7.4) | 3 (13) |
| MDS-EB1 | 10 (20) | 7 (26) | 3 (13) |
| MDS-EB2 | 17 (34) | 10 (37) | 7 (30) |
| MDS-MLD | 12 (24) | 4 (15) | 8 (35) |
| MDS/MPN-U | 1 (2.0) | 1 (3.7) | — |
| MPN/MDS | 2 (4.0) | 2 (7.4) | — |
| R-IPSS, n (%) | |||
| Intermediate | 14 (28) | 6 (22) | 8 (35) |
| Low | 6 (12) | 3 (11) | 3 (13) |
| High | 19 (38) | 12 (44) | 7 (30) |
| Very high | 11 (22) | 6 (22) | 5 (22) |
| Cytogenetic risk, n (%) | |||
| Good | 23 (46) | 13 (48) | 10 (43) |
| Intermediate | 17 (34%) | 8 (30) | 9 (39) |
| Poor | 8 (16) | 5 (19) | 3 (13) |
| Very good | 2 (4.0) | 1 (3.7) | 1 (4.3) |
| IDH2 isoform, n (%) | |||
| R140 | 47 (94) | 25 (93) | 22 (96) |
| R172 | 3 (6.0) | 2 (7.4) | 1 (4.3) |
| IDH2 variant n (%) | |||
| R140L | 2 (4.0) | 1 (3.7) | 1 (4.3) |
| R140Q | 43 (86) | 23 (85) | 20 (87) |
| R140W | 2 (4.0) | 1 (3.7) | 1 (4.3) |
| R172K | 3 (6.0) | 2 (7.4) | 1 (4.3) |
| Transfusion dependent, n (%) | 15 (30) | 4 (15) | 11 (48) |
| RBC dependent | 11 (22) | 2 (7.4) | 9 (39) |
| Platelet dependent | 1 (2.0) | 1 (3.7) | — |
| RBC and platelet dependent | 3 (6.0) | 1 (3.7) | 2 (8.7) |
| Number of prior therapies, median (range) | — | — | 1 (1-4) |
| Cycles of HMA received, median (range) | — | — | 8 (6-36) |
| Baseline hematologic parameters, median (range) | |||
| Absolute neutrophil count | 0.80 (0.01-21.41) | 0.78 (0.01-21.41) | 1.24 (0.10-6.35) |
| Hemoglobin | 9.10 (7.40-14.00) | 9.60 (7.40-14.00) | 8.60 (7.60-11.80) |
| Platelet | 117 (3-436) | 116 (19-368) | 123 (3-436) |
AZA, azacytidine; AML-MRC, AML with myelodysplasia related changes; CMML, chronic myelomonocytic leukemia; EB, excess blast; ENA, enasidenib; RAEB-T, refractory anemia with excess blasts in transformation; RBC, red blood cell; R-IPSS, revised international prognostic scoring system.
Values are presented as median (range) or number (%).
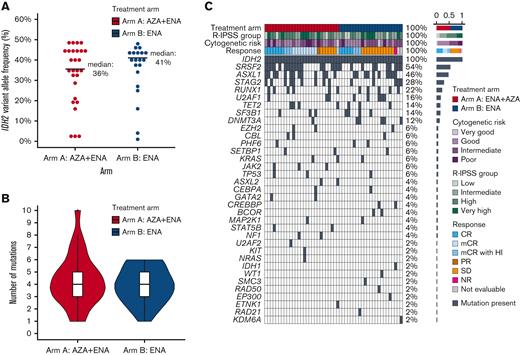
All patients harbored mIDH2: IDH2-R140 in 94% (n = 47) and IDH2-R172 in 6% (n = 3). Baseline mIDH2 VAF ranged from 1% to 49%, with a median of 39% (36% in arm A, 41% in arm B) (Figure 1A). Most patients had diploid (46%) or otherwise good or intermediate-risk cytogenetics (34%) per revised international prognostic scoring system. Median number of mutations per patient including mIDH2 was 4 in arm A (range, 1-10) and 4 in arm B (range. 1-6) (Figure 1B). The most common concomitant mutations in arm A were SRSF2 (63%), ASXL1 (48%), STAG2 (33%), and RUNX1 (22%). In arm B, SRSF2 (43%), ASXL1 (43%), RUNX1 (22%), U2AF1 (22%), STAG2 (22%), and DNMT3A (22%) represented the most common co-occurring mutations (Figure 1C).
Baseline mutational features of study cohort. (A) Dot plot showing the IDH2 variant allele frequency in each treatment arm. (B) Violin plot showing the frequency of genes mutated per patient in each treatment arm. (C) Complex heatmap of the entire cohort; each column represents a patient. Treatment arm, IPSS-R risk group, cytogenetic risk group, indicated mutations, and response are shown for each patient. Shown to the right of the plot are the percentage of patients in each labeled row. AZA, azacitidine; ENA, enasidenib; NR, not reported; SD, stable disease.
Baseline mutational features of study cohort. (A) Dot plot showing the IDH2 variant allele frequency in each treatment arm. (B) Violin plot showing the frequency of genes mutated per patient in each treatment arm. (C) Complex heatmap of the entire cohort; each column represents a patient. Treatment arm, IPSS-R risk group, cytogenetic risk group, indicated mutations, and response are shown for each patient. Shown to the right of the plot are the percentage of patients in each labeled row. AZA, azacitidine; ENA, enasidenib; NR, not reported; SD, stable disease.
Safety
Enasidenib therapy was generally well tolerated; treatment-related AEs are summarized in Table 2. The most common treatment-related AEs of any grade included neutropenia (40%), nausea (36%), constipation (32%), and fatigue (26%) (Figure 2). The most recognized enasidenib-related AE of hyperbilirubinemia, caused by off-target inhibition of the UGT1A1 enzyme, occurred in 14% (8% were grade 3 to 4). No events of tumor lysis syndrome were observed.
Adverse events
| Adverse event∗ . | All patients N = 50 . | Arm A: ENA + AZA n = 27 . | Arm B: ENA n = 23 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 1/2 . | Grade 3/4 . | Total (%) . | Grade 1/2 . | Grade 3/4 . | Total (%) . | Grade 1/2 . | Grade 3/4 . | Total (%) . | |
| Neutropenia | 1 | 19 | 20 (40) | — | 17 | 17 (63) | 1 | 2 | 3 (13) |
| Nausea | 17 | 1 | 18 (36) | 14 | 1 | 15 (56) | 3 | — | 3 (13) |
| Constipation | 16 | 0 | 16 (32) | 15 | — | 15 (56) | 1 | — | 1 (4) |
| Fatigue | 11 | 2 | 13 (26) | 3 | 1 | 4 (15) | 8 | 1 | 9 (39) |
| Thrombocytopenia | 0 | 11 | 11 (22) | — | 11 | 11 (40) | — | — | — |
| Differentiation syndrome | 3 | 5 | 8 (16) | 2 | 1 | 3 (11) | 1 | 4 | 5 (22) |
| Hyperbilirubinemia | 3 | 4 | 7 (14) | 1 | 4 | 5 (19) | 2 | — | 2 (9) |
| Anemia | 2 | 3 | 5 (10) | 1 | 3 | 4 (15) | 1 | — | 1 (4) |
| Leukopenia | 0 | 4 | 4 (8) | — | 4 | 4 (15) | — | — | — |
| Rash | 4 | 0 | 4 (8) | 1 | — | 1 (4) | 3 | — | 3 (13) |
| Vomiting | 3 | 1 | 4 (8) | 3 | 1 | 4 (15) | — | — | — |
| Diarrhea | 2 | 1 | 3 (6) | 2 | 1 | 3 (11) | — | — | — |
| Elevated alkaline phosphatase | 3 | 0 | 3 (6) | 2 | — | 2 (7) | 1 | — | 1 (4) |
| Elevated LFT | 2 | 1 | 3 (6) | 1 | — | 1 (4) | 1 | 1 | 2 (9) |
| Febrile neutropenia | 0 | 3 | 3 (6) | — | 3 | 3 (11) | — | — | — |
| Anorexia | 2 | 0 | 2 (4) | 2 | — | 2 (7) | — | — | — |
| Bruising | 2 | 0 | 2 (4) | 2 | — | 2 (7) | — | — | — |
| Dysgeusia | 2 | 0 | 2 (4) | 1 | — | 1 (4) | 1 | — | 1 (4) |
| Dermatitis | 2 | 0 | 2 (4) | 2 | — | 2 (7) | — | — | — |
| Weight loss | 2 | 0 | 2 (4) | 1 | — | 1 (4) | 1 | — | 1 (4) |
| Adverse event∗ . | All patients N = 50 . | Arm A: ENA + AZA n = 27 . | Arm B: ENA n = 23 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 1/2 . | Grade 3/4 . | Total (%) . | Grade 1/2 . | Grade 3/4 . | Total (%) . | Grade 1/2 . | Grade 3/4 . | Total (%) . | |
| Neutropenia | 1 | 19 | 20 (40) | — | 17 | 17 (63) | 1 | 2 | 3 (13) |
| Nausea | 17 | 1 | 18 (36) | 14 | 1 | 15 (56) | 3 | — | 3 (13) |
| Constipation | 16 | 0 | 16 (32) | 15 | — | 15 (56) | 1 | — | 1 (4) |
| Fatigue | 11 | 2 | 13 (26) | 3 | 1 | 4 (15) | 8 | 1 | 9 (39) |
| Thrombocytopenia | 0 | 11 | 11 (22) | — | 11 | 11 (40) | — | — | — |
| Differentiation syndrome | 3 | 5 | 8 (16) | 2 | 1 | 3 (11) | 1 | 4 | 5 (22) |
| Hyperbilirubinemia | 3 | 4 | 7 (14) | 1 | 4 | 5 (19) | 2 | — | 2 (9) |
| Anemia | 2 | 3 | 5 (10) | 1 | 3 | 4 (15) | 1 | — | 1 (4) |
| Leukopenia | 0 | 4 | 4 (8) | — | 4 | 4 (15) | — | — | — |
| Rash | 4 | 0 | 4 (8) | 1 | — | 1 (4) | 3 | — | 3 (13) |
| Vomiting | 3 | 1 | 4 (8) | 3 | 1 | 4 (15) | — | — | — |
| Diarrhea | 2 | 1 | 3 (6) | 2 | 1 | 3 (11) | — | — | — |
| Elevated alkaline phosphatase | 3 | 0 | 3 (6) | 2 | — | 2 (7) | 1 | — | 1 (4) |
| Elevated LFT | 2 | 1 | 3 (6) | 1 | — | 1 (4) | 1 | 1 | 2 (9) |
| Febrile neutropenia | 0 | 3 | 3 (6) | — | 3 | 3 (11) | — | — | — |
| Anorexia | 2 | 0 | 2 (4) | 2 | — | 2 (7) | — | — | — |
| Bruising | 2 | 0 | 2 (4) | 2 | — | 2 (7) | — | — | — |
| Dysgeusia | 2 | 0 | 2 (4) | 1 | — | 1 (4) | 1 | — | 1 (4) |
| Dermatitis | 2 | 0 | 2 (4) | 2 | — | 2 (7) | — | — | — |
| Weight loss | 2 | 0 | 2 (4) | 1 | — | 1 (4) | 1 | — | 1 (4) |
LFT, liver function test.
Values are presented as n or n (%).
AEs attributable to study therapy by grade and treatment arm. AZA, azacitidine; ENA, enasidenib.
AEs attributable to study therapy by grade and treatment arm. AZA, azacitidine; ENA, enasidenib.
IDH inhibitor–associated differentiation syndrome (IDH-DS) occurred in 8 patients (16%) after a median of 1.3 months (range, 1-8 months); 5 patients experienced grade 3 to 4 IDH-DS. IDH-DS was described more frequently with enasidenib monotherapy (n = 5; 22%, including 4 grade 3-4 events) than with enasidenib plus azacitidine (n = 3; 11%, including 1 grade 3-4 event) combination therapy. Events were managed according to IDH-DS treatment guidelines, including administration of systemic corticosteroids and enasidenib dose interruption. Permanent drug discontinuation was not required in any patient, and no deaths were attributed to IDH-DS.
Three responding patients in combination arm A (all with best response of mCR) experienced an infection-related death during cycle 2 or 3 of therapy (at 73, 78, and 102 days) in the setting of grade 4 neutropenia (1 patient with multifocal pneumonia at completion of cycle 2; 1 patient with bilateral lower lobe pneumonia during cycle 2; and 1 patient with MRSA bacteremia, pneumonia with pleural effusion, and congestive heart failure during cycle 3). After review of these events, the protocol was modified to administer standard azacitidine with a reduced duration of enasidenib on days 1 to 14 (instead of continuously for days 1-28) for the first 3 cycles. If a patient had persistent disease after 3 cycles of combination therapy or if deemed in the best interest of the patient, enasidenib could then be administered as continuous oral therapy with the start of the fourth cycle.
Response
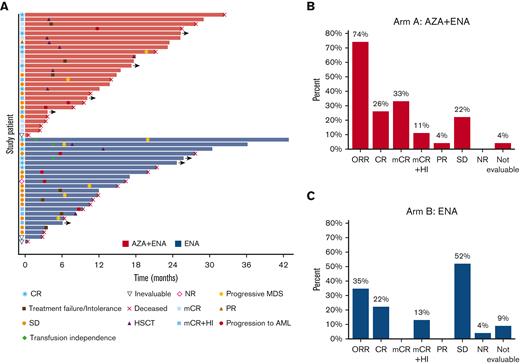
Response outcomes of the entire study population are summarized in Table 3. For the frontline combination of enasidenib plus azacitidine (arm A), the ORR (CR + mCR + PR) was 74% (n = 20), with composite CR (CRc: CR + mCR) in 70% (n = 19) and CR in 26% (n = 7) (Figure 3B). Median time to best response was 1.3 months (range, 0.9-3.8 months). Median time on study was 5 months, and median number of cycles received was 4 (range, 1-32). Seven patients (26%) discontinued study treatment to undergo allogeneic stem cell transplantation (Figure 3A).
Response outcomes
| Characteristic∗ . | Overall N = 50 . | Arm A: AZA + ENA n = 27 . | Arm B: ENA n = 23 . |
|---|---|---|---|
| ORR | 28 (56) [42-69] | 20 (74) [53-88] | 8 (35) [17-57] |
| CR | 12 (24) [14-38] | 7 (26) [12-47] | 5 (22) [9-45] |
| mCR | 9 (18) [9-32] | 9 (33) [18-54] | — |
| mCR + HI | 6 (12) [5-25] | 3 (11) [3-31] | 3 (13) [4-36] |
| Partial response | 1 (2.0) [0-14] | 1 (3.7) [1-25] | — |
| Stable disease | 18 (36) [24-51] | 6 (22) [10-43] | 12 (52) [31-73] |
| Not evaluable | 3 (6.0) [1-18] | 1 (3.7) [1-25] | 2 (8.7) [2-31] |
| No response | 1 (2.0) [0-14] | — | 1 (4.3) [1-28] |
| Characteristic∗ . | Overall N = 50 . | Arm A: AZA + ENA n = 27 . | Arm B: ENA n = 23 . |
|---|---|---|---|
| ORR | 28 (56) [42-69] | 20 (74) [53-88] | 8 (35) [17-57] |
| CR | 12 (24) [14-38] | 7 (26) [12-47] | 5 (22) [9-45] |
| mCR | 9 (18) [9-32] | 9 (33) [18-54] | — |
| mCR + HI | 6 (12) [5-25] | 3 (11) [3-31] | 3 (13) [4-36] |
| Partial response | 1 (2.0) [0-14] | 1 (3.7) [1-25] | — |
| Stable disease | 18 (36) [24-51] | 6 (22) [10-43] | 12 (52) [31-73] |
| Not evaluable | 3 (6.0) [1-18] | 1 (3.7) [1-25] | 2 (8.7) [2-31] |
| No response | 1 (2.0) [0-14] | — | 1 (4.3) [1-28] |
HI, hematologic improvement.
Values are presented as n (%) [95% CI].
Swim plot and response outcomes of the study population by treatment arm. (A) Swim plot showing individual patient, treatment duration, response, and patient disposition. (B) Proportion of responses in arm A (ENA + AZA). (C) Proportion of responses in arm B (ENA). AZA, azacitidine; ENA, enasidenib; SD, stable disease.
Swim plot and response outcomes of the study population by treatment arm. (A) Swim plot showing individual patient, treatment duration, response, and patient disposition. (B) Proportion of responses in arm A (ENA + AZA). (C) Proportion of responses in arm B (ENA). AZA, azacitidine; ENA, enasidenib; SD, stable disease.
In the enasidenib monotherapy cohort (arm B), ORR was 35% (n = 8); CRc was also 35% (n = 8) with CR in 22% (n = 5) (Figure 3C). Median time to first response was 27 days, and time to best response was 4.6 months (range, 2.7-7.6 months). Median time on study was 7 months, with a median of 7 cycles received (range, 1-29). One patient (4%) transitioned to allogeneic hematopoietic stem cell transplantation (HSCT). Eleven patients in the enasidenib arm were transfusion dependent at baseline (RBC: n = 9, RBC and platelets: n = 2), and 3 patients achieved RBC transfusion independence at 1.8, 4.6, and 4.6 months, respectively (Figure 3A).
Forty-three patients (86%) discontinued study treatment. The most common reason for study discontinuation was disease progression (24%, n = 12), including 6 patients (12%) with transformation to secondary AML. Seventeen patients (34%) received additional postprotocol therapies, including 11 patients (65%) who went on to receive subsequent venetoclax-based regimens (Table 4).
Treatment characteristics
| Characteristic∗ . | Overall N = 50 . | Arm A: AZA + ENA n = 27 . | Arm B: ENA n = 23 . |
|---|---|---|---|
| Time to best response, mo | 2 (1-8) | 1 (1-4) | 5 (3-8) |
| Cycles received | 6 (1-32) | 4 (1-32) | 7 (1-29) |
| Study disposition | |||
| On study | 7 (14) | 4 (15) | 3 (13) |
| HSCT | 8 (16) | 7 (26) | 1 (4.3) |
| Treatment failure/intolerance | 9 (18) | 3 (11) | 6 (26) |
| Progressive MDS | 6 (12) | 1 (3.7) | 5 (22) |
| Progression to AML | 6 (12) | 3/27 (11) | 3 (13) |
| Death | 9 (18) | 6/27 (22) | 3 (13) |
| Patient decision/withdrew consent | 4 (8.0) | 3/27 (11) | 1 (4.3) |
| Unknown | 1 (2.0) | — | 1 (4.3) |
| HSCT (any time) | 11 (22) | 7/27 (26) | 4 (17) |
| Postprotocol therapy | 17 (34) | 6/27 (22) | 11 (48) |
| Median number of subsequent therapies | 1 (1-2) | 1 (1-2) | 2 (1-2) |
| Venetoclax therapy | 11/17 (65) | 2/6 (33) | 9/11 (82) |
| Reason for change in therapy | |||
| Progression to AML | 5 (29) | 3 (50) | 2 (18) |
| Progressive MDS | 4 (24) | — | 4 (36) |
| Treatment failure/intolerance | 8 (47) | 3 (50) | 5 (45) |
| Time to next therapy (d) | 10 (0-214) | 9 (0-214) | 10 (0-125) |
| Characteristic∗ . | Overall N = 50 . | Arm A: AZA + ENA n = 27 . | Arm B: ENA n = 23 . |
|---|---|---|---|
| Time to best response, mo | 2 (1-8) | 1 (1-4) | 5 (3-8) |
| Cycles received | 6 (1-32) | 4 (1-32) | 7 (1-29) |
| Study disposition | |||
| On study | 7 (14) | 4 (15) | 3 (13) |
| HSCT | 8 (16) | 7 (26) | 1 (4.3) |
| Treatment failure/intolerance | 9 (18) | 3 (11) | 6 (26) |
| Progressive MDS | 6 (12) | 1 (3.7) | 5 (22) |
| Progression to AML | 6 (12) | 3/27 (11) | 3 (13) |
| Death | 9 (18) | 6/27 (22) | 3 (13) |
| Patient decision/withdrew consent | 4 (8.0) | 3/27 (11) | 1 (4.3) |
| Unknown | 1 (2.0) | — | 1 (4.3) |
| HSCT (any time) | 11 (22) | 7/27 (26) | 4 (17) |
| Postprotocol therapy | 17 (34) | 6/27 (22) | 11 (48) |
| Median number of subsequent therapies | 1 (1-2) | 1 (1-2) | 2 (1-2) |
| Venetoclax therapy | 11/17 (65) | 2/6 (33) | 9/11 (82) |
| Reason for change in therapy | |||
| Progression to AML | 5 (29) | 3 (50) | 2 (18) |
| Progressive MDS | 4 (24) | — | 4 (36) |
| Treatment failure/intolerance | 8 (47) | 3 (50) | 5 (45) |
| Time to next therapy (d) | 10 (0-214) | 9 (0-214) | 10 (0-125) |
AZA, azacitidine; ENA, enasidenib.
Values are presented as median (range), n (%), or n/N (%).
Of the 11 patients who received subsequent venetoclax-based regimens, survival data were available for 91% (n = 10 of 11), including 2 patients in arm A, and 8 patients in arm B. Median OS was 9 months (95% CI: 4.2-NA) and 8 months (95% CI: 2-NA) after initiation of venetoclax-based therapy for patients in arm A (n = 2) and arm B (n = 8), respectively.
DOR and survival
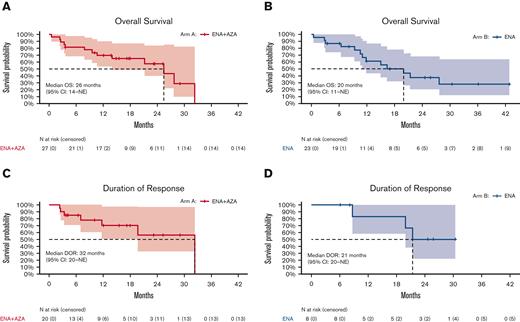
In patients receiving the frontline combination of enasidenib+azacitidine (arm A), median OS was 26 months (range, 14 to not evaluable [NE]) (Figure 4A). Among responding patients (n = 20), median DOR was 32 months (95% CI: 20-NE) (Figure 4C), and median OS was 28 months (95% CI: 26-NE). Median EFS was 11.7 months (95% CI: 6.5-NE) (supplemental Figure 2). In the 7 patients transitioning to allogeneic SCT, median OS was not reached (estimated 24-month OS: 100%) and was 26 months (95% CI: 14-NE) in the 13 responding patients who did not transition to allogeneic HSCT.
OS and DOR by treatment arm. (A) Kaplan-Meier curves showing OS in arm A (ENA + AZA). (B) Kaplan-Meier curves showing OS in arm B (ENA). (C) Kaplan-Meier curves showing the DOR in arm A (ENA + AZA). (D) Kaplan-Meier curves showing the DOR in arm B (ENA). AZA, azacitidine; ENA, enasidenib.
OS and DOR by treatment arm. (A) Kaplan-Meier curves showing OS in arm A (ENA + AZA). (B) Kaplan-Meier curves showing OS in arm B (ENA). (C) Kaplan-Meier curves showing the DOR in arm A (ENA + AZA). (D) Kaplan-Meier curves showing the DOR in arm B (ENA). AZA, azacitidine; ENA, enasidenib.
In the R/R enasidenib monotherapy cohort (arm B), median OS was 20 months (range, 11 to NR) (Figure 4B), and median EFS was 6.5 months (95% CI: 5.4-21.4). In the 8 patients achieving CR or mCR, median DOR was 21 months (95% CI 20-NE) (Figure 4D), and median OS had not been reached (95% CI: 20-NE; estimated 24-month OS: 67% [95% CI: 38-100]).
With a median duration of follow-up of 23 months (95% CI: 18 to NE), in the 8 patients who transitioned to allogeneic HSCT (7 in arm A, 1 in arm B), estimated 24-month OS was 100%.
Correlates of efficacy and/or resistance
Correlates of efficacy and resistance were assessed within the respective treatment arms. Similar to previous reports, the baseline mIDH2 VAF level did not predict for response or survival in either study arm (supplemental Figures 3 and 4). Interestingly, in the frontline combination (arm A), no significant OS difference was observed based on additional comutational burden (0-4 vs ≥5; HR: 0.69 [95% CI: 0.21-2.27], P = .55) or when assessed as a continuous variable (HR: 1.022 [95% CI: 0.79-1.32], P = .87). However, in patients treated with enasidenib monotherapy (arm B), patients with <5 mutations (including IDH2) had a trend toward improved survival (28 [95% CI: 11-NE] vs 15 months [95% CI: 11-NE]) compared with patients with ≥5 mutations (HR: 0.33 [95% CI: 0.098-1.1], P = .07). This was confirmed when comutations were analyzed as a continuous variable, where an increasing mutation burden was associated with an increased risk of death with enasidenib monotherapy (HR: 1.7 [95% CI: 1.11-2.7], P = .017).
The revised international prognostic scoring system risk group did not predict for survival in either cohort. Furthermore, the presence or absence of splicing factor mutations (SRSF2, U2AF1, SF3B1, and ZRSR2) did not predict for response or survival in either arm. ASXL1 mutations, although considered to confer adverse risk in both MDS and AML scoring systems, did not significantly correlate with inferior outcomes in either arm; however, a trend for inferior survival was observed in patients with ASXL1 mutations treated with enasidenib monotherapy. In arm A, patients with mutated ASXL1 (n = 13) experienced an OS of 28 months [95% CI: 21-NE] vs 14 months [95% CI: 8-NE] in patients with wild-type ASXL1 (HR: 0.32 [95% CI: 0.083-1.27], P = .11). In arm B, patients with ASXL1-mutated MDS treated with enasidenib monotherapy (n = 10) experienced an OS of 15 months (95% CI: 9.4-NE) vs NR (95% CI: 11-NE) in ASXL1 wild-type patients (HR: 3.1 [95% CI: 0.98-9.77], P = .052).
In addition, 4 patients with poor risk cytogenetics had complex karyotypes, including 2 patients with TP53 mutations identified on the NGS panel. The 2 patients with TP53 mutations identified at screening were both newly diagnosed patients treated on arm A, with a median OS of 12.3 months (95% CI: 3.4-NA). The 2 patients with complex karyotypes without identified TP53 mutations on NGS were in arm B and treated with enasidenib monotherapy, and both were alive at the time of data cutoff.
Discussion
Although frontline HMA treatment (ie, azacitidine, decitabine, oral decitabine/cedazuridine) for high-risk MDS is standard, primary or secondary resistance to HMA therapy is inevitable.14,15 More effective frontline treatment options that can evoke a deeper and more durable response, or are active at the time of HMA failure, represent critical areas of unmet clinical need. Following HMA failure, no approved treatment strategies exist; average expected survival is ≤6 months.16,17
To our knowledge, this study is the first to report on the safety and efficacy of the combination of azacitidine and enasidenib for treatment-naïve, higher-risk mIDH2 MDS, as well as enasidenib monotherapy in mIDH2 MDS after prior HMA therapy. In this older patient population with a median age of 73 years, enasidenib therapy was overall well tolerated. The frequency of indirect hyperbilirubinemia (14%) and IDH-DS (16%) was similar to that in previous reports of enasidenib therapy, and IDH-DS was effectively managed with initiation of systemic corticosteroids and supportive care measures.8,18
In the cohort of treatment-naïve mIDH2 MDS (arm A), the ORR was 74%, with composite CR of 70%; 37% of patients attained either full CR (n = 7) or mCR with hematologic improvement (n = 3). These results compare favorably with anticipated outcomes with azacitidine monotherapy (ORR: 35%-40%) and correlated well with long-term survival of 28 months in responding patients.19 Responses occurred quickly with a time to best response of 1 cycle, and 7 patients (representing 26% of the arm A cohort) transitioned to HSCT. Remarkably, all patients who transitioned to allogeneic transplant continue in ongoing remission after SCT with a median follow-up of 2 years, suggesting this may be an effective combination for patients with high-risk MDS in whom an allogeneic SCT is planned. The 3 infection-related deaths that occurred in patients with a best response of mCR, without peripheral count recovery and in the setting of severe neutropenia at 73, 78, and 102 days, were notable and led to a protocol modification reducing the enasidenib duration to days 1 to 14 in combination with azacitidine for the first 3 cycles. Only 5 patients enrolled to arm A after the amendment was approved, and thus, the safety and efficacy of this modified treatment schedule was not possible to determine.
The results of the enasidenib monotherapy cohort in the setting of primary or secondary HMA failure (arm B) were particularly encouraging given the unmet need in this high-risk clinical population, with a composite CR rate of 35%, including 22% of patients attaining a full CR. Of importance, even though fewer patients were enrolled than originally planned, arm B of the study still “met” the final efficacy goal with 8 responding patients (5 CR and 3 mCR + HI) as was defined by the original Simon 2-stage design, confirming the effectiveness of enasidenib in previously treated mIDH2 MDS. Consistent with the noncytotoxic mechanism of action, best responses evolved over time with continued therapy, with a time to best response of 4.6 months, although the median time to first response occurred within the first month. This once-daily oral outpatient therapy was well tolerated with a median of 7 cycles received; several patients remained on study for ≥2 years at the time of data cutoff. In responding patients, the median DOR of 21 months and estimated 24-month OS of 67% were particularly encouraging and suggest a clear role of targeted IDH2 inhibition for this patient population.
In conclusion, this study demonstrated that enasidenib is an effective treatment option for mIDH2 MDS, both in combination with azacitidine for treatment-naïve high-risk MDS and as a single agent after prior HMA therapy, leading to durable clinical responses in both study populations. This study further highlights the importance of molecular profiling to inform treatment strategies for patients with MDS.
Acknowledgments
This study was funded in part by research funding from Celgene and Bristol Myers Squibb (BMS) (C.D.D.) and in part by the University of Texas MD Anderson Cancer Center Support grant CA016672 and the University of Texas MD Anderson Cancer Center MDS/AML Moon Shot.
C.D.D. is supported by a V Foundation Lloyd Family Clinical Scholar and LLS Scholar in Clinical Research Award.
Authorship
Contribution: C.D.D., H.C., M.S., A.D., H.M.K., G.R., and G.G.M. designed the study; S.L., A.S., K.T., G.M-B., D.H., K.C., A.M., L.M., K.S., Y.A., T.K., N.J.S., N.D., G.B., F.R., and B.P. contributed patients; C.D.D., S.V., C.L., and X.W. analyzed the data; C.D.D. wrote the first draft; and all authors reviewed and approved the final manuscript.
Conflict-of-interest disclosure: C.D.D. reports research grants from AbbVie, Servier, Astex, BMS, Cleave, Daiichi-Sankyo, ImmuneOnc, and Loxo, and receives honoraria/consulting fee from AbbVie, Servier, Astellas, BMS, Celgene, Cleave, Foghorn, Genentech, Gilead, Novartis, Notable Labs, and Takeda. K.T. reports consultancy and an advisory fee from Symbio Pharmaceuticals, Novartis, Celegene/BMS, Agios, GSK, and Otsuka, and received honoraria from Mission Bio and Illumina. S.L. owns stock in AbbVie. N.J.S. reports receiving research grants from Takeda Oncology, Astellas Pharma Inc., Stemline Therapeutics Inc., and Xencor, and has received honoraria from Amgen, Jazz Pharmaceuticals, Novartis, and Pfizer. F.R. reports research funding, consultancy, and honoraria from Celgene/BMS, Astex/Taiho, Genentech, Syros, and Astellas; research funding from Prelude and Hutchison Pharma; and consultancy/honoraria from Novartis, AstraZeneca, and Mablytics. A.D. has previously acted as a consultant and served in advisory roles for BMS, Novartis, Takeda, Geron, Taiho, and CTI Biopharma. G.G.M. reports funding from BMS. The remaining authors declare no competing financial interests.
Correspondence: Courtney D. DiNardo, Department of Leukemia, UT MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: cdinardo@mdanderson.org.
References
Author notes
Interim results were presented in the form of an abstract at the 2019 annual meeting of the American Society of Hematology, the 2021 annual meeting of the American Society of Clinical Oncology, and the 2021 annual meeting of the European Hematology Association.
Individual participant data will not be shared. Deidentified data may be shared with individuals who have appropriate institutional review board approvals and data safeguards in place. Interested researchers should reach out to the corresponding author, Courtney D. DiNardo (cdinardo@mdanderson.org), for additional details.
The full-text version of this article contains a data supplement.