TO THE EDITOR:
Finding safe and effective treatment strategies for patients with TP53-mutated acute myeloid leukemia (AML) remains an important unmet need.1,2 Among all somatic mutations found in AML, TP53 mutations are associated with intrinsic resistance to cytotoxic therapy, shorter overall survival (OS), and inferior outcomes with allogeneic hematopoietic cell transplantation.3-6 The NEDD8-activating enzyme inhibitor pevonedistat (PEVO) has been shown to induce apoptosis in AML via increased reactive oxygen species7 as well as accumulation of the oncoprotein MYC and transactivation of the gene encoding the BH3-only protein NOXA in AML cell lines and primary AML samples.8 Additional studies have demonstrated that PEVO enhances the cytotoxicity of hypomethylating agents in preclinical AML models.9 Consistent with these observations, a phase 1b study of PEVO with azacitidine (AZA) demonstrated a composite complete remission (CCR) rate of 30%, with an additional 11% partial remission rate. Responses occurred irrespective of poor-risk disease features and were observed in 6 of 8 patients with TP53-mutated AML.10 Here, we report the results of a phase 2 study of PEVO + AZA in older patients with previously untreated TP53-mutated AML that builds on the previous results. This was a substudy of the Beat AML “umbrella” Master trial (www.clinicaltrials.gov, #NCT03013998) evaluating targeted therapies using prospective genomic profiling of previously untreated older patients with AML.11
The primary objective of this open-label phase 2 study was to evaluate the CCR rate (complete remission [CR] + CR with incomplete hematologic recovery [CRi]) and tolerability of PEVO + AZA combination therapy in patients ≥60 years of age with untreated TP53-mutated AML (variant allele frequency [VAF] ≥30%). Inclusion criteria are listed in supplemental Table 1. This study was approved by a central institutional review board and by local institutional review boards and was conducted according to the Declaration of Helsinki. Four sites enrolled ≥1 patient(s) between April 2018 and December 2019. AZA (75 mg/m2) was administered subcutaneously or intravenously on days 1 to 7 (or days 1-5 and days 8 and 9) of every 28-day cycle for up to 12 cycles. PEVO (20 mg/m2) was given as a 1-hour infusion on days 1, 3, and 5 of each cycle for up to 24 cycles after administration of AZA. Bone marrow aspiration and biopsy were performed before therapy and after 1, 2, and 4 cycles to assess clinical response using the 2017 European LeukemiaNet criteria.12 The primary end point was the proportion of patients with CCR by the end of cycle 4. Secondary end points included response duration, OS, and proportion of patients transitioning to allogeneic hematopoietic cell transplantation. This study used Simon’s Optimal 2-stage design. The statistical methods are summarized in Supplemental Table 2.
After the enrollment, 10 patients received treatment with both the study drugs. The baseline demographics/characteristics of these patients are summarized in Table 1 and supplemental Table 3. Patients completed a median of 3.5 (range, 2-13) cycles of PEVO + AZA over a median of 81 days. Three patients (30%) completed more than 6 cycles.
Patient demographics and baseline characteristics
| . | Total, N = 10 . |
|---|---|
| Age, y | |
| Median (range) | 72.0 (68.0-85.0) |
| Sex, n (%) | |
| Male | 5 (50.0) |
| Female | 5 (50.0) |
| Race, n (%) | |
| White | 9 (90.0) |
| Other | 1 (10.0) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 0 |
| Not Hispanic or Latino | 10 (100.0) |
| Body mass index (kg/m2) | |
| Median (range) | 28.42 (20.7-38.6) |
| Previous exposure to chemotherapy for an indication other than AML, n (%) | 3 (30.0) |
| Previous exposure to radiation therapy, n (%) | 4 (40.0) |
| . | Total, N = 10 . |
|---|---|
| Age, y | |
| Median (range) | 72.0 (68.0-85.0) |
| Sex, n (%) | |
| Male | 5 (50.0) |
| Female | 5 (50.0) |
| Race, n (%) | |
| White | 9 (90.0) |
| Other | 1 (10.0) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 0 |
| Not Hispanic or Latino | 10 (100.0) |
| Body mass index (kg/m2) | |
| Median (range) | 28.42 (20.7-38.6) |
| Previous exposure to chemotherapy for an indication other than AML, n (%) | 3 (30.0) |
| Previous exposure to radiation therapy, n (%) | 4 (40.0) |
n, number of patients in subset.
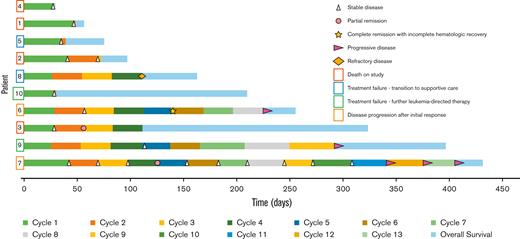
All patients were clinically followed up for response (Figure 1). Nine patients were assessed for the primary end point; none attained CCR by the end of cycle 4. Two patients attained partial remission (patients 3 and 7, shown in supplemental Table 3) and 7 patients (70%) had stable disease by the end of cycle 4. Because the ≥40% CCR rate required for continuing enrollment after stage 1 was not reached, the futility of the PEVO + AZA combination was declared, and enrollment was stopped. Notably, however, 1 patient (patient 6) attained CR with incomplete hematologic recovery by the beginning of cycle 6 but subsequently progressed at cycle 9, day 1. When compared with the other 9 patients, unique characteristics of this patient’s AML included mutations in BRCA2 (VAF, 45.71%), TET2 (VAF, 49.34%), CSF1R (VAF, 53.73%), MDM2 (VAF, 50.54%), and ASXL1 (42.71%). The median OS was 187.5 days (95% confidence interval, 31.0-326.0) (supplemental Figure 1).
Swimmer’s plot of the 10 patients who received study treatment with PEVO and AZA. Rectangles surrounding patient numbers indicate the reason for the discontinuation of study treatment. Created with www.biorender.com.
Swimmer’s plot of the 10 patients who received study treatment with PEVO and AZA. Rectangles surrounding patient numbers indicate the reason for the discontinuation of study treatment. Created with www.biorender.com.
All patients treated in the study experienced at least 1 treatment-emergent adverse event (TEAE). Overall, 9 patients (90%) experienced grade 3 or higher TEAEs. Two patients (20%) had treatment-emergent serious adverse events (SAEs) that resulted in death (grade 5 respiratory failure and an unknown cause) and were considered unrelated to the study therapy. The most common grade 3 or higher TEAE was grade 4 neutropenia in 2 patients (20%). Three patients experienced the following 6 additional treatment-emergent SAEs: grade 3 pleural effusion, grade 2 posterior tibial and peroneal vein thrombosis, grade 3 pneumonia, grade 3 cellulitis, grade 3 small intestine obstruction, and grade 4 sepsis. All patients died during the study; the most common causes of death were AML progression (60%) and SAEs which resulted in the death of 2 patients (20%).
The response rate in this study was lower than in the phase 1b study.10 One reason may be that this study enrolled patients with TP53-mutated AMLs with a VAF ≥30% to assure treatment of dominant clones, whereas a lower VAF was used to classify the disease as TP53-mutated in the phase 1b study.10 Importantly, a higher TP53 VAF has been associated with similarly poor survival outcomes with hypomethylating agent treatment.13
Among the planned correlative studies was immunoblotting to examine changes in BCL2 family members and DNMT1 throughout the treatment. These proteins were studied in the 12 available samples from 5 patients because (1) BCL2 family members, which mediate most chemotherapy-induced killing,14 are modulated by the study drugs8,15 and (2) DNMT1, the most abundantly expressed DNA methyltransferase in proliferating cells and a potential oncoprotein in AML,16 has been reported to be higher in AZA-resistant AML cells17 and lower in AML that responds to decitabine.18 Consistent with preclinical observations,8 the proapoptotic BCL2 family member NOXA was upregulated at the end of cycle 1 in 4 of 5 patients (supplemental Figure 2). NOXA upregulation was associated with the upregulation of additional BH3-only proteins, including PUMA, BIM, and BID. Importantly, though BCL2 was unchanged, upregulation of the antiapoptotic proteins BCLXL and MCL1 was also observed at the end of cycle 1 compared with baseline in all patients, providing a potential explanation for the ability of blasts to tolerate increased BH3-only protein levels. Given the small sample size and low response rate, it is difficult to draw definitive conclusions about any differences between patients. Although PEVO and AZA have both been reported to increase NOXA expression,8,15 the short half-life of these drugs (<10 hours) makes it important to look at the changes in pro- and antiapoptotic BCL2 family members at time points closer to therapy with those agents in future studies. In this study, DNMT1 levels also increased at the end of cycle 1 in 4 of 5 patients. However, conclusions about the predictive value of baseline DNMT1 levels cannot be made based on our study because most patients did not achieve a response.
In summary, in this phase 2 Beat AML substudy, treatment with AZA + PEVO did not induce responses (CCR) in older patients with TP53-mutated AML. These observations mirror recent results with the same combination in myelodysplatic syndrome,19 stand in contrast to previous results in AML,10 and do not support the combination as targeted therapy in TP53-mutated AML. Reports of studies (#NCT04172844 and #NCT03862157) examining the addition of venetoclax to AZA + PEVO in AML are eagerly awaited.
Acknowledgments: The work was supported by the Leukemia & Lymphoma Society and by grants from the National Cancer Institute, National Institutes of Health (R01 CA225996) (S.H.K.) and the National Cancer Institute, National Institutes of Health (R35 CA198183 and support grant P30 CA008748) (J.C.B.).
Contribution: A.N.S. and S.H.K. wrote the manuscript; A.N.S., S.H.K., A.B.S., A.O.Y., T.C., M.S., A.S.M., U.B., A.B., B.J.D., R.L.L., J.C.B., and J.M.F. designed research; A.N.S., S.H.K., E.M.S., P.A.P., M.R.B., W.S., M.D., W.B., G.J.S., R.L.O., M.R.L., T.L.L., B.J.B., M.M.B., E.T., O.O., M.L.A., A.W., V.H.D., T.K., R.H.C., M.C.F., K.L.P., P.A.S., A.S.M., U.B., B.J.D., R.L.L., J.C.B., and J.M.F. performed research; A.N.S., E.M.S., P.A.P., M.R.B., W.S., M.D., W.B., G.J.S., R.L.O., M.R.L., T.L.L., B.J.B., M.M.B., E.T., O.O., M.L.A., A.W., V.H.D., T.K., R.H.C., M.C.F., K.L.P., P.A.S., M.M., L.R., S.M., A.O.Y., T.C., M.S., A.S.M., U.B., B.J.D., R.L.L., J.C.B., and J.M.F. collected data; A.N.S., S.H.K., N.A.H., M.M., T.J.G., L.R., S.M., A.O.Y., T.C., and M.S. analyzed and interpreted data; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: S.H.K. has previously received funding from Eli Lilly and Takeda. E.M.S. has served on the advisory boards of Astellas Pharma, AbbVie, Genentech, Daiichi Sankyo, Novartis, Amgen, Seattle Genetics, Syros Pharmaceuticals, Syndax Pharmaceuticals, Agios Pharmaceuticals, and Celgene and is an equity holder in Auron Therapeutics. P.A.P. has served on the advisory board of Agios Pharmaceuticals. M.D. has served on the advisory boards of Blueprint Medicines, Takeda, Incyte Corporation, and Sangamo Therapeutics; has been a consultant for Blueprint Medicines, Fusion Pharmaceuticals, Medscape, Novartis, Sangamo Therapeutics, and DisperSol Technologies; has received research funding from Blueprint Medicines, Takeda, Novartis, Incyte Corporation, Sun Pharma Advanced Research Company, and Pfizer; and has served on the study management committees of Blueprint Medicines and Takeda. G.J.S. reports research funds paid for medical research (eg, collaborative research, funded research, and clinical trial) by 1 commercial entity, if the sum of which a presenter (presenters) is in the position to decide how to use is 1 million yen or more per year for Takeda. R.L.O. receives research funding from Cellectis, Astellas, and Pfizer and is a consultant for Actinium, Astellas, and AbbVie. M.M.B. is employed by Genentech. E.T. has served on the advisory boards of AbbVie, Astellas Pharma, Genentech, Agios Pharmaceuticals, and Daiichi Sankyo; has received clinical trial support from Janssen Pharmaceuticals; receives clinical trial funding and sponsored research from Incyte Corporation; and holds equity in notable laboratories. O.O. has served on the advisory boards of AbbVie, Celgene, and Impact Biomedicines and has received research funding from AbbVie, AstraZeneca, Astex, Agios Pharmaceuticals, Celgene, CTI BioPharma, Incyte Corporation, Janssen Pharmaceuticals, Kartos Therapeutics, NS Pharma, and OTS. M.C.F. receives consulting fees from Daiichi Sankyo, Macrogenics, and Zentalis Pharmaceuticals and research funding from Bellicum Pharmaceuticals, Bristol-Myers Squibb/Celgene, Rafael Pharmaceuticals, LOXO/Lilly Oncology, Newave Pharmaceuticals, and Macrogenics. T.J.G. is a clinical consultant for Leukemia & Lymphoma Society, which receives funding from AbbVie, Agios Pharmaceuticals, Alexion Pharmaceuticals, Amgen, Astellas Pharma, AstraZeneca, Boehringer Ingelheim International, Boston Biomedical, Bristol-Myers Squibb, Celgene, Genentech, Gilead Sciences, ImmunoGen, Jazz Pharmaceuticals, Johnson & Johnson, Novartis, Pfizer, Pharmacyclics, RTI Health Solutions, Shire, and Takeda. L.R., S.M., A.O.Y., and A.B. are employees of Leukemia & Lymphoma Society. A.S.M. has served on the advisory boards of Jazz Pharmaceuticals, AbbVie/Genentech, Astellas Pharma, PTC Therapeutics, Novartis, Agios Pharmaceuticals, and Syndax Pharmaceuticals. U.B. has been a consultant for Genetech, Daiichi Sankyo, Takeda, Pfizer, AbbVie/Genetech, and Novartis. B.J.D. has served on the scientific advisory boards for Adela Bio, Aileron Therapeutics, Therapy Architects (ALLCRON), Cepheid, Celgene, RUNX1 Research Program, Nemucore Medical Innovations, Novartis, and Vivid Biosciences (inactive); has served on the scientific advisory boards and stock for Aptose Biosciences, Blueprint Medicines, Enliven Therapeutics, Iterion Therapeutics, GRAIL, and Recludix Pharma; has served on the board of directors and stock for Amgen and Vincerx Pharma; has served on the board of directors for Burroughs Wellcome Fund, and CureOne; has served on the joint steering committee for Beat AML LLS; is the founder of VB Therapeutics; is in a sponsored research agreement with Enliven Therapeutics and Recludix Pharma; received clinical trial funding from Novartis and AstraZeneca; received royalties from patent 6958335 (Novartis exclusive license) and Oregon Health & Science University and Dana-Farber Cancer Institute (1 Merck exclusive license, 1 CytoImage, Inc. exclusive license, and 1 Sun Pharma Advanced Research Company nonexclusive license); and US patents 4326534, 6958335, 7416873, 7592142, 10473667, 10664967, and 11049247. R.L.L. is on the supervisory board of Qiagen; is a scientific advisor to Imago, Mission Bio, Syndax. Zentalis, Ajax, Bakx, Auron, Prelude, C4 Therapeutics, and Isoplexis for which he receives equity support; receives research support from Ajax and AbbVie; has been a consultant for Incyte, Janssen, Morphosys, and Novartis; and has received honoraria from AstraZeneca and Kura for invited lectures and from Gilead for grant reviews. J.C.B. has received research support from Janssen Pharmaceuticals, Genentech, Acerta Pharma, and Pharmacyclics and has served on the advisory board of Syndax Pharmaceuticals. J.M.F. receives research support (to the institution) from Takeda. The remaining authors declare no competing financial interests.
Correspondence: Antoine N. Saliba, Mayo Clinic, 200 1st St SW, Rochester, MN 55905; e-mail: saliba.antoine@mayo.edu.
References
Author notes
Data are available on request from the corresponding author, Antoine N. Saliba (antoine.nabil.saliba@gmail.com and saliba.antoine@mayo.edu).
The full-text version of this article contains a data supplement.