Key Points
Underweight children aged 5 to 12 years and with SCA are at risk for early death in a low-resource setting.
Weight-for-age z score is a simple measure to screen children older than 5 years with SCA at risk for death in a low-resource setting.
Abstract
Undernutrition is a risk factor for under-5 mortality and is also postulated to be a risk factor for mortality in older children and adults with sickle cell anemia (SCA). We tested the hypothesis that underweight is associated with mortality in children aged 5 to 12 years with SCA. We performed a secondary analysis of participants in the Primary Prevention of Stroke in Children with Sickle Cell Disease in Nigeria trial, a double-blind, parallel-group randomized controlled trial for low-dose or moderate-dose hydroxyurea in children with abnormal transcranial Doppler velocities and a comparison group of participants with nonelevated transcranial Doppler velocities in northern Nigeria. Nutritional status was classified as underweight (weight-for-age z score), stunting (height-for-age z score), and wasting (body mass index z score) using the World Health Organization growth reference. The mean weight-for-age z score was lower in children who died during the study than in those who survived. Otherwise, the baseline characteristics of children who died during the study were not significantly different from those of the children who survived. A pooled analysis of participants demonstrated that a lower weight-for-age z score was associated with an increased hazard of death. Underweight participants (weight-for-age z score <−1) had a greater probability of death during follow-up than those who were not underweight. Underweight status in school-aged children with SCA is a previously unrecognized risk factor for early mortality in Nigeria and can be easily applied to screen children at risk for death. This trial was registered at www.clinicaltrials.gov as #NCT02560935.
Introduction
Undernutrition and sickle cell anemia (SCA) are common occurrences in children living in sub-Saharan Africa, where 75% of all children with SCA are born.1-3 Initial efforts to model the mortality rates in children with SCA indicated 50% to 90% early-life mortality.4 Through improved medical therapies and access to care, the survival of children with SCA has been substantially enhanced.5 A recent multicenter and multicountry retrospective study of children with SCA living in sub-Saharan Africa estimated a significant decline in childhood mortality.5 However, there continues to be excess mortality and morbidity in children with SCA living in sub-Saharan Africa.1,4-6 In the Primary Prevention of Stroke in Children with Sickle Cell Disease in Nigeria (Stroke Prevention in Nigeria [SPRING]) trial, the mortality in children aged 5 to 12 years with SCA was 2.38 per 100 person-years, with no significant difference between participants on low-dose or moderate-dose treatment or those in the treatment or comparison group.7 In contrast, children with SCA aged 5 to 14 years in the United States have a death rate of <0.5 per 100 000 person-years.8-10
We had not identified specific risk factors in the SPRING trial for the high mortality rate.7 Moreover, in our primary analysis we did not explore the 3 standard indicators of undernutrition (underweight, stunting, and wasting) associated with death in children under 5 years.11,12 Of these 3 indicators, weight-for-age z score (underweight) is a biomarker for acute and chronic undernutrition.13 Decreased weight-for-age z score is associated with death in children under 5 years of age without SCA, with ∼45% of deaths in this age group attributable to undernutrition.11,14,15 Additionally, weight-for-age z scores in children under 5 have high sensitivity and specificity for identifying those children who are concurrently stunted and wasted.16
Whether weight-for-age z score is associated with excess mortality in children with SCA older than 5 years in the region has not been established. As a secondary analysis, we tested the primary hypothesis that weight-for-age z score (continuous measurement) and the corresponding underweight (weight-for-age z score <−1; categorical value) are independent risk factors for mortality in children aged 5 to 12 years old with SCA in northern Nigeria. We also evaluated whether the other 2 standard undernutrition indicators, stunting and wasting, were associated with mortality in the same cohort.
Methods
Study design and participants
We performed a secondary data analysis of deidentified data sets of children with SCA enrolled in the SPRING trial (NCT02560935).7 The SPRING trial was a National Institute of Health-funded multicenter phase III double-blind, parallel-group randomized controlled trial conducted in a low-income region of northern Nigeria from July 2016 to April 2020.7,17 The trial was approved by the institutional review board of Vanderbilt University Medical Center in Nashville, TN, and the respective ethics committees of the local participating sites: Aminu Kano Teaching Hospital and Murtala Mohammad Specialist Hospital, with referrals from Hasiya Bayero Pediatric Hospital and Muhammad Abdullahi Wase Specialist Hospital, all in Kano, Nigeria and Barau Dikko Teaching Hospital in Kaduna, Nigeria. The parent or legal guardian of all participating children provided written informed consent.
Eligible children aged 5 to 12 years with confirmed hemoglobin SS or hemoglobin Sβ0 thalassemia (referred to as SCA) and abnormal transcranial Doppler measurements of the terminal portion of the internal carotid, middle cerebral artery, or both vessels above 200 cm/s were randomized to either low-dose (10 mg/kg) or moderate-dose (20 mg/kg) hydroxyurea by mouth daily. A comparison group of children with SCA with normal or conditional transcranial Doppler velocities was also prospectively followed. The comparison group did not receive hydroxyurea therapy.
Data collection and definitions
Demographics, anthropometrics, and baseline laboratory values were collected. Hematological variables, including baseline hemoglobin and white blood cell count, were included when the potential participant was in a steady state during study screening when the participants were not acutely ill or had not been recently hospitalized.
Following standard anthropometric methods, study administrators, nurses, or physicians noted weight (kg) and height (cm) details. Body mass index (BMI) (kg/m2) was calculated. Anthropometric measurements were converted to age- and sex-specific z scores based on the World Health Organization (WHO) growth reference.12,18 The WHO charts do not provide weight-for-age z scores for children older than the age of 10 years. The Canadian Pediatric Endocrine Group growth charts were used for weight-for-age z scores of children beyond the age of 10 years. The Canadian Pediatric Endocrine Group growth charts complement the WHO growth charts by extending the weight-for-age z score using the same core data set as the WHO reference for school-aged children and adolescents.19 Undernutrition was defined as z scores <−1, with degrees of anthropometric deficits further delineated as mild with z scores between ≥−2 and <−1, moderate between ≥−3 and <−2, and severe <−3. Underweight, stunting, and wasting were determined by weight-for-age, height-for-age, and BMI z scores, respectively.12,18
The follow-up of the pooled participants from the treatment and comparison groups was planned for a minimum of 3 years and the primary outcome was death. Causes of death were categorized into infectious and noninfectious causes or unknown because previous research has only established the relationship between undernutrition and mortality for infectious disease-related deaths.14 Infectious causes included causes of death that were most likely related to an infection, including but not limited to febrile illnesses, sepsis, and presumed malaria. The end date for follow-up was either the date of last contact with the participant at the previous clinic visit or the date of death or stroke.
Statistical analysis
Summary statistics for continuous variables were summarized as means and standard deviations or as medians and interquartile ranges for variables not normally distributed. Categorical variables and prevalence were reported as numbers and percentages. A χ2 or Fisher exact test was used for percentages, a t test for means, and a Mann-Whitney U test for medians.
We used multivariate Cox regression to assess the association between continuous anthropometric z scores with death, adjusted for age, sex, and hemoglobin levels. The hazard ratio (HR) with a 95% confidence interval (CI) was used to assess the risk of death associated with each covariate. Kaplan-Meier curves were generated to assess the association between underweight (weight-for-age z score <−1) and death. As this is a secondary analysis, we used a 2-sided P value of .05 as potential evidence of a significant result, but < .01 as stronger evidence for significance.20 Data analysis was performed using SPSS 27.0 (IBM, Armonk, NY).
Results
Baseline characteristics of the study participants
There were 431 participants in the SPRING trial, all of whom had complete anthropometric values at baseline. The median follow-up time was 2.6 years (interquartile range 2.0-3.0, Table 1). During the trial, 2.7% (6 of 220) of participants withdrew from the therapy group, 3 participants from each arm. A total of 8.6% (18 of 209) of the participants withdrew from the comparison group. Participants that did not follow up for the routine visits were called on the primary and secondary phones to assess their scheduled follow-up visits. There were 24 deaths among the SPRING trial participants during follow-up, with 15 and 9 deaths in the treatment and comparison groups, respectively (supplemental Table 1). Of the deaths, 50% (12 of 24) were classified as an infectious cause (supplemental Table 1). The proportion of deaths from presumed infections was not statistically different between the treatment and comparison groups (P = 1.000).
Baseline characteristics of the SPRING trial participants (n = 431) in the therapy (n = 220) and comparison groups (n = 211)
| Characteristics∗ . | Combined population (n = 431) . | Therapy group (n = 220) . | Comparison group (n = 211) . | P value† . |
|---|---|---|---|---|
| Age at enrollment, y | 7.7 (6.0-10.0) | 7.2 (5.5-8.9) | 8.4 (6.4-10.7) | <.001 |
| Female sex | 224 (52.0) | 114 (51.8) | 110 (52.1) | .948 |
| Follow-up time (y), median (IQR) | 2.6 (2.0-3.0) | 2.4 (2.0-2.8) | 2.8 (1.5-3.1) | .004 |
| Transcranial Doppler velocity at screening, cm/sec | 200.0 (131.0-207.0) | 206.5 (203.0-221.0) | 130.0 (115.0-146.0) | <.001 |
| Total hemoglobin, g/dL | 7.3 (6.6-8.1) | 7.1 (6.5-7.6) | 7.7 (6.8-8.4) | <.001 |
| Height (cm) | 117.5 (109.5-126.0) | 114.5 (107.0-123.0) | 121.5 (11.5-131.0) | <.001 |
| Weight (kg) | 18.0 (16.0-22.0) | 17.0 (15.0-20.0) | 20.0 (16.0-24.0) | <.001 |
| BMI (kg/m2) | 13.5 (12.6-14.5) | 13.4 (12.7-14.3) | 13.5 (12.6-14.7) | .280 |
| Height-for-age z score, mean (SD) | −1.5 (1.3) | −1.5 (1.3) | −1.4 (1.3) | .320 |
| Weight-for-age z score, mean (SD) | −2.1 (1.1) | −2.2 (1.0) | −2.1 (1.1) | .432 |
| BMI z score, WHO standard, mean (SD) | −1.8 (1.2) | −1.8 (1.2) | −1.9 (1.3) | .385 |
| Characteristics∗ . | Combined population (n = 431) . | Therapy group (n = 220) . | Comparison group (n = 211) . | P value† . |
|---|---|---|---|---|
| Age at enrollment, y | 7.7 (6.0-10.0) | 7.2 (5.5-8.9) | 8.4 (6.4-10.7) | <.001 |
| Female sex | 224 (52.0) | 114 (51.8) | 110 (52.1) | .948 |
| Follow-up time (y), median (IQR) | 2.6 (2.0-3.0) | 2.4 (2.0-2.8) | 2.8 (1.5-3.1) | .004 |
| Transcranial Doppler velocity at screening, cm/sec | 200.0 (131.0-207.0) | 206.5 (203.0-221.0) | 130.0 (115.0-146.0) | <.001 |
| Total hemoglobin, g/dL | 7.3 (6.6-8.1) | 7.1 (6.5-7.6) | 7.7 (6.8-8.4) | <.001 |
| Height (cm) | 117.5 (109.5-126.0) | 114.5 (107.0-123.0) | 121.5 (11.5-131.0) | <.001 |
| Weight (kg) | 18.0 (16.0-22.0) | 17.0 (15.0-20.0) | 20.0 (16.0-24.0) | <.001 |
| BMI (kg/m2) | 13.5 (12.6-14.5) | 13.4 (12.7-14.3) | 13.5 (12.6-14.7) | .280 |
| Height-for-age z score, mean (SD) | −1.5 (1.3) | −1.5 (1.3) | −1.4 (1.3) | .320 |
| Weight-for-age z score, mean (SD) | −2.1 (1.1) | −2.2 (1.0) | −2.1 (1.1) | .432 |
| BMI z score, WHO standard, mean (SD) | −1.8 (1.2) | −1.8 (1.2) | −1.9 (1.3) | .385 |
IQR, interquartile range; SD, standard deviation.
The therapy group comprised of children with abnormal transcranial Doppler velocities on hydroxyurea therapy, and the comparison group was children with nonelevated transcranial Doppler velocities.
IQR, interquartile range; SD, standard deviation.
Median (IQR) for continuous variables and counts (percentages) for categorical variables unless otherwise noted.
χ2 test for counts, t test for means, and Mann-Whitney U test for medians between the therapy and comparison group.
Excluding anthropometric measurements, the baseline characteristics of children who died during the study were not significantly different from those of children who survived (Table 2). The median age of children at enrollment was 7.7 (6.0-10.1) and 7.5 (6.3-9.0) years for those who survived and died (P = .778), respectively. Participants in the comparison group were older with higher baseline hemoglobin (P < .001, Table 1).
Baseline characteristics of the SPRING trial participants based on survival status at follow-up (n = 431)
| Characteristics∗ . | Survived (n = 407) . | Died (n = 24) . | P value† . |
|---|---|---|---|
| Age at enrollment, median (IQR) | 7.7 (6.0-10.1) | 7.5 (6.3-9.0) | .778 |
| Female | 211 (51.8) | 13 (54.2) | .825 |
| Total hemoglobin (g/dL), median (IQR) | 7.3 (6.7-8.1) | 7.2 (6.4-7.2) | .547 |
| White blood cell count (103/mm3), median (IQR) | 13.5 (11.2-16.9) | 14.1 (12.5-17.3) | .580 |
| Transcranial Doppler velocity at screening (cm/s), median (IQR) | 200.0 (130.0-206.0) | 203.5 (146.3-219.0) | .113 |
| Height (cm) | 118.5 (11.8) | 115.1 (10.9) | .168 |
| Weight (kg) | 19.2 (4.5) | 17.3 (3.7) | .038 |
| BMI (kg/m2) | 13.6 (1.5) | 13.0 (1.6) | .070 |
| Height-for-age z score | −1.5 (1.3) | −1.8 (1.2) | .186 |
| Weight-for-age z score | −2.1 (1.1) | −2.6 (1.2) | .016 |
| BMI z score | −1.9 (1.3) | −2.2 (1.6) | .161 |
| Characteristics∗ . | Survived (n = 407) . | Died (n = 24) . | P value† . |
|---|---|---|---|
| Age at enrollment, median (IQR) | 7.7 (6.0-10.1) | 7.5 (6.3-9.0) | .778 |
| Female | 211 (51.8) | 13 (54.2) | .825 |
| Total hemoglobin (g/dL), median (IQR) | 7.3 (6.7-8.1) | 7.2 (6.4-7.2) | .547 |
| White blood cell count (103/mm3), median (IQR) | 13.5 (11.2-16.9) | 14.1 (12.5-17.3) | .580 |
| Transcranial Doppler velocity at screening (cm/s), median (IQR) | 200.0 (130.0-206.0) | 203.5 (146.3-219.0) | .113 |
| Height (cm) | 118.5 (11.8) | 115.1 (10.9) | .168 |
| Weight (kg) | 19.2 (4.5) | 17.3 (3.7) | .038 |
| BMI (kg/m2) | 13.6 (1.5) | 13.0 (1.6) | .070 |
| Height-for-age z score | −1.5 (1.3) | −1.8 (1.2) | .186 |
| Weight-for-age z score | −2.1 (1.1) | −2.6 (1.2) | .016 |
| BMI z score | −1.9 (1.3) | −2.2 (1.6) | .161 |
IQR, interquartile range; SD, standard deviation.
The therapy group comprised of children with abnormal transcranial Doppler velocities on hydroxyurea therapy, and the comparison group was children with nonelevated transcranial Doppler velocities.
Mean (SD) for continuous variables and counts (percentages) for categorical variables, unless otherwise noted.
χ2 test for counts, t test for means, Mann-Whitney U test for medians.
Baseline prevalence of undernutrition
The mean weight-for-age, height-for-age, and BMI z scores were all negative, at −1.5 or lower for those who survived and died during study participation (Table 2). All participants had a weight-for-age z score <1, signifying no overweight participants, and therefore participants with a weight-for-age z score >−1 were referred to as normal weight. The participants who died during the SPRING trial had a weight-for-age z score significantly lower than that of those who survived (−2.6 and −2.1, respectively; P = .016, Table 2). There was no significant difference between baseline anthropometric z scores in the therapy and comparison groups (Table 1).
The baseline prevalence of at least moderate (z score <−2) underweight, stunting, and wasting were 52.3%, 33.6%, and 42.7%, respectively (supplemental Table 2). All moderately stunted participants were also underweight; however, 2 children were severely stunted and did not reach the severe category in other domains. All the participants who were severely wasted and died during the study follow-up were also severely underweight at baseline.
Association of nutritional status and mortality in children with SCA
Of the participants who died (n = 24), 100% were underweight, and 54% (13 of 24) were stunted, underweight, and wasted (Figure 1). The proportion of children who survived but were underweight at baseline was 85.25% (n = 347). The proportion of deaths during follow-up was greater in those who were underweight vs normal weight, as determined by a Fisher exact test (6.5% vs 0.0%, respectively, P = .035, Table 3).
Venn diagram shows the overlap of stunted, underweight, and wasted children at baseline for those who died during study follow-up (n = 24). Stunted (height-for-age z score <−1.0), underweight (weight-for-age z score <−1.0), and wasted (BMI z score <−1.0) were defined using z scores calculated using the WHO growth references. The number and percentage of cases in each division are reported. The zero cells indicate that all stunted and/or wasted children were also underweight.
Venn diagram shows the overlap of stunted, underweight, and wasted children at baseline for those who died during study follow-up (n = 24). Stunted (height-for-age z score <−1.0), underweight (weight-for-age z score <−1.0), and wasted (BMI z score <−1.0) were defined using z scores calculated using the WHO growth references. The number and percentage of cases in each division are reported. The zero cells indicate that all stunted and/or wasted children were also underweight.
Survival stratified by baseline undernutrition measures categorized as not undernourished (greater than or equal to —1 z score) or undernourished (less than —1 z score) of the SPRING trial participants (n = 431) in the therapy (n = 220) and comparison groups (n = 211)
| Nutrition characteristics . | Therapy group (n = 220) . | Comparison group (n = 211) . | Combined population (n = 431) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Survived (n = 205) . | Died (n = 15) . | P value∗ . | Survived (n = 202) . | Died (n = 9) . | P value∗ . | Survived (n = 407) . | Died (n = 24) . | P value∗ . | |
| Underweight | .226 | .360 | .035 | ||||||
| No (weight-for-age z score ≥−1) | 27 (100.0) | 0 (0.0) | 33 (100.0) | 0 (0.0) | 60 (100.0) | 0 (0.0) | |||
| Yes (weight-for-age z score <−1) | 178 (92.2) | 15 (7.8) | 169 (94.9) | 9 (5.1) | 347 (93.5) | 24 (6.5) | |||
| Stunting | .779 | .733 | 1.000 | ||||||
| No (height-for-age z score ≥−1) | 67 (94.4) | 4 (5.6) | 76 (95.0) | 4 (5.0) | 143 (94.7) | 8 (5.3) | |||
| Yes (height-for-age z score <−1) | 138 (92.6) | 11 (7.4) | 126 (96.2) | 5 (3.8) | 264 (94.3) | 16 (5.7) | |||
| Wasting | .554 | .689 | 1.000 | ||||||
| No (BMI z score ≥−1) | 54 (91.5) | 5 (8.5) | 49 (98.0) | 1 (2.0) | 103 (94.5) | 6 (5.5) | |||
| Yes (BMI z score <−1) | 151 (93.8) | 10 (6.2) | 153 (95.0) | 8 (5.0) | 304 (94.4) | 18 (5.6) | |||
| Nutrition characteristics . | Therapy group (n = 220) . | Comparison group (n = 211) . | Combined population (n = 431) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Survived (n = 205) . | Died (n = 15) . | P value∗ . | Survived (n = 202) . | Died (n = 9) . | P value∗ . | Survived (n = 407) . | Died (n = 24) . | P value∗ . | |
| Underweight | .226 | .360 | .035 | ||||||
| No (weight-for-age z score ≥−1) | 27 (100.0) | 0 (0.0) | 33 (100.0) | 0 (0.0) | 60 (100.0) | 0 (0.0) | |||
| Yes (weight-for-age z score <−1) | 178 (92.2) | 15 (7.8) | 169 (94.9) | 9 (5.1) | 347 (93.5) | 24 (6.5) | |||
| Stunting | .779 | .733 | 1.000 | ||||||
| No (height-for-age z score ≥−1) | 67 (94.4) | 4 (5.6) | 76 (95.0) | 4 (5.0) | 143 (94.7) | 8 (5.3) | |||
| Yes (height-for-age z score <−1) | 138 (92.6) | 11 (7.4) | 126 (96.2) | 5 (3.8) | 264 (94.3) | 16 (5.7) | |||
| Wasting | .554 | .689 | 1.000 | ||||||
| No (BMI z score ≥−1) | 54 (91.5) | 5 (8.5) | 49 (98.0) | 1 (2.0) | 103 (94.5) | 6 (5.5) | |||
| Yes (BMI z score <−1) | 151 (93.8) | 10 (6.2) | 153 (95.0) | 8 (5.0) | 304 (94.4) | 18 (5.6) | |||
The therapy group comprised of children with abnormal transcranial Doppler velocities on hydroxyurea therapy, and the comparison group was children with nonelevated transcranial Doppler velocities.
Fisher exact test.
A total of 3 separate multivariate Cox regression models were constructed with continuous z scores for weight-for-age, height-for-age, and BMI. All model criteria included age, hemoglobin at screening, and sex. In the pooled analysis, in the model for weight-for-age z score, the HR for death was <1, so a lower z score was associated with an increased hazard of death (HR, 0.580; P = .004; 95% CI 0.399-0.843; Table 4). In the therapy group alone, the hazard of death with decreasing weight-for-age z score was also significant (HR, 0.593; P = .032; 95% CI, 0.300-0.956). However, height-for-age and BMI z scores were not associated with death in the pooled analysis (P = .092 and P = .117, respectively). When the treatment group (none, low- or moderate-dose) group was added to the model for weight-for-age z-score, there was no significant association with mortality (low dose: p=0.168; moderate dose: p=0.843, supplemental Table 3), and the HR for weight-for-age z-score remained significant (HR = 0.577, p = 0.005).
Baseline nutritional status z scores and risk of death
| Model . | Variable . | Therapy . | Comparison . | Combined population . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | ||
| Model 1: weight-for-age z score | Age | 0.912 | 0.700-1.118 | .494 | 0.912 | 0.677-1.156 | .546 | 0.886 | 0.729-1.076 | .222 |
| Female | 1.244 | 0.431-3.592 | .687 | 0.839 | 0.215-3.266 | .800 | 1.151 | 0.514-2.578 | .733 | |
| Hemoglobin (g/dL) | 1.343 | 0.679-2.656 | .396 | 0.595 | 0.704-2.714 | .190 | 0.873 | 0.546-1.396 | .570 | |
| Weight-for-age z score | 0.593 | 0.300-0.956 | .032 | 0.610 | 0.321-1.158 | .131 | 0.580 | 0.399-0.843 | .004 | |
| Model 2: height-for-age z score | Age | 0.929 | 0.713-1.211 | .586 | 0.976 | 0.719-1.323 | .875 | 0.916 | 0.756-1.111 | .373 |
| Female | 1.450 | 0.509-4.133 | .487 | 0.669 | 0.178-2.507 | .551 | 1.108 | 0.495-2.479 | .804 | |
| Hemoglobin (g/dL) | 1.275 | 0.640-2.543 | .490 | 0.563 | 0.253-1.248 | .157 | 0.864 | 0.535-1.396 | .551 | |
| Height-for-age z score | 0.692 | 0.446-1.074 | .101 | 0.950 | 0.547-1.650 | .855 | 0.753 | 0.541-1.047 | .092 | |
| Model 3: BMI z score | Age | 0.992 | 0.770-1.277 | .950 | 0.943 | 0.712-1.250 | .684 | 0.936 | 0.777-1.128 | .489 |
| Female | 1.364 | 0.475-3.912 | .564 | 0.970 | 0.238-3.963 | .966 | 1.128 | 0.503-2.530 | .771 | |
| Hemoglobin (g/dL) | 1.322 | 0.680-2.569 | .411 | 0.582 | 0.272-1.245 | .163 | 0.840 | 0.527-1.339 | .464 | |
| BMI z score | 0.891 | 0.569-1.396 | .615 | 0.629 | 0.386-1.026 | .063 | 0.778 | 0.568-1.065 | .117 | |
| Model . | Variable . | Therapy . | Comparison . | Combined population . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | ||
| Model 1: weight-for-age z score | Age | 0.912 | 0.700-1.118 | .494 | 0.912 | 0.677-1.156 | .546 | 0.886 | 0.729-1.076 | .222 |
| Female | 1.244 | 0.431-3.592 | .687 | 0.839 | 0.215-3.266 | .800 | 1.151 | 0.514-2.578 | .733 | |
| Hemoglobin (g/dL) | 1.343 | 0.679-2.656 | .396 | 0.595 | 0.704-2.714 | .190 | 0.873 | 0.546-1.396 | .570 | |
| Weight-for-age z score | 0.593 | 0.300-0.956 | .032 | 0.610 | 0.321-1.158 | .131 | 0.580 | 0.399-0.843 | .004 | |
| Model 2: height-for-age z score | Age | 0.929 | 0.713-1.211 | .586 | 0.976 | 0.719-1.323 | .875 | 0.916 | 0.756-1.111 | .373 |
| Female | 1.450 | 0.509-4.133 | .487 | 0.669 | 0.178-2.507 | .551 | 1.108 | 0.495-2.479 | .804 | |
| Hemoglobin (g/dL) | 1.275 | 0.640-2.543 | .490 | 0.563 | 0.253-1.248 | .157 | 0.864 | 0.535-1.396 | .551 | |
| Height-for-age z score | 0.692 | 0.446-1.074 | .101 | 0.950 | 0.547-1.650 | .855 | 0.753 | 0.541-1.047 | .092 | |
| Model 3: BMI z score | Age | 0.992 | 0.770-1.277 | .950 | 0.943 | 0.712-1.250 | .684 | 0.936 | 0.777-1.128 | .489 |
| Female | 1.364 | 0.475-3.912 | .564 | 0.970 | 0.238-3.963 | .966 | 1.128 | 0.503-2.530 | .771 | |
| Hemoglobin (g/dL) | 1.322 | 0.680-2.569 | .411 | 0.582 | 0.272-1.245 | .163 | 0.840 | 0.527-1.339 | .464 | |
| BMI z score | 0.891 | 0.569-1.396 | .615 | 0.629 | 0.386-1.026 | .063 | 0.778 | 0.568-1.065 | .117 | |
Multivariate Cox regression of factors associated with death among children with SCA in the SPRING Trial (n = 431). Separate models for height-for-age, weight-for-age, and BMI z scores.
Multivariate Cox regression models included age, sex, and hemoglobin at screening. The therapy group comprised children with abnormal transcranial Doppler velocities on hydroxyurea therapy, and the comparison group was children with nonelevated transcranial Doppler velocities.
Nutritional status is classified into weight-for-age, height-for-age, and BMI z scores using the WHO growth references.
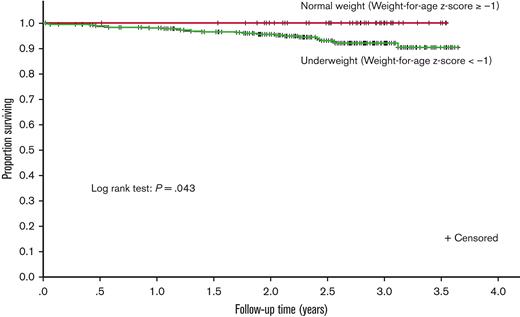
The Kaplan-Meier curve demonstrates that underweight participants had lower survival during follow-up than those who were normal weight (P = .043, Figure 2). The association with death persisted when the weight-for-age z score was grouped by severity into underweight categories as described in "Data collection and definitions" above (supplemental Table 2, P = .016). Of the children with severe underweight (weight-for-age z score <−3, 11.1% (10 of 80) died during follow-up.
Kaplan-Meier estimates of survival for participants during study follow-up. The survival curve compares the (unadjusted) survival of children who were underweight (weight-for-age z score <−1) at baseline to those who were not underweight (weight-for-age z score ≥−1). The weight-for-age z score used was the one at the index time (study enrollment).
Kaplan-Meier estimates of survival for participants during study follow-up. The survival curve compares the (unadjusted) survival of children who were underweight (weight-for-age z score <−1) at baseline to those who were not underweight (weight-for-age z score ≥−1). The weight-for-age z score used was the one at the index time (study enrollment).
In the subgroup analysis of only infectious disease mortality rather than all-cause mortality, a Cox regression model was constructed with the only significant covariate of weight-for-age z score. Because of the small number of participants with infectious-related deaths, the other covariates were not included. In the pooled analysis for weight-for-age z score, the HR for death was 0.506 (P = .008, 95% CI 0.304-0.840, supplemental Table 4).
Discussion
Undernutrition and SCA are independent risk factors for death for children in Nigeria, a country with an estimated 50% of the world’s newborns with SCA at ∼150 000 births per year.21 Although it is well established that in younger children decreasing weight-for-age z score is associated with death,11,14 to our knowledge, we have demonstrated the benefit of routine anthropometric measures to detect a modifiable risk factor for death in children with SCA, for the first time. In a prospective cohort of 431 children with SCA between 5 and 12 years of age, we demonstrated that a lower weight-for-age z score was associated with a higher hazard of death, and also that those simply being underweight (weight-for-age z score <−1) had a greater hazard of death than those not underweight. We also demonstrated that the relationship of weight-for-age z score and hazard of death remained in only infectious-related deaths.
Similar to children <5 years of age, we posit that underweight children with SCA >5 years of age die because of the increased incidence and even greater case fatality from infections.14,22 When including all deaths and in the subgroup analysis of only deaths attributable to infection, weight-for-age z score was predictive of death. BMI z score was not predictive of death. BMI identifies children with an acute decrease in weight16; whereas, severe underweight identifies acute, chronic, or combined undernutrition.13 Furthermore, given that children with SCA have lower heights, with increasing deficits in height-for-age z score with age,23-26 BMI may not optimally capture nutritional status. Another disadvantage of the BMI measurement is the requirement of both weight and height, a rate-limiting step for busy malnutrition treatment programs that are often understaffed.12 Importantly, in the SPRING study cohort, applying screening with weight-for-age z score <−1 would not have missed any child who died. The advantage of the weight-for-age z score as a screening measure for undernutrition is the ability to have a single, quickly obtained, and reproducible measure without the need for overly specialized training associated with additional anthropometric measurements.27 For these reasons, the Nigerian care team has implemented weight-for-age z score <−1, as a screening measure for undernutrition in at least 20 000 older children with SCA at the Kano SPRING trial study sites.
As expected, our study has limitations. Our study was a secondary data analysis of the nutritional status of participants from a clinical trial designed for stroke prevention. However, all-cause-deaths and death only in those with a history of fever or suspected infection were associated with weight-for-age z score at P values < .01, statistically significant after correcting for a secondary analysis. Thus we believe our results are biologically plausible24,28 and more likely to be a significant finding that will be replicated in future studies because they meet our prestated threshold of P < .01.20 We cannot exclude a biological difference between those with abnormal transcranial Doppler velocities receiving hydroxyurea and those without abnormal transcranial Doppler velocities not receiving hydroxyurea, although our regression models used relevant biological covariates to control for known differences between the 2 groups. Furthermore, the SPRING trial did not demonstrate any difference in the mortality rates between the 2 groups, and in our previous analysis we did not identify any relevant difference between the 2 groups associated with mortality.7 We do not have sufficient data to compare the growth trajectories of the children who died vs survived. The higher loss to follow-up rate among participants in the comparison group (8.6%) is a limitation. However, our loss to follow-up in the comparison group was only ∼6% higher than that of the therapy group, a proportion still considered small. We were unable to include direct measures of socioeconomic status; interviews during our previous studies revealed that mothers could not accurately assess household income. We could not analyze whether the participants were siblings, although none of the participants who died were related. Despite these limitations, the opportunity to identify children at risk for near-term death based on underweight status is within the scope of standard care for all children and should be emphasized as a potentially modifiable risk factor for death. Our findings should reinforce the importance of detecting, monitoring, and treating underweight older children with SCA living in a low-resource setting.
A perceived limitation is that our results occur in an economically distressed portion of Nigeria; however, ∼40% of the country lives under the poverty line of 1.00 US dollar per day.29 Furthermore, in 2020, most regions of Africa were economically stressed, and growth impairment is a common and expected comorbidity in children with SCA living in Africa,26 the continent where 75% of all children with SCA are born.1 Despite the significant undernutrition prevalence in Africa30 and the well-established fact that growth impairment and SCA are common comorbidities in Africa, to our knowledge, limited investigation has addressed the impact of undernutrition on the increased incidence of death in children with SCA younger or older than 5 years of age.
The most important finding from our prospective cohort study is that the weight-for-age z score is a reproducible and simple clinical measure that can screen older children with SCA at risk for death in a low-resource setting. Additional studies are needed to elucidate why being underweight is associated with death in older children with SCA. Future studies must determine evidence-based strategies to prevent or mitigate undernutrition in older children with SCA. Results of the study have changed the clinical management in older children with SCA in northern Nigeria. Now, older children with weight-for-age z score <−1 to −2 receive nutrition counseling; children with weight-for-age z scores <−2 are referred to the nutrition clinic for nutritional counseling and treatment according to local guidelines for moderate and severe acute malnutrition.
Acknowledgments
The authors thank Bilya Sani Musa, Mustafa Nateqi, Khadijah Bulama, Murtala Umar, Charity Dooshima Agba-Dooyum, Fahad Usman, Abdulrasheed Sani, Awwal Gambo, Jamila Ibrahim, Gloria Bahago, Jamil Galadanci, Leshana Saint Jean, and Jennifer C. Beck for providing the successful conduct of this study.
Research reported in this article was supported by grants from the National Institutes of Health (NIH) (R01NS094041) and NIH/NCATS (UL1 TR000445), and the generous donation of the Phillips family. L.K. was supported by the Fogarty International Center and the National Heart, Lung, and Blood Institute (NHLBI) of the NIH (D43 TW009337) and the National Institute of Diabetes & Digestive & Kidney Diseases of the NIH (T32DK007673).
The sponsors did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The findings and conclusions in this article are those of the authors and do not represent the official position of the National Institutes of Health.
Authorship
Contribution: L.K., M.R., and M.R.D. conceptualized and designed the secondary analysis; M.R.D., M.R., and L.K. had full access to all the data in the study and took responsibility for the data’s integrity and the data analyses accuracy; M.R.D., S.U.A., and M.R. designed the trial; S.U.A. and S.G. conducted the trial; L.K. and M.R. performed the analyses; M.R.D. and L.K. interpreted the results; L.K., M.R., and M.R.D. wrote the initial manuscript; and all authors reviewed the manuscript and approved its submission.
Conflict-of-interest disclosure: M.R.D. and his institution are the sponsors of 2 externally funded research investigator-initiated projects. Global Blood Therapeutics (GBT) will provide funding for the cost of the clinical studies. GBT was not a cosponsor of either study. M.R.D. did not receive any compensation for the conduct of these 2-investigator–initiated observational studies. M.R.D. is a member of the GBT advisory board for a proposed randomized controlled trial for which he receives compensation; is on the steering committee for a Novartis-sponsored phase II trial to prevent priapism in men; was a medical adviser for developing the CTX001 Early Economic Model; provided medical input on the economic model as part of an expert reference group for Vertex/CRISPR CTX001 Early Economic Model in 2020; and provided a consultation to the Formal Pharmaceutical Company about sickle cell disease in 2021 and 2022. The remaining authors declare no competing financial interests.
Correspondence: Michael R. DeBaun, Vanderbilt-Meharry Center of Excellence in Sickle Cell Disease, Vanderbilt Institute for Global Health, 2525 West End Ave, Suite 750, Nashville, TN 37203-1738; e-mail: m.debaun@vumc.org.
References
Author notes
This article is a continuation of a previous report and is a secondary analysis; primary outcome results from the Primary Prevention of Stroke in Children with Sickle Cell Disease in Nigeria trial were previously published.7
Data are available on request from the corresponding author, Michael R. DeBaun (m.debaun@vumc.org).
Fully anonymized data can be shared on request from any qualified investigator with approved study and data transfer agreement between Vanderbilt University Medical Center and the Institution.
The full-text version of this article contains a data supplement.