Key Points
Patients with sickle cell disease have weakened brain white matter connectivity compared with control subjects.
Weakened connectivity mediates the relationship between oxygen saturation and processing speed in patients.
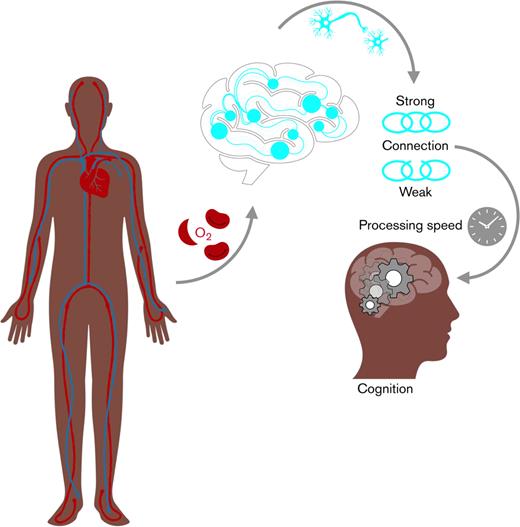
Visual Abstract
Oxygen saturation, brain white matte connectivity and processing speed in sickle cell disease
Oxygen saturation, brain white matte connectivity and processing speed in sickle cell disease
Abstract
In sickle cell disease, the relative importance of reduced hemoglobin (Hb) and peripheral oxygen saturation on brain structure remains uncertain. We applied graph-theoretical analysis to diffusion magnetic resonance imaging data to investigate the effect of structural brain connectivity on cognitive function, alongside the presence or absence, number, and volume of silent cerebral infarction. In patients, we investigated the relationships between network properties, blood oxygenation, and cognition (working memory and processing speed indices). Based on streamline counts and fractional anisotropy, we identified a subnetwork with weakened connectivity in 92 patients with sickle cell disease (91 homozygous for HbS [HbSS], 1 heterozygote with HbSβ0 thalassemia; 49 males; aged 8.0 to 38.8 y), compared with 54 control subjects (22 males; aged 6.7 to 30.6 y). Multiple regression analyses showed a significant effect of Hb on full-network edge density (P < .05) and of peripheral oxygen saturation on streamline-weighted subnetwork efficiency (P < .01). There were effects of fractional anisotropy-weighted full-network and subnetwork efficiency on working memory index (both P < .05), and of streamline-weighted subnetwork efficiency on processing speed index (P = .05). However, there were no effects of presence, number or volume of silent cerebral infarcts. Streamline-weighted efficiency was progressively lower with lower oxygen saturation, with a downstream effect on the processing speed index. In path analysis, indirect relationships between blood oxygenation and cognition, mediated by network properties, were better supported than direct alternatives, with an indirect relationship between low oxygen saturation and processing speed index in patients, mediated by structural connectivity efficiency in a subnetwork of the brain differing from control subjects. Our findings are consistent with the notion that cognitive impairment is primarily mediated by hypoxic–ischemic effects on normal-appearing white matter and highlight the utility of network-based methods in providing biomarkers of cognitive dysfunction in patients with sickle cell disease.
Introduction
The range of debilitating and often progressive neurological complications associated with homozygous sickle cell anemia (SCA, HbSS) and hemoglobin Sβ0 thalassemia (HbSβ0) includes cerebrovascular disease, cerebral infarction, global developmental delay, and cognitive dysfunction.1 Since screening and preventative treatments were introduced, the incidence of ischemic stroke has reduced substantially in children in high-resource countries.1 However, cognitive difficulties are increasingly recognized in patients with sickle cell disease (SCD) compared with control subjects, in specific domains including processing speed2 and working memory,3 as well as global measures such as full-scale intelligence quotient (IQ).4
Magnetic resonance imaging (MRI) shows a high prevalence of silent cerebral infarction,5 associated with male sex, higher blood pressure, and increasing age, which may be associated with cognitive impairment.6,7 Quantitative MRI studies have reported diffuse microstructural white matter injury8 and, more controversially, variability in regional brain volumes9-11 and cortical thickness.12,13 Low hemoglobin (Hb) and low peripheral oxygen saturation are associated with both microstructural injury and cognitive complications in SCD, suggesting a role for reduced oxygen delivery without hemodynamic compensation.14
To better understand the mechanisms underlying cognitive dysfunction, further advanced quantitative neuroimaging studies are warranted. Cognitive function is thought to be the emergent property of distributed, parallel neural networks,15 including white matter connections underpinning processing speed and a network involving the frontal, anterior cingulate, and parietal cortices involved in executive functions, including working memory. These can be modeled using advanced MRI by reconstructing the macroscopic structural or functional “connectome.” Compared with voxel-based analysis techniques, connectome approaches combine several sources of imaging information to offer a better representation of the interdependent nature of neural networks.
In patients with SCD, functional connectivity studies using resting-state functional MRI have revealed both decreased and increased connectivity compared with data from control populations of similar ages but various ethnicities who were healthy or anemic from other causes.16-19 Data on associations with cognitive function conflict,17,19,20 perhaps related to age or tests selected, while there have been few studies on the structural connectome, which involves mapping the physical white matter tracts connecting regions of cortical gray matter together. Global graph metrics, such as path length and global efficiency (representing the strength of connectivity or ease of communication within the network), correlate with intelligence and other cognitive measures, such as processing speed and working memory, in healthy populations of adults, adolescents, and children,21-24 as well as in adults with cognitive decline.25 In addition to age and sex,26 effects on the structural connectome of small vessel disease,27 anemia,25 and adaptation to hypoxia28 have been demonstrated in adults. An effect of socioeconomic status has been reported in children.29 In the SCD population, despite the evidence for an adverse effect on educational attainment30 and social and economic mobility,31,32 the possibility that structural connectivity underpins the cognitive difficulties, and is affected by potentially preventable exposure to hypoxia related to the anemia and oxygen desaturation, has received little attention.33,34
Two commonly used measures of structural connection strength are the number of streamlines or pathways forming the reconstructed white matter tract and the weighted mean anisotropy (ie, directional dependence of diffusion along it). These measures may be broadly interpreted as, respectively, the weight of evidence for the existence of a connection between each pair of cortical regions and the structural integrity of that connection.
The aim of this study was to generate structural connectomes from diffusion MRI streamline and anisotropy data, to identify whether there is a subset of cortical regions whose connectivity differs between patients with SCD and control subjects, and to explore whether, after adjusting for age and sex, such a subnetwork may be preferentially affected by Hb or oxygen saturation, and, in turn, affect cognition. Path analysis was applied to simultaneously consider both the direct effects of the various predictors on cognition and any indirect effects mediated by brain connectivity. We also explored the effects of treatment with hydroxyurea and chronic blood transfusion.
Methods
Sample
Participants with SCD were recruited between August 2015 and October 2019 from the SAC (Sleep Asthma Cohort35) (London study phase 3) and from baseline screening in the POMS2b (Prevention of Morbidity in SCD) study (phase 2).36,37 For both studies, inclusion criteria were age 8 to 50 years, ability to speak English, and a diagnosis of HbSS or HbSβ0; see references35-37 and supplemental Material in the data supplement for further details. Race-matched (ie, Black British) siblings, family members, or peers of participants with SCD were recruited as control subjects without SCD whether or not they had sickle cell trait or silent cerebral infarction on MRI. All participants and/or parents provided informed consent, and studies were approved by West London (SAC; 15/LO/0347) and Yorkshire (POMS; 15/YH/0213) National Health Service research ethics committees. Data on treatment with blood transfusion or hydroxyurea were available.35,36 As an estimate of socioeconomic status, local educational attainment was obtained from United Kingdom postcodes using the English Indices of Deprivation (supplemental Material),38 which is associated with processing speed in SCD.2
Cognitive and hematological variables
IQ (population mean, 100; standard deviation 15) was estimated using the 2-subtest WASI (Wechsler Abbreviated Scale of Intelligence) (POMS patients), or full-scale IQ assessed from the WISC-IV (Wechsler Intelligence Scale for Children, fourth edition) (SAC patients and control subjects <16 years) and the WAIS-IV (Wechsler Adult Intelligence Scale, fourth edition) (SAC patients and control subjects ≥16 years). WISC-IV or WAIS-IV subtests measuring working memory (Digit Span, Arithmetic) and processing speed (Coding, Symbol Search) indices were administered to all. Daytime oxygen saturation was measured using a fingertip pulse oximeter on the day of cognitive testing when the subject was in a steady state. In patients, steady-state Hb was recorded from the closest available routinely conducted full blood count.
MRI and network construction
MRI was performed on a 3T Siemens Prisma scanner (Siemens Healthcare; Erlangen, Germany) with 80 mT/m gradients and a 64-channel receive head coil. For diagnostic radiology, scans included an axial T2-weighted turbo spin echo sequence (TR = 8420 ms; TE = 68 ms; voxel size = 0.51 × 0.51 × 5.6 mm) and a high-resolution 3D fluid-attenuated inversion recovery (FLAIR) sequence (TR = 5000 ms; TE = 395 ms; TI = 1600 ms; T2-Preparation 125 ms; voxel size = 0.65 × 0.65 × 1.0 mm). An experienced neuroradiologist (D.E.S.) read each scan and classified a silent cerebral infarct as a hyperintensity on FLAIR of >3 mm in diameter and present on 2 planes on axial T2-weighted MRI, the accepted definition from the Silent Infarct Transfusion trial.39 A generous region of interest was manually drawn around each lesion. To remove any normal appearing white matter from the lesion regions of interest, a minimum intensity threshold was derived from the mean FLAIR intensity across the cortex (1.02 × meanFLAIR CORTEX), and voxels with FLAIR intensities below this threshold were removed.40,41
For network reconstruction and graph analysis, scans included a high-resolution T1-weighted magnetization-prepared rapid acquisition gradient echo sequence (TR = 2300 ms; TE = 2.74 ms; TI = 909 ms; flip angle = 8°; voxel size = 1 × 1 × 1 mm), and a multishell multiband (factor of 2) diffusion-weighted spin–echo echo–planar sequence42 (TR = 3050 ms; TE = 60 ms; voxel size = 2 × 2 × 2 mm). The latter used 60 diffusion-sensitization directions on each of 2 shells at b = 1000 s/mm2 and b = 2200 s/mm2, with 13 interleaved b = 0 images; signal-to-noise ratio for this sequence is >30 on the Prisma. Diffusion images were visually screened for motion artifacts and preprocessed using the “topup” and “eddy” tools from the FMRIB Software Library (FSL) 5.0.1043,44 for susceptibility-induced distortion and eddy current artifact correction. FSL-BEDPOSTX (Bayesian Estimation of Diffusion Parameters Obtained using Sampling Techniques modelling crossing fibres) was used to fit a “ball-and-3-sticks” diffusion model in each voxel,45 using a gamma distribution of diffusivities to handle multiple b-value data,46 in addition to the standard diffusion tensor model. Parcellation of the T1-weighted images was performed using FreeSurfer v5.3,47 resulting in 68 cortical regions of interest per subject. Individual FreeSurfer reconstructions were visually inspected for irregularities and manually edited if required.
Structural connectomes were constructed from the images (supplemental Material)48,49 and compared statistically between the patient and control subjects to identify a subnetwork differing significantly with regard to streamline count or fractional anisotropy. Graph theory measures were extracted from the full networks and the identified subnetworks under each weighting measure.50 These included edge density, which is the proportion of possible white matter connections between regions that existed (irrespective of weight), and global efficiency, which reflects the overall strength of connections within each graph.51
Relationships with cognition and blood oxygenation
Within the patient cohort, multiple regression models were constructed to investigate the degree of influence of network metrics and physiological (Hb, oxygen saturation, and blood pressure) and treatment variables on processing speed index and working memory index. In each case, full-scale IQ was included as a covariate, along with age, sex, socioeconomic status, and presence of silent cerebral infarction, to improve the specificity of any relationship to the measure under test. We used separate regression models to investigate the influence of physiological and treatment variables on edge density and the efficiency of the subnetworks, in the latter case using whole-brain efficiency as a covariate to control for global factors. Path analysis was subsequently performed to jointly consider both direct influences of the predictors of cognition (ie, age, sex, Hb, oxygen saturation, and treatment) and indirect relationships mediated by structural connectivity.
Results
Sample
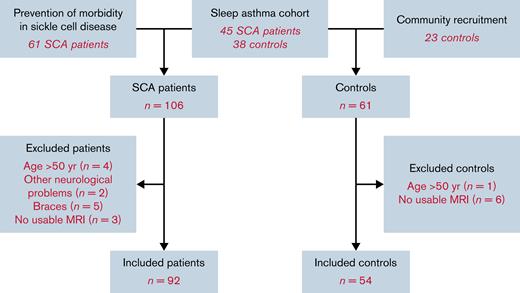
Of those recruited, 14 patients and 7 control subjects were excluded, as they were aged >50 or had neurological disorders or poor-quality imaging data (Figure 1). The remaining cohort (Table 1) included 92 patients with SCD and 54 control subjects (31 family members). Thirty-nine patients were receiving long-term treatment with hydroxyurea or blood transfusion (Table 1). The age and gender distributions of the 2 groups did not differ significantly, although patients demonstrated poorer cognitive performance, with a mean deficit of 6.6 processing speed index points and 5.2 working memory index points (Table 1).
Demographics and descriptive statistics for the patient and control subjects included in this study
| . | Control Subjects . | Patients . | P value . |
|---|---|---|---|
| Participants, N | 54 | 92 | |
| Age (y), mean ± SD (range) | 15.9 ± 5.2 (6.7 to 30.6) | 16.8 ± 6.0 (8.0 to 38.8) | .318 |
| Sex ratio | 32F:22M | 43F:49M | .197 |
| Genotype | 15 HbAA, 14 HbAS, 2 HbAC, 23 unknown | 91 HbSS, 1 HbSβ0 thalassemia | — |
| Seizures, n (%) | — | 3 (3) | — |
| Headaches, n (%) | — | 33 (36) | — |
| Systolic blood pressure >90th centile, n (%) | — | 26 (28) | — |
| Diastolic blood pressure >90th centile, n (%) | — | 3 (3) | — |
| Peripheral oxygen saturation (%), mean ± SD (range) | 98.4 ± 1.2 (96 to 100)∗ | 96.8 ± 2.6 (89 to 100) | .001 |
| Hb level (g/L), mean ± SD (range) | N/A | 87.2 ± 14.6 (51 to 134) | — |
| Hydroxyurea treatment, n (%) | — | 34 (37) | — |
| Chronic transfusion treatment, n (%) | — | 5 (5) | — |
| Penicillin and folic acid only, n (%) | — | 53 (58) | — |
| SCI, n (%) | 8 (15) | 38 (41) | <.0005 |
| SCI on hydroxyurea, n (%) | — | 14/34 (41) | — |
| SCI on no treatment, n (%) | — | 24/58 (41) | — |
| Number of SCIs, median (range) | 0 (0 to 8) | 0 (0 to 26) | .03 |
| Volume of SCIs, median (range) | 0 (0 to 1075) | 0 (0 to 5071) | .03 |
| Full-scale intelligence quotient, mean ± SD (range) | 101.4 ± 18.1 (75 to 112) | 92.6 ± 13.3 (58 to 122) | .011 |
| Processing speed index, mean ± SD (range) | 95.7 ± 4.2 (53 to 115) | 89.0 ± 13.2 (59 to 118) | .022 |
| Working memory index, mean ± SD (range) | 96.9 ± 9.7 (65 to 114) | 91.0 ± 13.0 (56 to 122) | .012 |
| Time between cognitive assessment and Hb (d), median (range) | — | −6.5 (−180 to 270) | — |
| . | Control Subjects . | Patients . | P value . |
|---|---|---|---|
| Participants, N | 54 | 92 | |
| Age (y), mean ± SD (range) | 15.9 ± 5.2 (6.7 to 30.6) | 16.8 ± 6.0 (8.0 to 38.8) | .318 |
| Sex ratio | 32F:22M | 43F:49M | .197 |
| Genotype | 15 HbAA, 14 HbAS, 2 HbAC, 23 unknown | 91 HbSS, 1 HbSβ0 thalassemia | — |
| Seizures, n (%) | — | 3 (3) | — |
| Headaches, n (%) | — | 33 (36) | — |
| Systolic blood pressure >90th centile, n (%) | — | 26 (28) | — |
| Diastolic blood pressure >90th centile, n (%) | — | 3 (3) | — |
| Peripheral oxygen saturation (%), mean ± SD (range) | 98.4 ± 1.2 (96 to 100)∗ | 96.8 ± 2.6 (89 to 100) | .001 |
| Hb level (g/L), mean ± SD (range) | N/A | 87.2 ± 14.6 (51 to 134) | — |
| Hydroxyurea treatment, n (%) | — | 34 (37) | — |
| Chronic transfusion treatment, n (%) | — | 5 (5) | — |
| Penicillin and folic acid only, n (%) | — | 53 (58) | — |
| SCI, n (%) | 8 (15) | 38 (41) | <.0005 |
| SCI on hydroxyurea, n (%) | — | 14/34 (41) | — |
| SCI on no treatment, n (%) | — | 24/58 (41) | — |
| Number of SCIs, median (range) | 0 (0 to 8) | 0 (0 to 26) | .03 |
| Volume of SCIs, median (range) | 0 (0 to 1075) | 0 (0 to 5071) | .03 |
| Full-scale intelligence quotient, mean ± SD (range) | 101.4 ± 18.1 (75 to 112) | 92.6 ± 13.3 (58 to 122) | .011 |
| Processing speed index, mean ± SD (range) | 95.7 ± 4.2 (53 to 115) | 89.0 ± 13.2 (59 to 118) | .022 |
| Working memory index, mean ± SD (range) | 96.9 ± 9.7 (65 to 114) | 91.0 ± 13.0 (56 to 122) | .012 |
| Time between cognitive assessment and Hb (d), median (range) | — | −6.5 (−180 to 270) | — |
HbAA, normal hemoglobin; HbAC, heterozygous for hemoglobins A & C; HbAS, heterozygous for hemoglobins A & S; HbSβ0, heterozygous sickle β0; HbSS,homozygous for hemoglobin S; SCI, silent cerebral infarct.
Difference statistics and P values from χ2 tests of equal proportions and two-sample t tests are given as appropriate, with significant P values (for α = 0.05) shown in bold.
Control subjects with peripheral oxygen saturation (n = 38).
Network differences between patients and control subjects
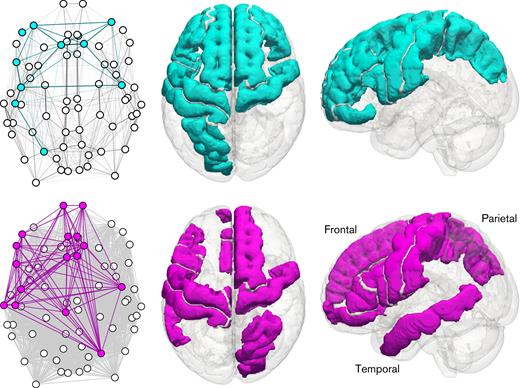
Within the streamline-weighted connectome, a densely interconnected subnetwork of 10 cortical regions of interest was found to differ between the patients and the control subjects, including the left and right precentral gyrus, left and right rostral middle frontal gyrus, left and right superior frontal gyrus, left inferior frontal gyrus (pars opercularis and pars orbitalis), left postcentral gyrus and left superior parietal gyrus. The equivalent fractional anisotropy-weighted subnetwork had a lower edge density, more similar to the full network, and contained 16 cortical regions of interest: the left and right precentral gyrus, left and right frontal pole, left and right caudal anterior cingulate cortex, left and right rostral anterior cingulate cortex, right superior frontal gyrus, right superior parietal gyrus, left frontal gyrus, left paracentral gyrus, left postcentral gyrus, and left middle temporal gyrus. In every region in the 2 identified subnetworks, median connectivity was lower in the patient group. The 2 subnetworks shared 6 regions of interest, a majority of the smaller network’s nodes (topologies in Figure 2).
Topological and standard-space representations of the subnetworks differing between patients and control subjects for the streamline-weighted case (top, cyan) and the fractional anisotropy-weighted case (bottom, magenta). Left column: axial topological view of the whole network, with subnetwork nodes and edges colored. Edge opacity is proportional to weight. Middle column: axial view of the brain, with regions involved in the subnetwork colored. Right column: sagittal view of the brain, with regions involved in the subnetwork colored. The left of the brain is shown on the left in axial views; sagittal views are from the left.
Topological and standard-space representations of the subnetworks differing between patients and control subjects for the streamline-weighted case (top, cyan) and the fractional anisotropy-weighted case (bottom, magenta). Left column: axial topological view of the whole network, with subnetwork nodes and edges colored. Edge opacity is proportional to weight. Middle column: axial view of the brain, with regions involved in the subnetwork colored. Right column: sagittal view of the brain, with regions involved in the subnetwork colored. The left of the brain is shown on the left in axial views; sagittal views are from the left.
Table 2 details key graph theory properties for the full network and identified subnetworks under each edge weighting scheme. The edge density in the full network is the same under both weightings because the presence or absence of edges is only determined by the tractography. Edge density and efficiency are, on average, lower in patients compared with control subjects, with moderate to large effect sizes. The exception is streamline-weighted efficiency in the full network, which is indistinguishable between the groups, indicating no change in the overall morphology of reconstructed tracts in aggregate across the whole brain. The group differences are larger in magnitude for the subnetworks, as expected since these networks are selected based on a contrast between the groups.
Graph properties for the full network under each weighting scheme, as well as the subnetworks composed of those cortical nodes whose strengths are significantly different between patients and control subjects
| . | Whole-brain network (streamline-weighted) . | Whole-brain network (FA-weighted) . | Selected subnetwork (streamline-weighted) . | Selected subnetwork (FA-weighted) . |
|---|---|---|---|---|
| Cortical ROIs (nodes), N | 68 | 68 | 10 | 16 |
| Mean (SD) edge density, % | ||||
| Control Subjects | 75.0 (4.5) | 75.0 (4.5) | 98.3 (1.7) | 79.9 (8.6) |
| Patients | 71.5 (5.1) | 71.5 (5.1) | 96.3 (3.1) | 70.9 (8.0) |
| Effect size (Cohen’s d) | −0.72 | −0.72 | −0.76 | −1.09 |
| Mean (SD) global efficiency | ||||
| Control Subjects | 0.314 (0.034) | 0.423 (0.016) | 0.384 (0.085) | 0.421 (0.024) |
| Patients | 0.314 (0.053) | 0.414 (0.017) | 0.315 (0.066) | 0.399 (0.023) |
| Effect size (Cohen’s d) | 0.01 | −0.53 | −0.94 | −0.97 |
| . | Whole-brain network (streamline-weighted) . | Whole-brain network (FA-weighted) . | Selected subnetwork (streamline-weighted) . | Selected subnetwork (FA-weighted) . |
|---|---|---|---|---|
| Cortical ROIs (nodes), N | 68 | 68 | 10 | 16 |
| Mean (SD) edge density, % | ||||
| Control Subjects | 75.0 (4.5) | 75.0 (4.5) | 98.3 (1.7) | 79.9 (8.6) |
| Patients | 71.5 (5.1) | 71.5 (5.1) | 96.3 (3.1) | 70.9 (8.0) |
| Effect size (Cohen’s d) | −0.72 | −0.72 | −0.76 | −1.09 |
| Mean (SD) global efficiency | ||||
| Control Subjects | 0.314 (0.034) | 0.423 (0.016) | 0.384 (0.085) | 0.421 (0.024) |
| Patients | 0.314 (0.053) | 0.414 (0.017) | 0.315 (0.066) | 0.399 (0.023) |
| Effect size (Cohen’s d) | 0.01 | −0.53 | −0.94 | −0.97 |
FA, fractional anisotropy; ROI, region of interest; SD, standard deviation.
An absolute Cohen’s d value of 0.5 is conventionally considered to be a medium effect, and 0.8 a large effect.
Correlation analyses with cognition and blood oxygenation
Figure 3 shows the full correlation matrix between the continuous graph properties, cognitive scores, blood oxygenation measures, and age. Strong relationships within the blocks of graph properties and cognitive scores are immediately visible, but there is also evidence of univariate relationships between these measures, age, Hb, and oxygen saturation.
Matrix of marginal Pearson correlation coefficients between blood oxygenation, network, and cognitive variables in patients. Pale colors indicate weak correlations; stronger negative correlations are shown in darker shades of blue, and stronger positive correlations in darker shades of red. Absolute r-values >0.2 are also annotated in the appropriate cell. fED, full-network edge density; fGE, full-network global efficiency; FSIQ, full-scale intelligence quotient; PSI, processing speed index; sGE, subnetwork global efficiency; SpO2, peripheral oxygen saturation; WMI, working memory index.
Matrix of marginal Pearson correlation coefficients between blood oxygenation, network, and cognitive variables in patients. Pale colors indicate weak correlations; stronger negative correlations are shown in darker shades of blue, and stronger positive correlations in darker shades of red. Absolute r-values >0.2 are also annotated in the appropriate cell. fED, full-network edge density; fGE, full-network global efficiency; FSIQ, full-scale intelligence quotient; PSI, processing speed index; sGE, subnetwork global efficiency; SpO2, peripheral oxygen saturation; WMI, working memory index.
Regression analyses
All regression analyses only included patients with SCD, assessing working memory index and processing speed index as dependent variables. Our multiple linear regression analysis with working memory index as the dependent variable found that higher scores were associated with higher fractional anisotropy-weighted full-network efficiency (standardized β = 0.34; t70 = 2.03; P < .05), but that this was partly counterbalanced by an opposite effect in the full fractional anisotropy subnetwork (β = −0.30; t70 = −2.17; P < .05), suggesting that it is not this subnetwork in particular which drives variability in working memory index between patients. With processing speed index as the dependent variable, there was a significant effect of age after controlling for full-scale IQ (β = −0.25; unstandardized coefficient, −0.53/y; t78 = −2.62; P = .01), but there was no significant effect of sex, silent cerebral infarct status, blood pressure, or full-network edge density. Silent cerebral infarct status, blood pressure, and socioeconomic status (index of multiple deprivation) had no effect in either model (all P > .35).
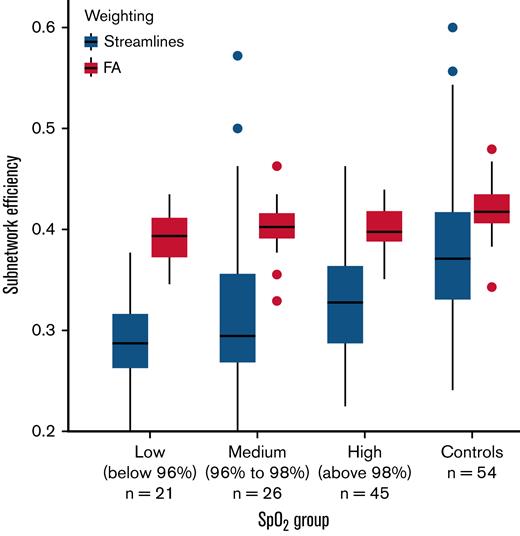
In further regression models considering hematological effects on the brain networks, Hb was marginally associated with full-network edge density (standardized β = 0.22; t79 = 1.91; P = .06), but oxygen saturation had no effect. By contrast, higher oxygen saturation was marginally associated with higher streamline-weighted subnetwork efficiency (β = 0.16; t78 = 1.95; P = .05), but Hb had no effect after controlling for full-network efficiency. Figure 4 illustrates that the relationship between oxygen saturation and streamline-weighted subnetwork efficiency is progressive between 3 subgroups of patients, split by oxygen saturation: low (oxygen saturation <96%; n = 21), medium (96% ≤ oxygen saturation < 98%; n = 26), and high (oxygen saturation ≥98%; n = 45). The control group is also shown for comparison, although the subnetwork was chosen specifically based on its difference relative to control subjects. There was no significant effect on fractional anisotropy-weighted subnetwork efficiency (Figure 4). Silent cerebral infarct status, blood pressure, and socioeconomic status showed no effects.
Boxplots of subnetwork efficiency in each of 3 patient subgroups, divided in order by measured SpO2, and for the control group for comparison. SpO2, peripheral oxygen saturation.
Boxplots of subnetwork efficiency in each of 3 patient subgroups, divided in order by measured SpO2, and for the control group for comparison. SpO2, peripheral oxygen saturation.
Multiple linear regression analysis, including hydroxyurea treatment as a predictor with working memory index as the dependent variable, found that lower scores were associated with hydroxyurea treatment (see supplemental Material).
Path analysis
Figure 5 shows our full path analysis, allowing for direct and indirect effects of blood oxygenation, age, and sex on structural connectivity and cognition. Silent cerebral infarct status, blood pressure, and socioeconomic status were not considered in this analysis, as they were not influential in the preceding regressions. The maximum likelihood fitted model was a good fit to the data (standardized root mean square residual = 0.045, root mean square error of approximation = 0.035) and reflected our conventional regressions, as the effects of the hematological variables on cognition were largely mediated by network measures of structural connectivity, with an additional direct link between Hb and full-scale IQ. The hydroxyurea and blood transfusion data are shown in the supplemental Material.
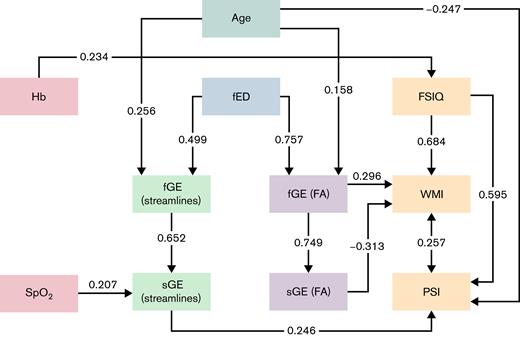
Path analysis diagram showing direct and indirect relationships between blood oxygenation measures (red boxes, left) and cognition (orange boxes, right) in patients. Mediation pathways via unweighted (blue), streamline-weighted (green), and fractional anisotropy-weighted (lavender) network measures appear toward the center of the diagram. Standardized coefficients are shown on solid lines for relationships with P < .05. The double-headed arrow between processing speed index and working memory index reflects a residual covariance term. FA, fractional anisotropy; fED, full-network edge density; fGE, full-network global efficiency; FSIQ, full-scale intelligence quotient; PSI, processing speed index; sGE, subnetwork global efficiency; SpO2, peripheral oxygen saturation; WMI, working memory index.
Path analysis diagram showing direct and indirect relationships between blood oxygenation measures (red boxes, left) and cognition (orange boxes, right) in patients. Mediation pathways via unweighted (blue), streamline-weighted (green), and fractional anisotropy-weighted (lavender) network measures appear toward the center of the diagram. Standardized coefficients are shown on solid lines for relationships with P < .05. The double-headed arrow between processing speed index and working memory index reflects a residual covariance term. FA, fractional anisotropy; fED, full-network edge density; fGE, full-network global efficiency; FSIQ, full-scale intelligence quotient; PSI, processing speed index; sGE, subnetwork global efficiency; SpO2, peripheral oxygen saturation; WMI, working memory index.
Discussion
Using graph-theoretical analysis to investigate the structural connectome, we have shown that white matter connectivity is disrupted in children and young adults with SCD. The degree of disruption in the full network for fractional anisotropy is related to poorer cognitive performance in working memory, while disruption to the streamline subnetwork is associated with slower processing speed. Further, we demonstrated that this connectivity mediates the relationship between measures of blood oxygenation and cognitive dysfunction. Our findings are consistent with the notion that cognitive deficits are mediated not primarily by the presence of silent cerebral infarction but instead by hypoxic–ischemic effects on connections in normal-appearing white matter.
Graph-theoretical analysis of brain connectivity is an increasingly popular framework for understanding the relationships between integrated neural information processing and functional outcomes. There are, however, methodological variations between studies taking this general approach.49 In this study, we sought convergent evidence of differences between patients with SCD and control subjects from networks constructed using 2 distinct measures of connection strength: the number of streamlines forming the reconstructed white matter tract and the weighted mean anisotropy along the tract. We then showed that fractional anisotropy-weighted network efficiency was linked to variation in working memory performance in patients, and streamline-weighted efficiency was progressively lower with lower peripheral oxygen saturation (Figure 4), with a downstream effect on processing speed (Figure 5). Finally, we demonstrated using a path analysis that this indirect relationship between blood oxygenation and cognition is generally better supported by our data than more direct alternatives (Figure 5).
Despite evidence for more efficient structural connectivity, there was a negative effect of hydroxyurea on working memory index in regression analyses, potentially because cognition was already compromised before treatment started. Recommendations to prescribe widely were introduced relatively recently in the United Kingdom.57 Age at the first prescription and prescription duration varied in our sample, and there were no data on adherence.
For chronic transfusion, numbers were limited, so the path analysis was recomputed with these patients excluded. With the negative effects of hydroxyurea and chronic transfusion excluded from the model, the effect of oxygen saturation on the subnetwork was slightly stronger, while in the path analysis, oxygen saturation remained a predictor of processing speed index and Hb became a predictor of full-scale IQ. To determine the effect of treatment on the structural connectome, longitudinal prospective studies will be essential.
The subnetworks identified using the 2 different weighting schemes overlapped substantially, with 6 of the 10 cortical regions of interest in the smaller network being shared with the larger one (Figure 2). This overlap supports the robustness of our findings to the choice of weighting and backs up the spatial specificity of the connectivity differences of interest. The streamline-weighted network, in particular, also displays a degree of symmetry, suggesting that those differences are likely to be bilateral. These findings are consistent with previous reports of widespread bilateral reductions in microstructural tissue integrity in normal-appearing white matter in patients with SCD.8
Our data (Figures 2 and 4) show that connection weights, and hence global efficiencies, are less variable in fractional anisotropy-weighted networks than streamline-weighted networks, as anisotropy is relatively consistent in white matter tracts from mid-childhood,58 whereas tractography-based streamline counts can vary widely based on factors such as the distance between their endpoints. Although the latter is sometimes a nuisance effect, in the current context, this is mitigated by comparing networks involving a fixed set of brain regions across all participants.
Our path analysis demonstrates that low oxygen saturation selectively affects processing speed (Figure 5). The moderately strong relationship between low oxygen saturation and processing speed index is mediated by the efficiency of structural connectivity in a subnetwork of the brain that differs from controls, on average. Hb directly affects general intelligence, measured by full-scale IQ, but also weakly influences the overall extent of connectivity in the brain. Working memory performance is affected by anisotropy in the brain, which is linked to microstructural integrity, but since the signs of the effects at full- and subnetwork levels were opposite, it is probable that this link is either global or tied to a different subnetwork to that detected in our patient vs control analysis. Indeed, while our data-driven subnetwork is obtained in an unbiased fashion, a useful avenue for future work would be to consider networks expected a priori to be important in patients with SCD.
Disproportionately reduced oxygen delivery,59 abnormal oxygen extraction in response to reduced vascular reserve,60 and reduced volume of gray as well as white matter61 are common in SCD. Silent cerebral infarcts typically occur in the white matter of the border zones between the large intracranial arteries.40,62 Networks between nodes in the gray matter traversing affected regions are potentially at risk, as shown in a recent functional connectivity study.19 In this data-driven structural connectome study, the networks include the gray matter of the frontal, parietal, and temporal lobes. We were not able to specifically address connectivity in border zone regions between the territories of the middle, anterior, and posterior cerebral arteries where the cerebral blood flow is relatively low and silent cerebral infarcts are most common.
We observed no independent or additional effects of silent cerebral infarct presence, number, or volume on either working memory index or processing speed index. Other high-resolution MRI studies have similarly found no differences in cognition between patients with and without silent cerebral infarcts,40 and no effects of silent cerebral infarction on relationships between cognition and regions of reduced white matter integrity or functional connectivity.2,17,19 Taken together, these findings suggest that cognitive impairment is primarily mediated by disruption of networks in normal-appearing white matter in SCD, with silent cerebral infarcts representing the “tip of the iceberg.” It is also possible that silent cerebral infarct position in relation to eloquent networks contributes to some patients.
In line with previous reports of associations in patients with SCD,8,62 our path analysis also indicates that the observed disruptions to connectivity are likely hypoxic–ischemic in nature, potentially driven by a failure to adequately compensate for acute and/or chronic reductions in oxygen delivery.14 Acute silent ischemic cerebral events occur in patients with SCD, although not all transition into later observable silent cerebral infarcts63; further work should establish whether these are associated with reduced structural connectivity. The observed differential effects of Hb and oxygen saturation on brain connectivity are consistent with a resting-state functional MRI study that reported associations between regions of increased functional connectivity and reduced oxygen saturation, but not Hb.17 A possible explanation is the different compensatory hemodynamic response to reduced oxygen saturation and Hb observed in non-SCD populations,64 with a stronger response to oxygen desaturation, which may place a greater strain on vascular reserve. The direct effect of Hb on full-scale IQ is in line with a recent study reporting improvement in executive function soon after, as opposed to immediately before, transfusion in patients with SCD on chronic regimens,65 potentially reflecting a direct but temporary improvement in oxygen delivery. Additional exploration of relationships between connectivity and acute and steady-state hemodynamic changes, the downstream and likely more proximate contributors to injury, may help shed light on some of these possibilities.
An important limitation of the present study was our reliance on Hb concentrations collected in routine clinical care in patients with SCD, with no Hb for the control subjects, as including a blood draw is typically refused by ethics committees in the United Kingdom, particularly in children, if there is no benefit to the individual. The available data in people with sickle cell trait suggests that Hb is normal, but we elected not to impute. We, therefore, could not include the relatively large, compared with previous studies of functional connectivity in SCD, population of age- and race-matched control subjects in the path analysis. There was also significant between-patient variation in the time between MRI and the closest blood draw, with measurements ranging from 1 week to close to a year before MRI. There are few data on the stability of routinely collected measures, and we cannot exclude the possibility that our ability to detect a stronger effect of Hb was reduced. A related limitation was the inclusion of patients prescribed different treatment regimens, although we confirmed that the overall structure of relationships between variables remained when patients receiving chronic transfusion were excluded from the path analysis. Moreover, both brain structure and cognitive function have been shown to be influenced by socioeconomic factors and poverty. We did not include income in the models as some families preferred not to answer this question.66,67 However, we did recruit many family members as control subjects and included an estimate of local educational attainment as a proxy. Future studies should consider modeling direct measures, including parental education and profession as well as income, alongside disease biomarkers. There were no prior data to inform a power calculation, this was a convenience sample, and we took the conservative approach of not correcting for multiple comparisons. There was only about half the number of control subjects as patients, but the data-driven comparison was clear, and the path analysis included only patients with SCD.
Young adults may have acquired silent cerebral infarcts and altered white matter connectivity during brain development many years before. The Wechsler scales are different for adults and children, and, as well as measuring intelligence, we estimated short-form IQ in some; however, the available evidence suggests that the WASI, WISC-IV, and WAIS-IV are closely correlated. We have not found differences between children and adults in the relationship between cognitive measures and presence, number, or volume of silent cerebral infarction,40 and there is no evidence that silent cerebral infarct tissue characteristics are different beyond the acute phase.63 The cross-sectional design meant that it was difficult to determine the effect of treatment. Longitudinal studies and randomized treatment trials using the structural connectome as an endpoint will be important, particularly if adherence to the prescribed management strategy can be established. Of interest, given the importance of pain to patients with SCD, is the significant degree of overlap between our subnetworks and the previously described network underlying pain perception.68,69 Altered functional connectivity has been observed in regions associated with pain processing,18,70 consistent with central sensitization to chronic daily pain and/or acute pain crises.71,72 However, we only had pain diary data for the POMS patients,71,72 so we did not investigate associations with pain.
Conclusions
By applying graph analysis to diffusion MRI in patients with SCD, we showed reductions in structural connectivity mediating relationships between measures of blood oxygenation and cognitive functioning. Our data suggest that cognitive dysfunction is mediated by potentially reversible hypoxic–ischemic effects on networks in normal-appearing white matter and highlight the need to move beyond a focus on the presence or absence of silent cerebral infarction in this vulnerable population. Measures of structural brain connectivity may serve as useful biomarkers to monitor disease trajectories and study therapeutic interventions.73
Acknowledgments
H.S. and M.K. were funded by Action Medical Research (GN2509). J.M.K. was funded by Great Ormond Street Children’s Charity (V4615). A.M.H. was supported in part by a grant from the National Heart, Lung, and Blood Institute (NHLBI) (1F32HL143915). The National Institute for Health Research (PB-PG-1112-29099) and NHLBI (R01HL079937) provided funding for patient recruitment. The work was supported by the National Institute for Health Research and Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and the Institute of Child Health (IS-BRC-1215-20012).
Authorship
Contribution: J.D.C., H.S., J.M.K., A.S., M.K., and D.E.S. collected data and performed experiments; O.W., M.L., B.I., M.P., S.C., D.C.R., J.H., and M.A. collected data; J.D.C. analyzed results and made the figures; J.D.C., J.M.K., H.S., F.J.K., and C.A.C. designed the research; J.D.C., H.S., A.M.H., C.L., F.J.K., and C.A.C. wrote the manuscript; and all authors read and approved the manuscript.
Conflict-of-interest disclosure: F.J.K. receives funding for transcranial Doppler training from Global Blood Therapeutics, and during 2019, received honoraria from Bluebird Bio, Novartis, and Johnson & Johnson. The remaining authors declare no competing financial interests.
Correspondence: Fenella Kirkham, Research, Developmental Neurosciences, UCL Great Ormond Street Institute of Child Health, 30 Guilford St, London WC1N 1EH, United Kingdom; e-mail: fenella.kirkham@ucl.ac.uk.
References
Author notes
∗J.D.C., H.S., and J.M.K. are joint first authors.
†F.J.K. and C.A.C. are joint senior authors.
Data are available on request from the corresponding author, Fenella Kirkham (fenella.kirkham@ucl.ac.uk).
The full-text version of this article contains a data supplement.