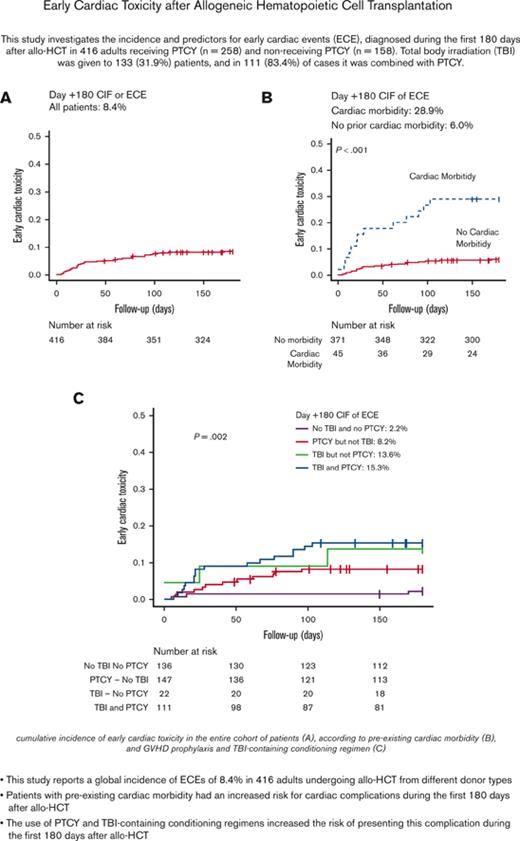
Key Points
Using PTCY for GVHD prevention and TBI-based conditioning regimens increased the risk for early cardiac toxicity in adults undergoing allo-HCT.
Abstract
This study investigates early cardiac events (ECEs) occurring during the first 180 days after allogeneic hematopoietic cell transplant (allo-HCT) in 416 adults receiving posttransplant cyclophosphamide (PTCY) (n = 258) or not receiving PTCY (n = 158). Total body irradiation (TBI) was given to 133 (31.9%) patients, of whom 111 (83.4%) received TBI combined with PTCY. The day +180 cumulative incidence function (CIF) of ECEs was 8.4%, with heart failure (n = 13) and pericardial complications (n = 11) being the most prevalent complications. The incidence of ECEs was higher in patients receiving PTCY, and receiving TBI. ECEs were more prevalent in haploidentical HCTs than in matched sibling donor, 10/10 HLA-matched unrelated donor, and 9/10 HLA-mismatched unrelated donor allo-HCTs. As for the ECE risk from the combination of PTCY and TBI, the multivariate analysis reported that patients receiving PTCY without TBI, TBI without PTCY, and TBI with PTCY were at higher risk for ECEs compared with patients receiving neither PTCY nor TBI. Pre-existing cardiac morbidity predicted ECEs. However, using high-dose CY-containing preparative regimens did not increase the risk for cardiac toxicity at +180 days after allo-HCT. ECEs were associated with higher nonrelapse mortality and lower overall survival. Considering that PTCY and TBI were predictors for ECEs, and the impact of this complication on transplant mortality, the implementation of cardiac monitoring plans could be appropriate in patients receiving these medications.
Introduction
Allogeneic hematopoietic cell transplant (allo-HCT) is a curative strategy for patients with high-risk hematological disorders.1 Moreover, the progressive reduction in transplant-related toxicity is expanding the indication of allo-HCT to older patients and to patients with comorbidities.2,3 Posttransplant cyclophosphamide (PTCY), combined with other immunosuppressant agents, has become the standard graft-versus-host disease (GVHD) prophylaxis for haploidentical HCT (haplo-HCT),4 and its use is being expanded to matched sibling donor (MSD), 10/10 HLA-matched unrelated (MUD), and 9/10 HLA-mismatched unrelated donor (MMUD) allo-HCT, with notable success.5-7
Cyclophosphamide (CY) is an alkylating agent of the nitrogen mustard class used to treat different malignant and autoimmune disorders, and is included as part of the preparative regimens in allo-HCT.8 CY-containing conditioning regimens have been associated with rates of cardiac toxicity that range from 1% to 17%.9,10 However, limited studies have investigated how PTCY prophylaxis interacts with the risk of cardiac toxicity.11-13
PTCY-based prophylaxis was implemented at our institution to perform haplo-HCT in 2013, and it was progressively expanded to all allo-HCTs performed independently of the selected donor type.14,15 Moreover, based on the number of cases in which PTCY was combined with total body irradiation (TBI), an independent risk factor for cardiac toxicity, we decided to investigate the association between PTCY and cardiac complications controlling for the effect of TBI.16,17 This study investigates the incidence and predictors for early cardiac events (ECEs) after allo-HCT, with particular attention to the effect of PTCY and donor type on the probability of presenting this complication.
Methods
Patient selection
This study included 416 adults with malignant hematological disorders who underwent their first allo-HCT at Hospital Clinic de Barcelona, Spain, between January 2014 and October 2021. Two hundred fifty-eight (62.0%) patients received PTCY-based prophylaxis. Data were collected retrospectively and updated in June 2022. This study was approved by the ethics committee of the Hospital Clinic de Barcelona and was conducted in accordance with the Declaration of Helsinki.
Main allo-HCT information
Myeloablative conditioning regimens mainly contained high-dose busulfan (3.2 mg/kg per day intravenously [IV] for 4 days) or 12 Gy of TBI, combined with fludarabine (30 mg/m2 per day IV for 4 days) or with CY (total dose: 120 mg/kg). Reduced-intensity conditioning regimens were composed mainly by lower doses of busulfan (3.2 mg/kg per day IV for 3 days), or 8 Gy of TBI combined with standard doses of fludarabine. All patients undergoing haplo-HCT received 2 Gy of TBI when TBI was not included as part of the conditioning regimen. Unmanipulated T-cell replete stem cell grafts were infused on day 0. Granulocyte colony-stimulating factor was not administered during the study period.
PTCY-based prophylaxis consisted of 50 mg/kg per day of IV CY on days +3 and +4, followed by tacrolimus initiated on day +5. Patients receiving grafts from haploidentical donors received mycophenolate mofetil from day +5 to day +35. None of the patients receiving PTCY received CY as part of the preparative regimen. Other prophylaxis combined calcineurin inhibitors (CNIs) with standard doses of methotrexate, mycophenolate mofetil, or sirolimus. No patient received antithymocyte globulin. Immunosuppressant medication was maintained therapeutic until day +90 and tapered down progressively to day +180 in patients receiving PTCY and to day +250 in those who did not.
Cardiac toxicity definition, monitoring, and study design
ECE was considered the main variable of interest. ECE was defined as any new episode of arrhythmia, heart failure, acute pulmonary edema, myocardial infarction or ischemia, pericarditis or pericardial effusion, or left ventricular systolic dysfunction (defined as a decrease in the left ventricular ejection fraction [LVEF] of >10% points), diagnosed within the first +180 days after the stem cell infusion. Cardiac complications were defined and graded according to the National Cancer Institute common terminology criteria for adverse events, version 4.0.14. Because all patients included in the study received CNI, hypertension attributed to CNI was not considered an adverse cardiac event. In addition, cardiac toxicities diagnosed before the administration of PTCY were not accounted as an event.
Pretransplant cardiac evaluation, monitoring, and supportive care were homogeneous during the study period. All patients with prior history of cardiac disease underwent an updated evaluation by their respective cardiologists before being cleared for allo-HCT. A transthoracic echocardiography (ECHO) and an electrocardiogram (ECG) were performed on all candidates during the pretransplant assessment. Patients with relevant abnormalities at the ECHO or ECG and without a history of cardiac disorders were considered patients with pre-existing cardiac morbidity. Patients with borderline LVEF (between 45% and 49%) and without prior history of cardiac disorder were considered patients with pre-existing cardiac morbidity only if additional abnormalities were documented in the pretransplant cardiac assessment.
During the admission for allo-HCT, daily anamnesis, physical examination, weight measurement, and fluid balance monitoring were routinely performed on all patients. Posttransplant cardiac function was not routinely monitored during the study period, and the cardiology department was consulted only in the presence of cardiac complications. Additional definitions have been incorporated into the supplemental Material.
Statistical analysis
The statistical analysis firstly explored the incidence and risk factors for ECEs. These risk factors included, among others, the use of PTCY and TBI. The cumulative incidence of ECEs was analyzed using Gray proportional hazard regression models for competing risk analyses and accounting for death as a competing event. A subanalysis was conducted exploring ECEs according to the donor type.
A second analysis investigated the impact of ECEs on posttransplant outcomes, including overall survival (OS) and nonrelapse mortality (NRM). Posttransplant follow-up was censored at 2 years, except for patients undergoing a second allograft, in whom the posttransplant follow-up was censored on the day of the second stem cell infusion. The variable ECEs was defined as a time-dependent variable. The impact of variables on OS and NRM was analyzed using univariate and multivariate Cox and Fine-Gray proportional hazard regression models. Those variables found to be statistically significant in the univariate model or considered clinically relevant were included in the multivariate model. All P values were 2-sided, and for the statistical analyses, P < .05 indicated a statistically significant result. Statistical analysis was performed using EZR.18
Results
General patient and allo-HCT information
Overall, the median age was 53 years (range, 18-70 years), and acute myeloid leukemia was the most prevalent baseline disease (37.5%). Pre-existing cardiac morbidity was documented in 45 patients (10.8%), with arrhythmias (n = 13), heart failure (n = 6), and pericardial complications (n = 6) being most frequent. Seven patients (1.7%) had a LVEF of <50%, and 5 of them had additional pretransplant cardiac morbidities. In total, 94 patients (22.6%) had an HCT-CI score > 3, 113 adults (26.9%) received grafts from MSDs, 168 (39.9%) from MUDs, 81 (19.5%) from MMUD, and 56 (13.5%) from haploidentical donors. Forty-seven patients (11.3%) received high-dose CY-containing conditioning regimens (17 CY/TBI1 2 Gy and 30 CY/Bu), and none of them received PTCY. TBI was given to 133 patients (31.9%), of which 62 (14.9%) received 12 Gy, 9 (2.1%) received 8 Gy, and 62 (14.9%) received 2 Gy.
The cohort was divided into 2 groups according to the GVHD prophylaxis (PTCY-based vs other). As shown in Table 1, the baseline characteristics were balanced between the 2 groups, except for the proportions of allo-HCTs performed from alternative donors (49.8% vs 4.4%, P = .001), and the use of TBI (43.0% vs 14.6%, P = .001). Notice that the indication of TBI was more prevalent in the PTCY group, because all patients undergoing haplo-HCT received TBI and PTCY.
Baseline patient characteristics and allo-HCT information
| . | Overall N = 416 . | Allo-HCT with PTCY-based prophylaxis n = 258 (62.0) . | Allo-HCT with other prophylaxis n = 158 (38.0) . | P value . |
|---|---|---|---|---|
| Age, y, median (range) | 53 (18-70) | 51 (18-70) | 53 (18-69) | .464 |
| ≥55 y | 192 (43.8) | 108 (41.9) | 74 (46.8) | .321 |
| Sex | ||||
| Female | 184 (44.2) | 108 (41.9) | 76 (48.1) | .214 |
| History of smoking | ||||
| Yes | 136 (32.7) | 76 (29.5) | 60 (38.0) | .072 |
| Relevant comorbidities | ||||
| History of HTN | 73 (17.5) | 55 (20.5) | 20 (12.7) | .040 |
| History of hyperlipidemia | 53 (13.0) | 32 (14.4) | 22 (13.9) | .654 |
| Diabetes mellitus | 29 (7.0) | 19 (7.4) | 10 (6.3) | .687 |
| Pre-existing cardiac morbidity | 45 (10.8) | 29 (11.2) | 16 (10.1) | .723 |
| Arrythmia | 12 (2.8) | 10 (3.8) | 2 (1.2) | .123 |
| Heart failure | 6 (1.4) | 3 (1.1) | 3 (1.8) | .736 |
| Coronary disease | 8 (1.9) | 5 (1.9) | 3 (1.8) | .989 |
| Pericardial disorders | 6 (1.4) | 3 (1.1) | 3 (1.8) | .678 |
| Other∗ | 13 (3.1) | 8 (3.1) | 5 (3.1) | 1 |
| LVEF < 50%† | 7 (1.6) | 4 (1.6) | 3 (1.8) | .201 |
| Baseline diagnosis | - | |||
| AML | 156 (37.5) | 93 (36.0) | 63 (39.9) | |
| ALL | 61 (14.7) | 43 (16.7) | 18 (11.4) | |
| MDS | 75 (18.0) | 44 (17.2) | 31 (19.6) | |
| MPN | 24 (5.8) | 13 (5.0) | 11 (7.0) | |
| Lymphoproliferative disorders | 61 (14.7) | 39 (15.1) | 22 (14.0) | |
| PCD | 16 (3.8) | 15 (5.8) | 1 (0.6) | |
| Other | 23 (5.5) | 11 (4.2) | 12 (7.5) | |
| Treatment with antracyclins before allo-HCT | ||||
| Yes | 288 (69.2) | 181 (70.2) | 107 (67.7) | .696 |
| Treatment with CY before allo-HCT‡ | ||||
| Yes | 75 (18.0) | 48 (18.6) | 28 (17.7) | .602 |
| HCT-CI | ||||
| >3 | 94 (22.6) | 59 (22.9) | 35 (22.2) | .849 |
| Karnofsky Performance Status | ||||
| 70%-80% | 101 (25.3) | 61 (23.6) | 40 (25.3) | .454 |
| Donor type | <.001 | |||
| MSD | 113 (27.2) | 26 (10.1) | 87 (55.1) | |
| MUD | 168 (39.9) | 102 (39.5) | 64 (40.5) | |
| MMUD | 81 (19.5) | 74 (28.7) | 7 (4.4) | |
| Haploidentical | 56 (13.5) | 56 (21.7) | 0 | |
| Conditioning | .971 | |||
| Myeloablative | 197 (47.4) | 122 (47.3) | 75 (47.5) | |
| Reduced intensity | 219 (52.6) | 136 (53.7) | 83 (52.5) | |
| TBI | ||||
| Yes (any dose) | 133 (32.2) | 111 (43.0) | 22 (13.9) | .001 |
| 2 Gy | 62 (14.9) | 62 (24.0) | 0 | |
| 8 Gy | 9 (2.1) | 9 (3.4) | 0 | |
| 12 Gy | 62 (14.9) | 40 (19.3) | 22 (13.9) | |
| GVHD prophylaxis | - | |||
| PTCY-MMF-TK | 76 (18.3) | 76 (29.5) | - | |
| PTCY-TK | 182 (43.7) | 182 (70.5) | - | |
| MTX-CNI | 68 (16.3) | - | 68 (43.1) | |
| MMF-CNI | 88 (21.2) | - | 88 (55.7) | |
| SIR-TK | 2 (0.5) | - | 2 (1.2) | |
| Stem cell source | ||||
| Peripheral blood | 390 (93.8) | 239 (92.6) | 151 (95.6) | .230 |
| . | Overall N = 416 . | Allo-HCT with PTCY-based prophylaxis n = 258 (62.0) . | Allo-HCT with other prophylaxis n = 158 (38.0) . | P value . |
|---|---|---|---|---|
| Age, y, median (range) | 53 (18-70) | 51 (18-70) | 53 (18-69) | .464 |
| ≥55 y | 192 (43.8) | 108 (41.9) | 74 (46.8) | .321 |
| Sex | ||||
| Female | 184 (44.2) | 108 (41.9) | 76 (48.1) | .214 |
| History of smoking | ||||
| Yes | 136 (32.7) | 76 (29.5) | 60 (38.0) | .072 |
| Relevant comorbidities | ||||
| History of HTN | 73 (17.5) | 55 (20.5) | 20 (12.7) | .040 |
| History of hyperlipidemia | 53 (13.0) | 32 (14.4) | 22 (13.9) | .654 |
| Diabetes mellitus | 29 (7.0) | 19 (7.4) | 10 (6.3) | .687 |
| Pre-existing cardiac morbidity | 45 (10.8) | 29 (11.2) | 16 (10.1) | .723 |
| Arrythmia | 12 (2.8) | 10 (3.8) | 2 (1.2) | .123 |
| Heart failure | 6 (1.4) | 3 (1.1) | 3 (1.8) | .736 |
| Coronary disease | 8 (1.9) | 5 (1.9) | 3 (1.8) | .989 |
| Pericardial disorders | 6 (1.4) | 3 (1.1) | 3 (1.8) | .678 |
| Other∗ | 13 (3.1) | 8 (3.1) | 5 (3.1) | 1 |
| LVEF < 50%† | 7 (1.6) | 4 (1.6) | 3 (1.8) | .201 |
| Baseline diagnosis | - | |||
| AML | 156 (37.5) | 93 (36.0) | 63 (39.9) | |
| ALL | 61 (14.7) | 43 (16.7) | 18 (11.4) | |
| MDS | 75 (18.0) | 44 (17.2) | 31 (19.6) | |
| MPN | 24 (5.8) | 13 (5.0) | 11 (7.0) | |
| Lymphoproliferative disorders | 61 (14.7) | 39 (15.1) | 22 (14.0) | |
| PCD | 16 (3.8) | 15 (5.8) | 1 (0.6) | |
| Other | 23 (5.5) | 11 (4.2) | 12 (7.5) | |
| Treatment with antracyclins before allo-HCT | ||||
| Yes | 288 (69.2) | 181 (70.2) | 107 (67.7) | .696 |
| Treatment with CY before allo-HCT‡ | ||||
| Yes | 75 (18.0) | 48 (18.6) | 28 (17.7) | .602 |
| HCT-CI | ||||
| >3 | 94 (22.6) | 59 (22.9) | 35 (22.2) | .849 |
| Karnofsky Performance Status | ||||
| 70%-80% | 101 (25.3) | 61 (23.6) | 40 (25.3) | .454 |
| Donor type | <.001 | |||
| MSD | 113 (27.2) | 26 (10.1) | 87 (55.1) | |
| MUD | 168 (39.9) | 102 (39.5) | 64 (40.5) | |
| MMUD | 81 (19.5) | 74 (28.7) | 7 (4.4) | |
| Haploidentical | 56 (13.5) | 56 (21.7) | 0 | |
| Conditioning | .971 | |||
| Myeloablative | 197 (47.4) | 122 (47.3) | 75 (47.5) | |
| Reduced intensity | 219 (52.6) | 136 (53.7) | 83 (52.5) | |
| TBI | ||||
| Yes (any dose) | 133 (32.2) | 111 (43.0) | 22 (13.9) | .001 |
| 2 Gy | 62 (14.9) | 62 (24.0) | 0 | |
| 8 Gy | 9 (2.1) | 9 (3.4) | 0 | |
| 12 Gy | 62 (14.9) | 40 (19.3) | 22 (13.9) | |
| GVHD prophylaxis | - | |||
| PTCY-MMF-TK | 76 (18.3) | 76 (29.5) | - | |
| PTCY-TK | 182 (43.7) | 182 (70.5) | - | |
| MTX-CNI | 68 (16.3) | - | 68 (43.1) | |
| MMF-CNI | 88 (21.2) | - | 88 (55.7) | |
| SIR-TK | 2 (0.5) | - | 2 (1.2) | |
| Stem cell source | ||||
| Peripheral blood | 390 (93.8) | 239 (92.6) | 151 (95.6) | .230 |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; HCT-CI, HCT comorbidity index; HTN, hypertension; MDS, myelodysplastic syndrome; MMF, mycophenolate mofetil; MPN, myeloproliferative neoplasm; PCD, plasma cell dyscrasia; SIR, sirolimus.
Other: degenerative aortic valve disease with severe insufficiency (n = 1), aneurysm of the ventricular septum (n = 1), arterial ductus in childhood, intervened (n = 1), atrial septal defect causing left to right shunt (n = 1), FVEF <50% with abnormalities in ECG and ECHO (n = 3), sinus bradycardia with ECHO abnormalities (n = 1), and right or left bundle branch block with impaired mobility on ECHO (n = 5).
Five of the 7 patients with FVEF <50% had echocardiographic abnormalities or history of cardiac disease and were additionally accounted in the pre-existing cardiac morbidity category (others).
Not accounted if patients received CY as part of the conditioning regimen or PTCY.
As reported in Table 2A, patients receiving PTCY had a more prolonged aplastic phase. Seventeen patients (4.0%) had graft failure, and 4 (0.9%) underwent a second allo-HCT. The cumulative incidence functions (CIFs) of grade II to IV, III to IV acute GVHD at day +100, and moderate/severe chronic GVHD at 2 years were 24.1%, 6.2%, and 12.0%, respectively, for patients receiving PTCY and 36.7% (P = .001), 13.3% (P = .007), and 37.1% (P < .001), respectively, for those who did not. The estimated 2-year OS and NRM were 65.5% (95% confidence interval [CI], 60.5-70.1) and 17.9% (95% CI, 14.2-21.8), respectively, with no differences in posttransplant outcomes depending on the GVHD prophylaxis.
Posttransplant information and incidence of cardiac toxicity
| (A) Main posttransplant information . | Overall N = 416 . | Allo-HCT with PTCY-based prophylaxis n = 258 . | Allo-HCT with other prophylaxis n = 158 . | P value . |
|---|---|---|---|---|
| Engraftment information | ||||
| Median days neutrophil engraftment (IQR) | 18 (15-22) | 20 (17-23) | 16 (14-18) | .001 |
| Median days platelet engraftment (IQR) | 14 (11-23) | 19 (13-28) | 12 (10-14) | .001 |
| Primary graft failure | 7 (1.6) | 6 (2.3) | 1 (0.6) | .268 |
| Second allograft∗ | 4 (0.9) | 4 (1.5) | 0 | .302 |
| Cumulative incidence GVHD | ||||
| Grade 2-4 acute GVHD at day +100 | 28.9 (24.6-33.3) | 24.1 (19.0-29.5) | 36.7 (29.2-44.2) | .001 |
| Grade 3-4 acute GVHD at day +100 | 8.9 (6.4-11.9) | 6.2 (3.7-9.6) | 13.3 (8.5-19.1) | .007 |
| Moderate/severe chronic GVHD at 2-y | 22.7 (18.4-27.3) | 12.0 (7.9-17.0) | 37.1 (29.3-45.0) | <.001 |
| Main outcome information∗ | - | |||
| Relapse | 105 (25.2) | 58 (22.4) | 47 (29.7) | - |
| Dead | 134 (32.2) | 74 (28.6) | 60 (37.9) | |
| Main causes of dead | ||||
| Relapse | 63 (15.1) | 29 (11.2) | 34 (21.5) | |
| Infection | 30 (7.2) | 22 (8.5) | 8 (5.0) | |
| Graft failure | 8 (1.9) | 6 (2.3) | 2 (1.2) | |
| GVHD | 18 (4.3) | 7 (2.7) | 11 (6.9) | |
| ECE | 2 (0.4) | 1 (0.3) | 1 (0.6) | |
| Other | 13 (3.1) | 9 (3.4) | 4 (2.5) | |
| Main posttransplant outcomes (% [95% CI])† | ||||
| 2-y OS | 65.5 (60.5-70.1) | 67.9 (61.3-73.6) | 61.7 (536-68.8) | .193 |
| 2-y relapse-free survival | 55.2 (50.1-60.1) | 55.8 (49.0-62.1) | 53.5 (45.4-61.0) | .528 |
| 2-y NRM | 17.9 (14.2-21.8) | 18.7 (14.0-23.9) | 16.5 (11.2-22.8) | .546 |
| 2-y cumulative incidence of relapse | 26.9 (22.6-31.4) | 25.5 (19.9-31.4) | 29.9 (22.9-37.2) | .207 |
| B) Early cardiac toxicity | ||||
| Total events: | 35 (8.4) | 29 (11.2) | 6 (3.7) | .010 |
| Cumulative incidence of tardiac toxicity (% [95% CI]) | ||||
| Day +180 | 8.4 (6.0-11.4) | 11.3 (7.8-15.5) | 3.8 (1.6-7.7) | .007 |
| Main related information: | N = 35 | N = 29 | N = 6 | |
| Type of cardiac toxicity | ||||
| Arrhythmia | 8 (22.8) | 7 (25.0) | 1 (16.7) | .580 |
| Pericarditis and/or pericardial Effusion | 11 (31.4) | 9 (31.0) | 2 (33.3) | .629 |
| Heart failure | 13 (37.1) | 11 (37.9) | 2 (33.3) | .608 |
| Ischemia | 1 (3.0) | 0 | 1 (16.7) | .177 |
| Other | 2 (5.7) | 2 (5.1) | 0 | .682 |
| Grade | ||||
| 1-2 | 21 (60.0) | 18 (62.0) | 3 (50) | .456 |
| 3-4 | 12 (34.2) | 10 (34.4) | 2 (33.3) | .311 |
| 5 | 2 (5.87) | 1 (3.4) | 1 (16.7) | .318 |
| Median of days to the event (IQR) | ||||
| Early cardiac toxicity | 27 (12-90) | 24 (13-67) | 59 (5-128) | .569 |
| Overall mortality (∗ any cause) | ||||
| 30-d mortality rate | 6 (17.1) | 3 (10.3) | 3 (50.0) | .268 |
| 100-d mortality rate | 14 (40.0) | 11 (37.9) | 3 (50.0) | .456 |
| (A) Main posttransplant information . | Overall N = 416 . | Allo-HCT with PTCY-based prophylaxis n = 258 . | Allo-HCT with other prophylaxis n = 158 . | P value . |
|---|---|---|---|---|
| Engraftment information | ||||
| Median days neutrophil engraftment (IQR) | 18 (15-22) | 20 (17-23) | 16 (14-18) | .001 |
| Median days platelet engraftment (IQR) | 14 (11-23) | 19 (13-28) | 12 (10-14) | .001 |
| Primary graft failure | 7 (1.6) | 6 (2.3) | 1 (0.6) | .268 |
| Second allograft∗ | 4 (0.9) | 4 (1.5) | 0 | .302 |
| Cumulative incidence GVHD | ||||
| Grade 2-4 acute GVHD at day +100 | 28.9 (24.6-33.3) | 24.1 (19.0-29.5) | 36.7 (29.2-44.2) | .001 |
| Grade 3-4 acute GVHD at day +100 | 8.9 (6.4-11.9) | 6.2 (3.7-9.6) | 13.3 (8.5-19.1) | .007 |
| Moderate/severe chronic GVHD at 2-y | 22.7 (18.4-27.3) | 12.0 (7.9-17.0) | 37.1 (29.3-45.0) | <.001 |
| Main outcome information∗ | - | |||
| Relapse | 105 (25.2) | 58 (22.4) | 47 (29.7) | - |
| Dead | 134 (32.2) | 74 (28.6) | 60 (37.9) | |
| Main causes of dead | ||||
| Relapse | 63 (15.1) | 29 (11.2) | 34 (21.5) | |
| Infection | 30 (7.2) | 22 (8.5) | 8 (5.0) | |
| Graft failure | 8 (1.9) | 6 (2.3) | 2 (1.2) | |
| GVHD | 18 (4.3) | 7 (2.7) | 11 (6.9) | |
| ECE | 2 (0.4) | 1 (0.3) | 1 (0.6) | |
| Other | 13 (3.1) | 9 (3.4) | 4 (2.5) | |
| Main posttransplant outcomes (% [95% CI])† | ||||
| 2-y OS | 65.5 (60.5-70.1) | 67.9 (61.3-73.6) | 61.7 (536-68.8) | .193 |
| 2-y relapse-free survival | 55.2 (50.1-60.1) | 55.8 (49.0-62.1) | 53.5 (45.4-61.0) | .528 |
| 2-y NRM | 17.9 (14.2-21.8) | 18.7 (14.0-23.9) | 16.5 (11.2-22.8) | .546 |
| 2-y cumulative incidence of relapse | 26.9 (22.6-31.4) | 25.5 (19.9-31.4) | 29.9 (22.9-37.2) | .207 |
| B) Early cardiac toxicity | ||||
| Total events: | 35 (8.4) | 29 (11.2) | 6 (3.7) | .010 |
| Cumulative incidence of tardiac toxicity (% [95% CI]) | ||||
| Day +180 | 8.4 (6.0-11.4) | 11.3 (7.8-15.5) | 3.8 (1.6-7.7) | .007 |
| Main related information: | N = 35 | N = 29 | N = 6 | |
| Type of cardiac toxicity | ||||
| Arrhythmia | 8 (22.8) | 7 (25.0) | 1 (16.7) | .580 |
| Pericarditis and/or pericardial Effusion | 11 (31.4) | 9 (31.0) | 2 (33.3) | .629 |
| Heart failure | 13 (37.1) | 11 (37.9) | 2 (33.3) | .608 |
| Ischemia | 1 (3.0) | 0 | 1 (16.7) | .177 |
| Other | 2 (5.7) | 2 (5.1) | 0 | .682 |
| Grade | ||||
| 1-2 | 21 (60.0) | 18 (62.0) | 3 (50) | .456 |
| 3-4 | 12 (34.2) | 10 (34.4) | 2 (33.3) | .311 |
| 5 | 2 (5.87) | 1 (3.4) | 1 (16.7) | .318 |
| Median of days to the event (IQR) | ||||
| Early cardiac toxicity | 27 (12-90) | 24 (13-67) | 59 (5-128) | .569 |
| Overall mortality (∗ any cause) | ||||
| 30-d mortality rate | 6 (17.1) | 3 (10.3) | 3 (50.0) | .268 |
| 100-d mortality rate | 14 (40.0) | 11 (37.9) | 3 (50.0) | .456 |
Posttransplant follow-up has been censored at the time of the second allograft.
Posttransplant follow-up has been censored at 2 years.
Incidence of early cardiac toxicity
As detailed in Table 2B, from day 0 to day +180, 35 (8.4%) patients had at least 1 ECE, for an overall incidence of 8.4% (95% CI, 6.0-11.4), and occurring at a median of 27 days (interquartile range [IQR], 12-90). The cardiac toxicities were heart failure (n = 13, 37.1%), pericardial complications (n = 11, 31.4%), arrhythmias (n = 8, 22.8%), and ischemia (n = 1, 2.9%).
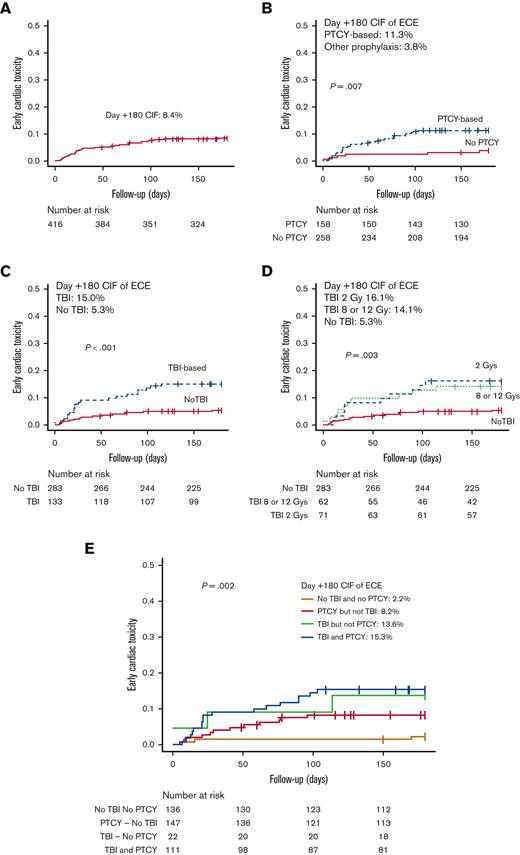
The incidence of ECEs was higher in patients receiving PTCY vs. those who did not (day +180 CIF of 11.3% [95% CI, 7.8-15.5] vs 3.8% [95% CI, 1.6-7.7], P = .007) (Table 2B; Figure 1). Twenty-nine of 258 patients (11.2%) who received PTCY had an ECE occurring at a median of 22 days (IQR, 13-67). In addition, 6 of 158 patients (3.7%) who received other prophylaxis presented a cardiac complication at a median of 59 days (IQR, 5-158). A higher incidence of ECEs was documented in patients receiving TBI compared with those who were not (day +180 CIF of 15.0% [95% CI, 9.6-21.7) vs 5.3% [95% CI, 3.1-8.5], P < .001), and with comparable incidences between patients receiving either 2 and 8 Gy or 12 Gy (day +180 CIF of 16.1% [95% CI, 8.2-26.4] vs 14.1% [95% CI, 7.2-23.3], P = .004).
Cumulative incidence of early cardiac toxicity. (A-D) Cumulative incidence of early cardiac toxicity in the entire cohort of patients (A), GVHD prophylaxis (B), and the administration of TBI (C-D). (E) Cumulative incidence of early cardiac toxicity according to the administration of TBI and PTCY-based prophylaxis. Notice that in plot E the y-axis has been limited to 0.5.
Cumulative incidence of early cardiac toxicity. (A-D) Cumulative incidence of early cardiac toxicity in the entire cohort of patients (A), GVHD prophylaxis (B), and the administration of TBI (C-D). (E) Cumulative incidence of early cardiac toxicity according to the administration of TBI and PTCY-based prophylaxis. Notice that in plot E the y-axis has been limited to 0.5.
Because 43% of the patients who received PTCY also received TBI, the question was whether PTCY and TBI interacted in the risk for ECEs. To answer this question, patients were grouped into 4 categories depending on whether they had received PTCY or not and on whether they had received TBI or not. As shown in Figure 1, the day +180 CIF of ECEs was 8.2% (95% CI, 4.1-13.7) in patients receiving PTCY without TBI (n = 147), 13.8% (95% CI, 3.2-31.3) in patients receiving TBI without PTCY (n = 22), 15.3% (95% CI, 9.3-22.7) in patients receiving both PTCY and TBI (n = 111), and 2.2% (95% CI, 0.6-5.9) in patients receiving neither PTCY nor TBI (n = 136). The null hypotheses of equal proportions were rejected (P = .002).
Impact of pre-existing cardiac morbidity on the risk for early cardiac toxicity
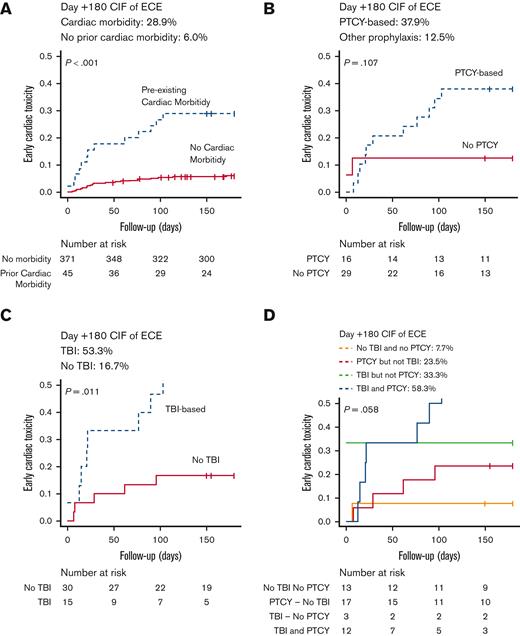
A higher incidence of ECEs was documented in the 45 patients (10.8%) with pre-existing cardiac morbidity (day +180 CIF 28.9% vs 6.0%, P < .001) (Figure 2). Seventeen of 45 patients (37.7%) received PTCY without TBI, 3 (6.6%) received TBI without PTCY, 12 (28.8%) received both TBI and PTCY (7 received 2 Gy, and 5 received 8 or 12 Gys), and 13 (17.7%) received neither PTCY nor TBI.
Cumulative incidence of early cardiac toxicity in patients with pre-existing cardiac morbidity. (A) Cumulative incidence of early cardiac toxicity in patients with pre-existing cardiac morbidity and those without this pretransplant condition . (B-D) Cumulative incidence of early cardiac toxicity in patients with pre-existing cardiac morbidity according to GVHD prophylaxis (B), the administration of TBI (C), and the administration of TBI and PTCY-based prophylaxis (D). Notice that in plot E the y-axis has been limited to 0.5.
Cumulative incidence of early cardiac toxicity in patients with pre-existing cardiac morbidity. (A) Cumulative incidence of early cardiac toxicity in patients with pre-existing cardiac morbidity and those without this pretransplant condition . (B-D) Cumulative incidence of early cardiac toxicity in patients with pre-existing cardiac morbidity according to GVHD prophylaxis (B), the administration of TBI (C), and the administration of TBI and PTCY-based prophylaxis (D). Notice that in plot E the y-axis has been limited to 0.5.
As reported in Table 3, 13 patients (28.8%) had ECEs, with a higher prevalence in patients with pre-existing pericardial disorders (50%), arrhythmias (41.6%), and heart failure (33.3%). As shown in Figure 1, the day +180 CIF of ECEs was 23.5% (95% CI, 6.9-45.7) in patients receiving PTCY but not TBI, 33.3% (95% CI, 0.1-83.2) in patients receiving TBI without PTCY, 58.3% (95% CI, 24.4-81.4) in patients receiving PTCY and TBI, and 7.7% (95% CI, 0.4-30.3) in patients receiving neither PTCY nor TBI (P = .058).
Impact of pre-existing cardiac morbidity on the risk for early cardiac toxicity
| . | Patients receiving PTCY . | Incidence of ECEs n (%) . | Received PTCY-based prophylaxis n (%) . | Type of cardiac complication: event: n (%) . | Risk for ECE regression analysis HR (95% CI) . | P value . | Day 30 mortality rate . |
|---|---|---|---|---|---|---|---|
| Patients without pre-existing cardiac morbidity (n = 371) | |||||||
| Patients without pre-existing cardiac morbidity (n = 371) | 229 (61.7) | 22 (5.9) | 18 (81.8) | Arrhythmia: 2 Pericardial disorders: 8 Heart failure: 9 Ischemia: 1 Other: 2 | Reference variable | - | 2 |
| Patients with pre-existing cardiac morbidity (n = 45) | |||||||
| Arrhythmia (n = 12) | 10 (83.3) | 5 (41.6) | 2 (40) | Arrhythmia: 3 Pericarditis: 1 Heart failure: 1 | 8.42 (3.30-21.46) | <.001 | 0 |
| Heart failure (n = 6) | 3 (50.0) | 2 (33.3) | 2 (100) | Arrhythmia: 2 | 5.77 (1.62-20.27) | .006 | 0 |
| Ischemia (n = 8) | 5 (62.5) | 1 (12.5) | 2 (100) | Arrhythmia: 1 | 2.24 (0.28-17.58) | .440 | 1 |
| Pericardial disorders (n = 6) | 3 (50.0) | 3 (50.0) | 1 (33.3) | Pericarditis: 1 Heart failure: 2 | 13.98 (3.37-57.88) | <.001 | 0 |
| Other† (n = 13) | 8 (61.5) | 2 (15.4) | 0 | Pericarditis: 1 Heart failure: 1 | 2.72 (0.64-11.50) | .170 | 1 |
| . | Patients receiving PTCY . | Incidence of ECEs n (%) . | Received PTCY-based prophylaxis n (%) . | Type of cardiac complication: event: n (%) . | Risk for ECE regression analysis HR (95% CI) . | P value . | Day 30 mortality rate . |
|---|---|---|---|---|---|---|---|
| Patients without pre-existing cardiac morbidity (n = 371) | |||||||
| Patients without pre-existing cardiac morbidity (n = 371) | 229 (61.7) | 22 (5.9) | 18 (81.8) | Arrhythmia: 2 Pericardial disorders: 8 Heart failure: 9 Ischemia: 1 Other: 2 | Reference variable | - | 2 |
| Patients with pre-existing cardiac morbidity (n = 45) | |||||||
| Arrhythmia (n = 12) | 10 (83.3) | 5 (41.6) | 2 (40) | Arrhythmia: 3 Pericarditis: 1 Heart failure: 1 | 8.42 (3.30-21.46) | <.001 | 0 |
| Heart failure (n = 6) | 3 (50.0) | 2 (33.3) | 2 (100) | Arrhythmia: 2 | 5.77 (1.62-20.27) | .006 | 0 |
| Ischemia (n = 8) | 5 (62.5) | 1 (12.5) | 2 (100) | Arrhythmia: 1 | 2.24 (0.28-17.58) | .440 | 1 |
| Pericardial disorders (n = 6) | 3 (50.0) | 3 (50.0) | 1 (33.3) | Pericarditis: 1 Heart failure: 2 | 13.98 (3.37-57.88) | <.001 | 0 |
| Other† (n = 13) | 8 (61.5) | 2 (15.4) | 0 | Pericarditis: 1 Heart failure: 1 | 2.72 (0.64-11.50) | .170 | 1 |
Other: degenerative aortic valve disease with severe insufficiency (n = 1), aneurysm of the ventricular septum (n = 1), arterial ductus in childhood, intervened (n = 1), atrial septal defect causing left to right shunt (n = 1), FVEF <50% with abnormalities in ECG and ECHO (n = 3), sinus bradycardia with ECHO abnormalities (n = 1), and right or left bundle branch block with impaired mobility on ECHO (n = 5).
The documented posttransplant cardiac complications were heterogeneous, with arrhythmia (6 out of 13) being the most prevalent one. Five patients (11.1%) had a clinically relevant adverse event (grade 3-4). No patient died secondary to the cardiac complication, but the day +30 overall mortality rate was 4.4%.
Risk factors for early cardiac toxicity in patients undergoing allo-HCT
Predisposing factors for ECEs are shown in Table 4. The univariate analysis revealed that PTCY-based prophylaxis (hazard ratio [HR], 3.08; P = .012), and the use of TBI (at any dose) (HR, 2.96; P = .001) increased the risk for presenting early cardiac complications. Pretransplant cardiac morbidity (HR, 5.56; P=.001), and receiving treatment with CY (HR, 2.20; P = .031) also increased the risk for ECEs. However, no association between receiving high-dose CY-containing conditioning regimens and risk for ECEs was documented (HR, 0.72; P = .60).
Risk factors for early cardiac toxicity
| Univariate analysis . | Cardiac toxicity first the 180 d HR (95% CI) . | P value . |
|---|---|---|
| PTCY-based prophylaxis (vs others) | 3.08 (1.28-7.42) | 0.012 |
| TBI (overall) | ||
| Yes (any dose) (vs no) | 2.96 (1.53-5.78) | 0.001 |
| TBI according to dose | ||
| 2 Gy (vs no) | 3.17 (1.43-7.01) | 0.004 |
| ≥8 Gy (vs no) | 2.78 (1.25-6.20) | 0.012 |
| Age at allo-HCT | ||
| Continuous | 1.01 (0.98-1.04) | 0.21 |
| Age ≥55 y (vs younger) | 1.53 (0.79-2.98) | 0.20 |
| Female (vs male) | 1.18 (0.60-2.33) | 0.62 |
| History of HTN (vs no) | 1.94 (0.94-4.03) | 0.073 |
| History of diabetes (vs no) | 1.79 (0.62-5.14) | 0.27 |
| History of hyperlipidemia (vs no) | 0.62 (0.18-2.06) | 0.44 |
| History of cardiac disease (vs no) | 5.56 (2.81-11.0) | <0.001 |
| LVEF <50% without pre-existing cardiac morbidity (vs others)‡ | 4.02 (0.96-16.77) | 0.056 |
| Prior treatment with anthracycline (vs no) | 0.76 (0.38-1.50) | 0.44 |
| Prior treatment with CY (vs no) | 2.20 (1.07-4.50) | 0.031 |
| High-dose CY-containing conditioning regimen (vs others) | 0.68 (0.20-2.24) | 0.53 |
| HCT-CI >3 (vs 0-3)† | 1.85 (0.92-3.71) | 0.084 |
| KPS ≤80% (vs 90%-100%) | 0.90 (0.41-0.98) | 0.80 |
| RIC (vs MAC) | 1.06 (0.55-2.07) | 0.81 |
| BM (vs PB) | 0.90 (0.21-3.72) | 0.88 |
| Univariate analysis . | Cardiac toxicity first the 180 d HR (95% CI) . | P value . |
|---|---|---|
| PTCY-based prophylaxis (vs others) | 3.08 (1.28-7.42) | 0.012 |
| TBI (overall) | ||
| Yes (any dose) (vs no) | 2.96 (1.53-5.78) | 0.001 |
| TBI according to dose | ||
| 2 Gy (vs no) | 3.17 (1.43-7.01) | 0.004 |
| ≥8 Gy (vs no) | 2.78 (1.25-6.20) | 0.012 |
| Age at allo-HCT | ||
| Continuous | 1.01 (0.98-1.04) | 0.21 |
| Age ≥55 y (vs younger) | 1.53 (0.79-2.98) | 0.20 |
| Female (vs male) | 1.18 (0.60-2.33) | 0.62 |
| History of HTN (vs no) | 1.94 (0.94-4.03) | 0.073 |
| History of diabetes (vs no) | 1.79 (0.62-5.14) | 0.27 |
| History of hyperlipidemia (vs no) | 0.62 (0.18-2.06) | 0.44 |
| History of cardiac disease (vs no) | 5.56 (2.81-11.0) | <0.001 |
| LVEF <50% without pre-existing cardiac morbidity (vs others)‡ | 4.02 (0.96-16.77) | 0.056 |
| Prior treatment with anthracycline (vs no) | 0.76 (0.38-1.50) | 0.44 |
| Prior treatment with CY (vs no) | 2.20 (1.07-4.50) | 0.031 |
| High-dose CY-containing conditioning regimen (vs others) | 0.68 (0.20-2.24) | 0.53 |
| HCT-CI >3 (vs 0-3)† | 1.85 (0.92-3.71) | 0.084 |
| KPS ≤80% (vs 90%-100%) | 0.90 (0.41-0.98) | 0.80 |
| RIC (vs MAC) | 1.06 (0.55-2.07) | 0.81 |
| BM (vs PB) | 0.90 (0.21-3.72) | 0.88 |
| Univariate analysis . | HR (95% CI) . | P value . |
|---|---|---|
| Effect of PTCY and TBI on early cardiac toxicity‡ | ||
| PTCY without TBI (vs no PTCY, no TBI) | 3.84 (1.08-13.61) | .037 |
| TBI (any dose) without PTCY (vs no TBI, no TBI) | 6.56 (1.32-32.50) | .021 |
| PTCY with TBI (vs no PTCY, no TBI) | 7.41 (2.17-25.27) | .001 |
| Univariate analysis . | HR (95% CI) . | P value . |
|---|---|---|
| Effect of PTCY and TBI on early cardiac toxicity‡ | ||
| PTCY without TBI (vs no PTCY, no TBI) | 3.84 (1.08-13.61) | .037 |
| TBI (any dose) without PTCY (vs no TBI, no TBI) | 6.56 (1.32-32.50) | .021 |
| PTCY with TBI (vs no PTCY, no TBI) | 7.41 (2.17-25.27) | .001 |
| Multivariate analysis . | HR (95% CI) . | P value . |
|---|---|---|
| Effect of PTCY and TBI on early cardiac toxicity‡ | ||
| PTCY without TBI (vs no PTCY, no TBI) | 3.79 (1.05-13.60) | .041 |
| TBI (any dose) without PTCY (vs no TBI, no TBI) | 6.0 (1.24-34.07) | .027 |
| PTCY with TBI (vs no PTCY, no TBI) | 6.98 (2.01-24.24) | .002 |
| Prior history of cardiac disease (vs no) | 5.28 (2.63-10.60) | <.001 |
| Prior treatment with CY (vs no) | 1.66 (0.78-3.52) | .190 |
| Multivariate analysis . | HR (95% CI) . | P value . |
|---|---|---|
| Effect of PTCY and TBI on early cardiac toxicity‡ | ||
| PTCY without TBI (vs no PTCY, no TBI) | 3.79 (1.05-13.60) | .041 |
| TBI (any dose) without PTCY (vs no TBI, no TBI) | 6.0 (1.24-34.07) | .027 |
| PTCY with TBI (vs no PTCY, no TBI) | 6.98 (2.01-24.24) | .002 |
| Prior history of cardiac disease (vs no) | 5.28 (2.63-10.60) | <.001 |
| Prior treatment with CY (vs no) | 1.66 (0.78-3.52) | .190 |
BM, bone marrow; HTN, hypertension; HCT-CI, HCT comorbidity index; KPS, Karnofsky Performance Status; MAC, myeloablative conditioning; PB, peripheral blood, RIC, reduced-intensity conditioning.
The administration of TBI and PTCY were found to be independent predictors of cardiac toxicity. Considering that 111 patients included in the study received TBI and PTCY, 4 explanatory variables were defined to be included in the univariate and multivariate model: not receiving neither PTCY nor TBI (n = 147); receiving PTCY but not TBI (n = 136); receiving TBI but not PTCY (n = 111); and receiving both, PTCY and TBI (n = 22) to independently explore the effect of TBI, PTCY, and TBI combined with PTCY.
Considering that pre-existing cardiac morbidity is one of the variables accounted in the HCT-CI score, the variable HCT-CI was not included in the multivariate model reported in Table 4.
The multivariate analysis, also reported in Table 4, confirmed that patients receiving PTCY without TBI (HR, 3.79; P = .041), TBI without PTCY (HR, 6.01; P = .027), and TBI and PTCY (HR, 6.98; P = .002) were at higher risk for presenting ECEs, compared with patients who did not receive PTCY or TBI. The HRs of the variables PTCY and no TBI and of receiving both PTCY and TBI were not statistically different; therefore, receiving PTCY and receiving TBI could be considered independent risk factors for ECEs. In addition, patients with pre-existing cardiac morbidity had an increased risk for ECEs (HR, 5.28; P < .001), compared with patients without this comorbidity.
Impact of cardiac toxicity in posttransplant outcomes: morbidity and mortality
Of the 37 patients (8.8%) with ECEs, 16 (43.2%) went through a clinically relevant adverse event (grade 3-4, 13 patients). Two patients (0.5%) died secondary to the cardiac complication in a median of 3 days. Moreover, the day +30 and day +100 mortality rates among these patients were 18.9% and 40.5%, respectively.
Risk factors for OS and NRM are reported in Table 5. The multivariate analysis showed that ECEs was a predictor for lower 2-year OS (HR, 3.03; P < .001) and of higher 2-year NRM (HR, 4.68; P < .001). Other risk factors for mortality were being older than 55 years (HR, 1.59; P = .012), a KPS <90% (HR ,1.82; P = .001), and the development of grade 3 to 4 acute GVHD (HR, 4.48; P < .001).
Impact of acute cardiac toxicity in posttransplant outcomes (multivariate analysis)
| Multivariate analysis . | Risk factors for OS . | Risk factors for NRM . | ||
|---|---|---|---|---|
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Early cardiac toxicity | ||||
| Time-dependent variable | 3.03 (1.81-5.13) | <.001 | 4.68 (2.58-8.46) | <.001 |
| GVHD prophylaxis | ||||
| PTCY-based (vs others) | 0.79 (0.52-1.21) | .295 | 1.01 (0.55-1.84) | .971 |
| TBI | ||||
| Yes (vs no) | 1.07 (0.70-1.63) | .745 | 1.28 (0.74-2.23) | .367 |
| Age at allo-HCT | ||||
| ≥55 y (vs <55) | 1.59 (1.10-2.30) | .012 | 1.74 (1.04-2.94) | .049 |
| KPS | ||||
| ≤80% (vs 90%-100%) | 1.82 (1.24.2.65) | .001 | 1.89 (1.15-3.10) | .119 |
| HCT-CI score | ||||
| ≥3 (vs <3) | 1.52 (1.02-2.26) | .355 | 0.99 (0.53-1.84) | .982 |
| Donor selection | ||||
| Haplo and MMUD (vs others) | 1.20 (0.77-1.87) | .406 | 1.41 (0.78-2.53) | .244 |
| Grade 3-4 acute GVHD | ||||
| Time-dependent variable | 4.48 (2.86-7.03) | <.001 | 6.20 (3.50-10.97) | <.001 |
| Multivariate analysis . | Risk factors for OS . | Risk factors for NRM . | ||
|---|---|---|---|---|
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Early cardiac toxicity | ||||
| Time-dependent variable | 3.03 (1.81-5.13) | <.001 | 4.68 (2.58-8.46) | <.001 |
| GVHD prophylaxis | ||||
| PTCY-based (vs others) | 0.79 (0.52-1.21) | .295 | 1.01 (0.55-1.84) | .971 |
| TBI | ||||
| Yes (vs no) | 1.07 (0.70-1.63) | .745 | 1.28 (0.74-2.23) | .367 |
| Age at allo-HCT | ||||
| ≥55 y (vs <55) | 1.59 (1.10-2.30) | .012 | 1.74 (1.04-2.94) | .049 |
| KPS | ||||
| ≤80% (vs 90%-100%) | 1.82 (1.24.2.65) | .001 | 1.89 (1.15-3.10) | .119 |
| HCT-CI score | ||||
| ≥3 (vs <3) | 1.52 (1.02-2.26) | .355 | 0.99 (0.53-1.84) | .982 |
| Donor selection | ||||
| Haplo and MMUD (vs others) | 1.20 (0.77-1.87) | .406 | 1.41 (0.78-2.53) | .244 |
| Grade 3-4 acute GVHD | ||||
| Time-dependent variable | 4.48 (2.86-7.03) | <.001 | 6.20 (3.50-10.97) | <.001 |
Posttransplant follow-up has been censored at 2 years.
HCT-CI, HCT–specific comorbidity index; KPS, Karnofsky Performance Status.
The OS (HR, 0.79; P = .295) and NRM (HR, 1.01; P = .971) in patients receiving PTCY were not statistically different from the OS and NRM of patients not receiving PTCY. Similarly, the OS (HR, 1.07; P = .745) and NRM (HR, 1.28; P = .367) were similar in patients who received TBI and in those who did not, while controlling for the rest of the risk factors.
Early cardiac toxicity according to donor type
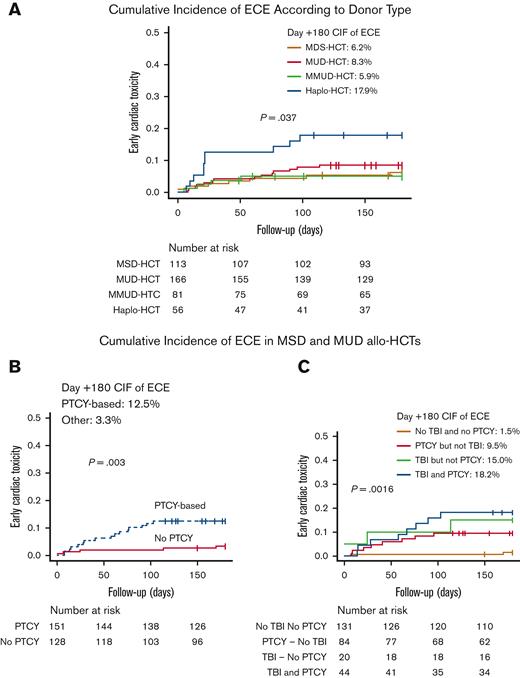
The incidences and predictors for ECEs were also explored across donor types. As shown in Figure 3, the incidence of ECEs was higher in patients undergoing haplo-HCTs than in patients receiving grafts from MSDs, MUDs, and MMUDs (day +180 CIF of 17.9% [95% CI, 9.1-29.0], 6.2% [95% CI, 2.7-11.7], 8.4% [95% CI, 4.8-13.3], and 5.9% [95% CI, 4.6-13.3]; P = .037).
Incidence of early cardiac toxicity according to donor type. (A) Cumulative incidence of early cardiac toxicity according to donor type. (B) Cumulative incidence of early cardiac toxicity according to GVHD prophylaxis in patients undergoing allo-HCTs from MSD and MUDs. (C) Cumulative incidence of early cardiac toxicity in patients undergoing allo-HCTs from MSD and MUDs according to the administration of TBI and PTCY-based prophylaxis. Notice that in plot E the y-axis has been limited to 0.5.
Incidence of early cardiac toxicity according to donor type. (A) Cumulative incidence of early cardiac toxicity according to donor type. (B) Cumulative incidence of early cardiac toxicity according to GVHD prophylaxis in patients undergoing allo-HCTs from MSD and MUDs. (C) Cumulative incidence of early cardiac toxicity in patients undergoing allo-HCTs from MSD and MUDs according to the administration of TBI and PTCY-based prophylaxis. Notice that in plot E the y-axis has been limited to 0.5.
Considering that all patients undergoing haplo-HCT received 2 Gy of TBI and PTCY, and that the majority of patients receiving grafts from MMUDs received PTCY-based prophylaxis, the risk for ECEs was investigated separately in patients undergoing MSD and MUD allo-HCT (n = 279). The study cohort was divided into 2 groups according to GVHD prophylaxis, and the baseline characteristics were balanced in between them (supplemental Table 2).
As reported in Figure 3, among patients undergoing MSD and MUD allo-HCT, the incidence of documented ECEs was higher in the PTCY group than in the other one (day +180 CIF 12.5% [95% CI, 7.5-18.9] vs 3.3% [95% CI, 1.2-7.1]; P = .003). Moreover, compared with patients who had received neither PTCY nor TBI (n = 131 [47.0%]), patients who had received PTCY without TBI (n = 84 [30.1%]) (HR, 6.54; P = .017), TBI without PTCY (n = 20 [7.1%]) (HR, 10.63; P = .009), and TBI and PTCY (n = 44 [15.8%]) (HR, 12.86; P = .001) had a higher risk for cardiac toxicity during the first 6 months after allo-HCT.
Discussion
This study reports an incidence of ECEs of 8.9% in 416 adults undergoing allo-HCT from different donor types. The diagnosis of pre-existing cardiac morbidity, using PTCY- and TBI-containing conditioning regimens, independently increased the risk of presenting this complication. Moreover, ECEs were statistically associated with higher posttransplant mortality.
This study reports a higher incidence of ECEs in patients receiving PTCY. CY undergoes hepatic metabolism, producing many metabolites, including acrolein, which become the main responsible factors for cardiac toxicities.19 CY-related cardiac toxicity includes cardio-myocyte apoptosis, endothelial dysfunction, calcium deregulation, endoplasmic reticulum, and mitochondrial damage. At a clinical level, these changes translate into structural/mechanical, vascular, or electric-conduction cardiac disorders.19,20 On the other hand, PTCY induces apoptosis of rapidly proliferating alloreactive T cells by sparing regulatory T cells inducing an effective GVHD prevention.19-22 However, these PTCY-derived immunologic events may induce myocardial damage contributing to the increased cardiotoxicity associated with PTCY.21-24 In contrast with the evidence reported in the literature,9,10 pretransplant exposition to high-dose CY chemotherapies or the use of high-dose CY-conditioning regimens were not associated with higher risk for ECEs in our study. The lack of association between CY-containing regimes and cardiac toxicity in our study could be explained by the fact that the study only explored the risk for cardiac toxicity occurring during the first 180 days after allo-HCT, by the small number of patients receiving CY-containing conditioning regimens, and by the baseline characteristics of this subsample of patients. The median age of patients receiving these regimens was 43 years; only 1 patient (2.1%) had a prior history of hypertension, 4 (8.5%) had pre-existing cardiac morbidity, and none received haploidentical donor grafts.
Other investigators explored the effect of PTCY on cardiac toxicity, with contradictory findings.11-13 Dulery et al13 reported an increased risk for ECEs in patients receiving PTCY compared with adults who did not, with a day +100 CIF of 19% vs 6%. By donor type, rates of cardiotoxicity were higher in haplo-HCTs than in MSD and MUD allo-HCTs (day +100 CIF, 21% vs 13%). In contrast, Yeh et al 12 did not find a significant association between PTCY and day +100 ECEs in a cohort of adults undergoing MSD and MUD allo-HCTs. Our study included a heterogeneous cohort of patients who underwent matched and mismatched, related and unrelated donor transplants, and the risk for ECEs was evaluated during the first 180 days after the stem cell infusion. The findings were similar to those of Dulery et al,13 although differences in sample compositions, together with the higher proportion of patients who had received TBI, limit the comparison of our results with those of previous studies. In order to gain comparability with the results by Yeh et al,12 the association between PTCY and ECEs was investigated separately in the subsample of patients undergoing MSD and MUD allo-HCTs. The results were similar to those found in the whole sample, that is, a positive association was found between PTCY and ECEs in the sample of allo-HCTs. Although this study controlled for the effect of receiving TBI in ECEs when evaluating the association between PTCY and ECEs, the higher proportion of patients who received TBI, together with the fact that ECEs were evaluated at 180+ days after the transplant, could explain the differences in the results from Yeh et al12 In any case, the risk for ECEs in patients receiving PTCY deserves further investigation.
The use of TBI alone, or in combination with PTCY, increased the risk for early cardiotoxicity in our analysis, in line with results reported in previous research.16,17 Radiation-induced cardiotoxicity comprises a broad spectrum of cardiac complications, ranging from cardiomyopathy to conduction system abnormalities. The derived pathomechanisms include endothelial, mitochondrial, and endoplasmic reticulum injury, and cytokine-mediated and oxidative-stress damage.25,26 Although TBI/CY preparative regimens have shown to contribute to cardiac toxicity in patients with and without pre-existing cardiac dysfunction,27-32 the effect of TBI combined with PTCY on ECEs has not been widely investigated. The results showed that the joint combination of PTCY and TBI did not significantly increase the risk of ECEs, compared with the risk from TBI alone. Moreover, the shared cardiac toxicity pathomechanisms attributed to TBI and CY could explain why, in our study, CY and TBI were independent predictors for ECEs, with similar risk profiles.19,25,26
Notice that, contrary to solid evidence reported in the literature,33-35 the increased risk for ECEs attributed to radiation was not dose-dependent in our analysis. The reduced number of patients receiving high-dose TBI and PTCY, together with the selection of adverse cardiac events occurring only during the first 6 months after allo-HCT could have underestimated the effect of high-dose TBI on the risk for cardiac toxicity. Moreover, the increased risk for ECEs in patients receiving low-dose TBI could be secondary to a synergic effect between the administration of 2 Gys of TBI, haploidentical donor grafts, and PTCY.
An additional finding was that patients undergoing haplo-HCT had a higher likelihood of ECEs than those undergoing allo-HCT from other donors and receiving PTCY. Dulery et al13 also reported a higher risk for ECEs in haplo-HCT, a result attributed to the inherent immunologic changes that occur after infusion of haploidentical stem cell grafts. From this explanation and the results provided by our analysis, the presence of a synergistic effect between the infusion of peripheral blood haploidentical donor grafts and the use of PTCY, potentially caused by cytokine release, endothelial activation, and the higher incidence of infectious complications attributed to PTCY-based haplo-HCTs could explain the increased risk for early cardiac toxicity documented in patients receiving a low-dose of TBI and undergoing haplo-HCTs.36-38 Nevertheless, additional analysis will be conducted to investigate ECEs in the haplo-HCT setting and to address whether the use of TBI to enhance engraftment should be avoided in patients with additional risk factors for ECEs.
In line with the data reported by Dulery et al13, heart failure was the most prevalent ECEs documented in our analysis, and prior history of hypertension, diabetes, and hyperlipidemia were not predictors for ECEs. Notably, patients with pre-existing cardiac morbidity, especially pericardial disorders, arrhythmias, and heart failure were at higher risk for presenting early cardiotoxicity, in line with results reported by other investigators.12,13 To prevent negative transplant outcomes, patients with pre-existing cardiac morbidity should be carefully evaluated and monitored after allo-HCT, especially if they present a history of pericardial disorders, arrhythmias, and heart failure. Special attention may be required for patients with arrhythmias and heart failure receiving PTCY, although more research is needed before more specific recommendations can be made about the care of patients with pre-existing cardiac toxicity undergoing PTCY-based allo-HCT.
In summary, this analysis reports increased ECEs related to PTCY and TBI. Nevertheless, a word of caution is needed with the reported results because the compared groups of patients were not balanced in terms of donor status and hypertension, 2 factors that could contribute to higher risk of cardiac events in the PTCY group. The relatively low incidences of ECEs documented among these patients support that TBI can still be combined with PTCY. However, because ECEs increased the risk of mortality,12,13 the implementation of posttransplant cardiac monitoring plans and the design of pre-emptive interventions in patient at high-risk seem recommendable.39-41
The retrospective design, the heterogeneity of the sample of patients receiving PTCY, including subsets of patients with reduced the sample size in the analysis, and the lack of posttransplant routine cardiac function monitoring are considered the main limitations in this study. In addition, the fact that all patients undergoing haplo-HCTs received 2 Gys of TBI limited the ability to investigate the impact of low-dose TBI on the probability of early cardiac complications in this subgroup of patients.
This study reports a relatively low incidence of ECEs in patients who underwent allo-HCT, although the presence of this complication negatively affected transplant survival. Because the use of PTCY, which is becoming prevalent in allo-HTC across all donor types, and the administration of TBI were identified as predictors for ECEs, the implementation of posttransplant cardiac monitoring plans among these patients would be highly recommendable, and even more so in patients with pre-existing cardiac morbidity. The results reported in this study are in line with those found in 1 of the published papers on the topic but differ from those found in another.12,13 Given the reduced and noncoincident evidence on the association between PTCY use and ECEs, and the fact that the evidence presented herein comes from nonhomogeneous patient populations, further studies are needed to determine the link between PTCY and risk for ECEs.
Acknowledgments
The authors thank the patients, the nursing and support staff in the HCT program, and the cardiology medical department at Hospital Clínic de Barcelona.
Authorship
Contribution: M.Q.S. designed the study and performed statistical analysis; M.Q.S. and A.I.P.-V. wrote the paper; E. Cascos helped with the interpretation and discussion dissertation; A.I.P.-V., S.C.-O., P.C., M.G., and J.A. collected the data; and E. Carreras, S.C.-O., P.C., L.G.R.-L., M.S.-L., N.M.-C., M.G.A., M.G., M.T.S., J.A., M.N., J.C., M.L., M.D.-R., L.R., J.E., A.U.-I., E. Cascos, E.M., C.M., F.F.-A., and M.R. provided valuable input into the study and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: María Queralt Salas, Hematopoietic Cell Transplantant Unit, Hematology Department, Clinical Institute of Hematology-Oncology, Hospital Clínic de Barcelona, C/ Vilarroel 190, CP 08036 Barcelona, Spain;.
References
Author notes
Data are available on request from the corresponding author, María Queralt Salas (queralt.salas87@outlook.es).
The full-text version of this article contains a data supplement.