Key Points
Subsequent neoplasms observed in recipients of umbilical cord blood transplantation are rare, although associated with poor survival.
Determining risk factors and lifelong screening for early detection of subsequent neoplasms are mandatory to improve survival.
Abstract
Subsequent neoplasms (SNs) compromise long-term survivors after hematopoietic cell transplantation. We performed a retrospective analysis of SNs in 10 358 recipients of umbilical cord blood transplantation (UCBT) from 1988 to 2018. SNs developed in 233 patients and 84 were of pediatric age. Indications for UCBT were malignant hematological diseases in 199 patients (85%). Three groups of SNs were observed. Posttransplant lymphoproliferative disorders (PTLD) were reported in 145 patients in a median of 4 months after UCBT. Of these, 9 patients died from relapse, 83 from PTLD, and 24 from transplant-related causes. At last follow-up, 29 were alive; 5-year overall survival (OS) after PTLD diagnosis was 21%. Acute leukemia/myelodysplasia (AL/MDS) was diagnosed in 23 patients in a median of 28 months after UCBT and included 3 donor-cell AL. Four of 23 patients died from relapse of primary disease, 8 from progression of SNs, and 4 from TRM. Seven patients remain alive; the 5-year OS after AL/MDS diagnosis was 36%. Solid tumors (ST) were reported in 65 patients in a median of 54 months after UCBT. Most common tumor sites were lung, thyroid, bone, and soft tissue. A total of 33 patients died (26 owing to ST, 6 to relapse of primary disease, and 1 cause missing). At last follow-up, 32 of 65 patients were alive; the 5-year OS after the diagnosis of ST was 51%. In conclusion, despite their poor outcomes, SNs that occur after UCBT are extremely rare. Identification of risk factors and early detection may help to improve OS.
Introduction
Long-term survivors after hematopoietic cell transplantation (HCT) are at higher risk for developing subsequent neoplasms (SNs), with a twofold to fivefold increased risk compared with their age-matched controls.1 The cumulative incidence (CI) of SNs continues to increase with longer follow-up after transplantation with no apparent plateau, and it is estimated to be 1% to 6% and 2% to 15% at 10 and 15 years after HCT, respectively.1 These malignant posttransplant complications are the result of the interactions of a large panel of transplant-related factors (conditioning regimen, T-cell depletion, antithymocyte globulin [ATG] administration, graft-versus-host disease [GVHD] immunosuppression [IS], infections, among others), patient-related factors (recipient age, gender, genetic predisposition, lifestyle aspects), and disease-related factors (disease type, disease stage, pretransplant treatment).2,3
Malignant complications in posttransplant patients can be stratified into 3 groups: posttransplant lymphoproliferative disorders (PTLD), acute leukemia/myelodysplasia (AL/MDS, recipient cell–derived, or donor cell–derived), and solid tumors (ST).
The time of occurrence after HCT varies according to the type of SNs: PTLD peaks in the first year after transplant, AL/MDS occurs, usually, between 1 and 4 years after transplant, and ST starts to develop around 5 years after transplant, increasing in incidence with longer survival.1,4
Reported incidences of SNs after HCT are based largely on registry data and show no difference according to donor type or degree of HLA matching. The prognosis is poor as SN-related death is observed, respectively, in 25% and 15% of adult recipients of allogeneic and autologous transplants who survived at least 5 years after the procedure.5 However, published studies assessing the incidence of SNs after umbilical cord blood transplantation (UCBT) and characterizing their outcomes are scarce.6-10
The objective of the present study is to provide a general overview and highlight the outcomes of SNs in UCB recipients transplanted in European Group for Blood and Marrow Transplantation (EBMT) affiliated centers over the last 3 decades. The findings of this study will help to better characterize the impact of SNs on overall survival (OS) after UCBT.
Patients and methods
Study design and definitions
We performed a retrospective analysis of SNs in a cohort of 10 358 UCBT recipients reported to Eurocord/EBMT registries from 6 October 1988 to 31 December 2018. Eligible graft sources were single, double, and expanded UCB units. We excluded transplants with UCB coinfused with adult-donor grafts. We also excluded patients with primary diagnosis of nonmalignant disease known to increase the risk of developing malignancies (Fanconi anemia and Blackfan-Diamond anemia).
HLA disparity was calculated based on the number of mismatches between the patient and the UCB for single UCBT, considering the HLA antigen level for HLA-A and HLA-B and the allele level for HLA-DRB1, and based on the UCB with the worst match with the recipient for double UCBT (dUCBT). Conditioning regimen intensity was defined according to the current EBMT criteria.11 The diagnosis and grading of acute12 and chronic13 GVHD were performed by transplant centers using standard published criteria.
Data were based on transplant center reports. Centers were contacted to complete missing data when needed. Cytogenetic and pathology reports were reviewed to confirm the diagnosis of SN and exclude relapse.
All patients or their legal guardians provided informed consent according to the Declaration of Helsinki to use transplantation data for research purposes. The Institutional Review Board of EBMT/Eurocord approved this study.
End points
The primary end point was OS, calculated from the date of diagnosis of the SNs until death or last follow-up for survivors. Secondary end points included the description of the subtypes of SNs occurring after UCBT and their associated risk factors and the evaluation of all causes of death.
Statistical analysis
Median values and ranges (or interquartile ranges [IQR]) were used for numerical variables and frequencies and percentages for categorical variables. Time to diagnosis of SNs was calculated as the time interval between UCBT and the occurrence of SNs. The probabilities of OS from the diagnosis of SNs were determined using Kaplan-Meier estimates and the log-rank test for bivariate comparisons. For patients who developed more than 1 SN after HCT, the outcome was assessed considering the first diagnosis. SPSS and R software packages were used for the statistical analyses.
Results
Patients, disease, and transplant characteristics
Between October 1988 (date of the first UCBT) and December 2018, 10 358 eligible patients received their first UCBT in 311 EBMT-affiliated centers. Among these recipients, 233 (123 males and 110 females) developed SNs (2.2%). The median age at UCBT was 31 years (range, 0.3-69), and 84 (36%) were pediatric patients. Primary diagnoses were 83% malignant (n = 199) and 15% (n = 34) nonmalignant hematological diseases. Myeloablative (MAC) regimens were administered in 54% (n = 126), and ATG in 67% (n = 157) of the UCBT recipients. Fifty-one percent (n = 118) of the patients received total body irradiation (TBI) as part of their conditioning with either low doses (≤8 Gy; 70/118) or high doses (>8 Gy; 48/118) regimen. Graft sources included single (n = 154), single-expanded (n = 2), and double (n=77) UCB. All patients received unrelated UCB grafts. The median cell dose at cryopreservation was 4.7 × 107/kg (IQR, 3.6-6.3) of total nucleated cells and 1.9 × 105/kg (IQR, 1.3-3.1) of CD34+ cells. Graft-recipient pairs had 0 to 1/6 HLA mismatch in 47%, and they had 2/6 and 3/6 HLA mismatches in 39% and 3% of the cases, respectively. UCBTs analyzed in this study were performed in 101 transplant centers. Patients, primary disease, and graft characteristics are summarized in Tables 1 and 2.
Patient and disease characteristics
| Number of patients, N (%) | 233 | (100%) |
| Median age at diagnosis of primary disease, y (range) | 28 | (0.1-68) |
| Median age at UCBT, y (range) | 31 | (0.3-69) |
| Children (<18 y), n (%) | 84 | (36) |
| Adults (≥18 y), n (%) | 149 | (64) |
| Median age at diagnosis of SN, y (range) | 32.4 | (1-73) |
| Median age at last follow-up, y (range) | 36.2 | (1.6-75.4) |
| Males, n (%) | 123 | (53) |
| Positive recipient CMV serology, n (%) | 120 | (52) |
| Type of primary disease, n (%) | ||
| Nonmalignant | 34 | (15) |
| Malignant | 199 | (83) |
| Primary diagnosis, n (%) | ||
| Acute leukemia | 121 | (52) |
| MDS/MPD | 32 | (14) |
| Lymphoproliferative disorders | 45 | (19) |
| Histiocytic disorder | 3 | (1) |
| ST | 1 | (<1) |
| BM failure syndrome | 13 | (6) |
| Hemoglobinopathy | 3 | (1) |
| Primary immune deficiency | 7 | (3) |
| Inborn error of metabolism | 8 | (4) |
| Previous HCT, n (%) | 52 | (22) |
| MAC, n (%) | 126 | (54%) |
| ATG, n (%) | 157 | (67) |
| TBI, n (%) | 118 | (51) |
| Number of patients, N (%) | 233 | (100%) |
| Median age at diagnosis of primary disease, y (range) | 28 | (0.1-68) |
| Median age at UCBT, y (range) | 31 | (0.3-69) |
| Children (<18 y), n (%) | 84 | (36) |
| Adults (≥18 y), n (%) | 149 | (64) |
| Median age at diagnosis of SN, y (range) | 32.4 | (1-73) |
| Median age at last follow-up, y (range) | 36.2 | (1.6-75.4) |
| Males, n (%) | 123 | (53) |
| Positive recipient CMV serology, n (%) | 120 | (52) |
| Type of primary disease, n (%) | ||
| Nonmalignant | 34 | (15) |
| Malignant | 199 | (83) |
| Primary diagnosis, n (%) | ||
| Acute leukemia | 121 | (52) |
| MDS/MPD | 32 | (14) |
| Lymphoproliferative disorders | 45 | (19) |
| Histiocytic disorder | 3 | (1) |
| ST | 1 | (<1) |
| BM failure syndrome | 13 | (6) |
| Hemoglobinopathy | 3 | (1) |
| Primary immune deficiency | 7 | (3) |
| Inborn error of metabolism | 8 | (4) |
| Previous HCT, n (%) | 52 | (22) |
| MAC, n (%) | 126 | (54%) |
| ATG, n (%) | 157 | (67) |
| TBI, n (%) | 118 | (51) |
CMV, cytomegalovirus; MDS/MPD, myelodysplastic/myeloproliferative disease.
Graft characteristics
| Number of patients, N | 233 | |
| Median transplant year (range) | 2009 | (1992-2020) |
| Transplant period, n (%) | ||
| 1988-2000 | 15 | (6%) |
| 2001-2005 | 32 | (14%) |
| 2006-2010 | 110 | (47%) |
| 2011-2015 | 68 | (29%) |
| ≥2016 | 8 | (3%) |
| Graft type, n (%) | ||
| Unrelated UCB | 233 | (100%) |
| Graft source, n (%) | ||
| Single UCB unmanipulated | 147 | (63%) |
| Single UCB expanded | 2 | (1%) |
| Double UCB | 77 | (33%) |
| Intrabone injection of 1 of the UCB | 7 | (3%) |
| HLA mismatch, n (%) | ||
| 0-1/6 | 109 | (47%) |
| 2/6 | 90 | (39%) |
| 3/6 | 7 | (3%) |
| Missing | 27 | (11%) |
| Cell dose, median (IQR) | ||
| Median TNC at cryopreservation (×107/kg) | 4.7 | (3.6-6.3) |
| Median CD34+ cell at cryopreservation (×105/kg) | 1.9 | (1.3-3.1) |
| Number of patients, N | 233 | |
| Median transplant year (range) | 2009 | (1992-2020) |
| Transplant period, n (%) | ||
| 1988-2000 | 15 | (6%) |
| 2001-2005 | 32 | (14%) |
| 2006-2010 | 110 | (47%) |
| 2011-2015 | 68 | (29%) |
| ≥2016 | 8 | (3%) |
| Graft type, n (%) | ||
| Unrelated UCB | 233 | (100%) |
| Graft source, n (%) | ||
| Single UCB unmanipulated | 147 | (63%) |
| Single UCB expanded | 2 | (1%) |
| Double UCB | 77 | (33%) |
| Intrabone injection of 1 of the UCB | 7 | (3%) |
| HLA mismatch, n (%) | ||
| 0-1/6 | 109 | (47%) |
| 2/6 | 90 | (39%) |
| 3/6 | 7 | (3%) |
| Missing | 27 | (11%) |
| Cell dose, median (IQR) | ||
| Median TNC at cryopreservation (×107/kg) | 4.7 | (3.6-6.3) |
| Median CD34+ cell at cryopreservation (×105/kg) | 1.9 | (1.3-3.1) |
TNC, total nucleated cells.
SNs characteristics
Three main subgroups of SNs were identified (Table 3): posttransplant lymphoproliferative disorders (PTLD, n = 145, 62%), AL/MDS (n = 23, 10%), and STs (n = 65 patients, 28%, who developed 72 STs).
Subtypes of SNs
| . | PTLD . | AL/MDS . | ST∗ . | . | ||
|---|---|---|---|---|---|---|
| Patients with SNs, n (%) | 145 | 100% | 23 | 100% | 65∗ | 100% |
| Transplant period | ||||||
| 1988-2000 | 8 | 6 | 1 | 5 | 6 | 9 |
| 2001-2005 | 19 | 13 | 8 | 35 | 5 | 8 |
| 2006-2010 | 66 | 45 | 9 | 39 | 35 | 54 |
| 2011-2015 | 46 | 32 | 4 | 17 | 18 | 28 |
| ≥2016 | 6 | 4 | 1 | 4 | 1 | 1 |
| Median age at UCBT, y (range) | 26 | (0.3-68) | 23 | (1-62) | 50 | (0.5-67) |
| Age <18 | 56 | 39 | 10 | 43 | 18 | 28 |
| Age ≥18 | 89 | 61 | 13 | 57 | 47 | 72 |
| Median interval (UCBT-SN), mo (IQR) | 3.7 | (2-7) | 28 | (16-54) | 54 | (30-83) |
| Nonmalignant diseases | 29 | 20 | 2 | 9 | 3 | 5 |
| Malignant diseases | 116 | 80 | 21 | 91 | 62 | 95 |
| MAC | 85 | 59 | 12 | 52 | 29 | 45 |
| TBI | 65 | 45 | 10 | 43 | 43 | 66 |
| ATG | 114 | 79 | 12 | 52 | 31 | 48 |
| Alemtuzumab | 3 | 2 | 3 | 13 | 1 | 2 |
| Recipient EBV-seropositivity | 82 | 57 | 12 | 52 | 34 | 52 |
| Collected TNC at cryopreservation (×107/kg), median (IQR) | 4.5 | 3.5-6.0 | 5.0 | 3.4-7.3 | 5.1 | 4.3-6.4 |
| HLA disparity, n (%) | ||||||
| 0/6 MM | 18 | 12 | 1 | 4 | 8 | 12 |
| 1/6 MM | 55 | 38 | 7 | 30 | 20 | 31 |
| 2/6 MM | 55 | 38 | 9 | 39 | 26 | 40 |
| 3/6 MM | 3 | 2 | 3 | 13 | 1 | 2 |
| Missing | 14 | 10 | 3 | 13 | 10 | 15 |
| Survivors, n | 29 | 7 | 32 | |||
| Median follow-up for survivors after UCBT, mo | 96 | 7-225 | 72 | 29-161 | 125 | 55-292 |
| Median follow-up for survivors after SN, mo | 92 | 5-223 | 35 | 16-110 | 58 | 14-179 |
| . | PTLD . | AL/MDS . | ST∗ . | . | ||
|---|---|---|---|---|---|---|
| Patients with SNs, n (%) | 145 | 100% | 23 | 100% | 65∗ | 100% |
| Transplant period | ||||||
| 1988-2000 | 8 | 6 | 1 | 5 | 6 | 9 |
| 2001-2005 | 19 | 13 | 8 | 35 | 5 | 8 |
| 2006-2010 | 66 | 45 | 9 | 39 | 35 | 54 |
| 2011-2015 | 46 | 32 | 4 | 17 | 18 | 28 |
| ≥2016 | 6 | 4 | 1 | 4 | 1 | 1 |
| Median age at UCBT, y (range) | 26 | (0.3-68) | 23 | (1-62) | 50 | (0.5-67) |
| Age <18 | 56 | 39 | 10 | 43 | 18 | 28 |
| Age ≥18 | 89 | 61 | 13 | 57 | 47 | 72 |
| Median interval (UCBT-SN), mo (IQR) | 3.7 | (2-7) | 28 | (16-54) | 54 | (30-83) |
| Nonmalignant diseases | 29 | 20 | 2 | 9 | 3 | 5 |
| Malignant diseases | 116 | 80 | 21 | 91 | 62 | 95 |
| MAC | 85 | 59 | 12 | 52 | 29 | 45 |
| TBI | 65 | 45 | 10 | 43 | 43 | 66 |
| ATG | 114 | 79 | 12 | 52 | 31 | 48 |
| Alemtuzumab | 3 | 2 | 3 | 13 | 1 | 2 |
| Recipient EBV-seropositivity | 82 | 57 | 12 | 52 | 34 | 52 |
| Collected TNC at cryopreservation (×107/kg), median (IQR) | 4.5 | 3.5-6.0 | 5.0 | 3.4-7.3 | 5.1 | 4.3-6.4 |
| HLA disparity, n (%) | ||||||
| 0/6 MM | 18 | 12 | 1 | 4 | 8 | 12 |
| 1/6 MM | 55 | 38 | 7 | 30 | 20 | 31 |
| 2/6 MM | 55 | 38 | 9 | 39 | 26 | 40 |
| 3/6 MM | 3 | 2 | 3 | 13 | 1 | 2 |
| Missing | 14 | 10 | 3 | 13 | 10 | 15 |
| Survivors, n | 29 | 7 | 32 | |||
| Median follow-up for survivors after UCBT, mo | 96 | 7-225 | 72 | 29-161 | 125 | 55-292 |
| Median follow-up for survivors after SN, mo | 92 | 5-223 | 35 | 16-110 | 58 | 14-179 |
MM, mismatch; TNC, total nucleated cell.
Seven patients developed 2 STs, which corresponded to a total of 72 ST reported.
Posttransplant lymphoproliferative disorders
A total of 145 patients (56 children and 89 adults) developed PTLD in a median of 3.7 months (range, 0.8-67; IQR, 2-7) after UCBT. Fifteen of the PTLD cases occurred after 1 year after transplant, including 1 late-onset Hodgkin lymphoma (HL). The median age at UCBT was 26 (0.3-68) years (Table 3) Primary disease was hematological malignancy in 116 patients (80%). Eighty-one patients (56%) had received a minimum of 2 lines of therapy before UCBT and 34 (23%) had undergone a previous autologous or/and allogeneic transplant (1 patient had previous auto + alloHCT). Eighty-five patients (59%) had received MAC regimens, 65 patients (45%) TBI (36 patients with doses ≤8 Gy, 29 patients with doses 10-14 Gy), and 117 patients (81%) had received ATG or alemtuzumab. One to 3 alkylating agents had been administered as part of the conditioning regimens in 135 patients (93%) and fludarabine in 99 patients (68%). Fifty-eight (40%) of the graft-recipient pairs had 2/6 HLA mismatches (Table 4). Before PTLD diagnosis, 39 and 48 patients had developed acute GVHD (aGVHD) and chronic GVHD (cGVHD) (23 extensive), respectively.
Risk factors for SNs
| . | PTLD (N = 145) . | AL/MDS (N = 23) . | STs (N = 65) . | |||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | |
| ATG | 114 | 79 | 12 | 52 | 31 | 48 |
| Alemtuzumab | 3 | 1 | 3 | 13 | 1 | 2 |
| ≥2/6 HLA mismatches | 58 | 40 | 12 | 52 | 27 | 42 |
| aGVHD II-IV | 38 | 26 | 6 | 26 | 24 | 37 |
| cGVHD | 48 | 33 | 3 | 13 | 26 | 40 |
| TBI | 65 | 45 | 10 | 43 | 43 | 66 |
| 2-8 Gys | 36 | 7 | 27 | |||
| 10-14 Gys | 29 | 3 | 16 | |||
| Previous autoHCT | 25 | 17 | 4 | 17 | 12 | 18 |
| Previous alloHCT | 10 | 7 | 1 | 4 | 1 | 2 |
| New alloHCT after UCBT | 16 | 4 | 12 | 52 | 2 | 3 |
| Previous therapy lines ≥ 2 | 81 | 56 | 15 | 65 | 48 | 74 |
| Conditioning regimen | ||||||
| RIC | 60 | 41 | 11 | 48 | 36 | 55 |
| No alkylating agent | 10 | 7 | 0 | 0 | 3 | 4 |
| One alkylating agent | 72 | 50 | 14 | 61 | 44 | 68 |
| Two alkylating agents | 59 | 41 | 8 | 35 | 18 | 28 |
| Three alkylating agents | 4 | 2 | 1 | 4 | 0 | 0 |
| Fludarabine | 99 | 68 | 15 | 65 | 50 | 77 |
| . | PTLD (N = 145) . | AL/MDS (N = 23) . | STs (N = 65) . | |||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | |
| ATG | 114 | 79 | 12 | 52 | 31 | 48 |
| Alemtuzumab | 3 | 1 | 3 | 13 | 1 | 2 |
| ≥2/6 HLA mismatches | 58 | 40 | 12 | 52 | 27 | 42 |
| aGVHD II-IV | 38 | 26 | 6 | 26 | 24 | 37 |
| cGVHD | 48 | 33 | 3 | 13 | 26 | 40 |
| TBI | 65 | 45 | 10 | 43 | 43 | 66 |
| 2-8 Gys | 36 | 7 | 27 | |||
| 10-14 Gys | 29 | 3 | 16 | |||
| Previous autoHCT | 25 | 17 | 4 | 17 | 12 | 18 |
| Previous alloHCT | 10 | 7 | 1 | 4 | 1 | 2 |
| New alloHCT after UCBT | 16 | 4 | 12 | 52 | 2 | 3 |
| Previous therapy lines ≥ 2 | 81 | 56 | 15 | 65 | 48 | 74 |
| Conditioning regimen | ||||||
| RIC | 60 | 41 | 11 | 48 | 36 | 55 |
| No alkylating agent | 10 | 7 | 0 | 0 | 3 | 4 |
| One alkylating agent | 72 | 50 | 14 | 61 | 44 | 68 |
| Two alkylating agents | 59 | 41 | 8 | 35 | 18 | 28 |
| Three alkylating agents | 4 | 2 | 1 | 4 | 0 | 0 |
| Fludarabine | 99 | 68 | 15 | 65 | 50 | 77 |
PTLD occurred within the first year post-UCBT in 130 recipients. From those, 105 had received ATG and 56 had received prolonged IS to treat their aGVHD II-IV (n = 31) and/or cGVHD (n = 40); 14 of the 130 did not receive rituximab to treat the PTLD (6 were diagnosed before year the 2000 and 8 died before rituximab administration). A total of 103 of the 130 patients died; the causes of death were PTLD (n = 73), relapse of primary disease (n = 9), nonrelapse mortality (n = 20 [9 GVHD, 9 infections, 1 idiopathic pneumonia, 1 hemorrhage]), and 1 cause unknown.
A total of 15 patients developed PTLD 1 year after UCBT; 9 of the 15 had received ATG before transplant, and 8 of the15 had received heavy and prolonged IS therapy for aGVHD (n = 4) and/or cGVHD (n = 6). PTLD was not treated with rituximab in 4 of 15 patients (2 who developed PTLD before year 2000, 1 who died before initiation of rituximab and 1 HL); only 2 of 15 patients survived regardless of PTLD treatment with rituximab ± chemotherapy. The causes of death were PTLD (n = 10), infection (n = 2), and gastrointestinal (GI) bleeding (n = 1).
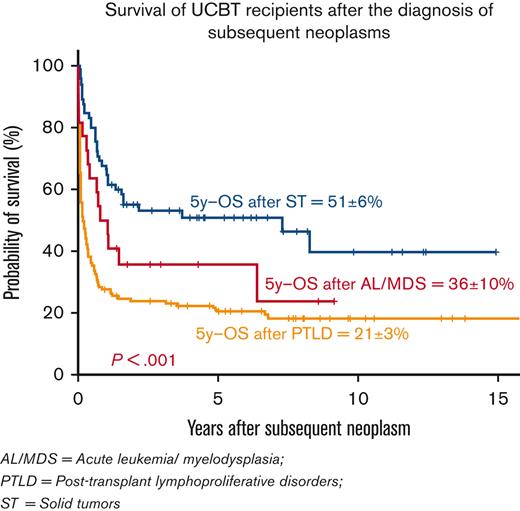
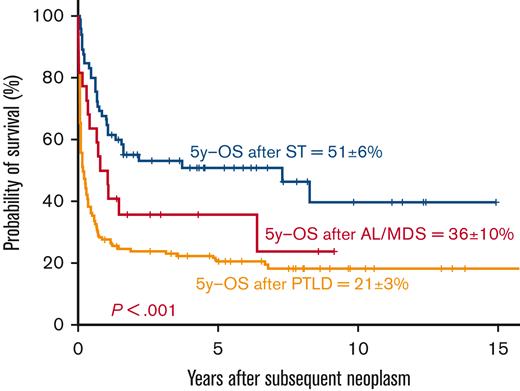
At last follow-up, 29 of 145 patients with PTLD were alive; the probability of 5-year survival after the diagnosis of PTLD was 21% ± 3%. (Figure 1)
Survival after diagnosis of SN: 5-year OS after diagnosis of PTLD (21% ± 3%), AL/MDS (36% ± 10%), and STs (51% ± 6%).
Survival after diagnosis of SN: 5-year OS after diagnosis of PTLD (21% ± 3%), AL/MDS (36% ± 10%), and STs (51% ± 6%).
One case of late-onset HL, diagnosed 3 years after dUCBT, was reported in our cohort in a male patient with primary acute myeloid leukemia (AML) in second complete remission. The patient had positive pretransplant Epstein-Barr virus (EBV) serology. Conditioning regimen included fludarabine, cyclophosphamide, and low-dose (2 Gy) TBI with no ATG administration. Prolonged IS was used because of the development of chronic GVHD. HL was treated with appropriate chemotherapy. The patient was in complete remission of both the leukemia and the lymphoma when he died from a nonhematological cause (GI bleeding), 3.5 years after the diagnosis of the SNs.
Acute leukemia/myelodysplasia
AL/MDS was observed in 23 patients within a median of 28 months (range, 8-103; IQR, 16-54) after UCBT, including 12 with AML, 3 with donor-cell AML, 3 with acute lymphoblastic leukemia (ALL), and 5 with MDS (Tables 3 and 5). The median recipient age at UCBT was 23 years (range, 1-62). Twenty-one patients (91%) had been transplanted for malignant diseases (ALL [n = 9], AML [n = 3], myeloproliferative diseases [n = 3], and lymphoma [n = 6]), 1 patient for bone marrow (BM) failure and 1 patient for thalassemia. Fifteen patients had received a minimum of 2 treatment lines before UCBT, and 5 had received previous autologous or/and allogeneic HCT. (Tables 3 and 4) Twelve patients had received MAC regimens, 10 patients had received TBI (7 patients with doses ≤8 Gy, 3 patients with doses >8 Gy), and 15 patients ATG or alemtuzumab. Alkylating agents had been administered to all the patients as part of the conditioning regimen, and fludarabine was used in 15 patients. The UCB unit had ≥2/6 HLA mismatches with the recipients in 12 patients. Acute GVHD and cGVHD were observed in 6 and 3 recipients, respectively. Twelve subsequent allogeneic transplants were performed in 12 UCBT recipients to treat AL/MDS (n = 7), graft failure (n = 1), or relapse (n = 4). Sixteen patients died; the causes of death were relapse of primary disease (n = 4), progression of the SN (n = 8), GVHD (n = 2), and infections (n = 2). At last follow-up, only 7 patients were alive (6 after a second allogeneic HCT). The 5-year survival probability after the diagnosis of AL/MDS was 36% ± 10%. (Figure 1)
Types of SNs
| PTLD . | N = 145 . |
|---|---|
| Early PTLD | 130 |
| Late PTLD | 15 |
| AL/MDS | N = 23 |
| ALL | 3 |
| AML | 12 |
| Donor-derived AML | 3 |
| MDS | 5 |
| STs | N = 72∗ |
| Lung | 10 |
| Thyroid | 8 |
| Bone sarcoma | 1 |
| Soft tissue sarcoma | 6 |
| Oral cavity | 3 |
| Upper GI | 2 |
| Colorectal | 3 |
| Skin (BCC) | 4 |
| Skin (non-BCC) | 4 |
| Breast | 3 |
| Cervix | 2 |
| Kidney | 2 |
| Bladder | 2 |
| CNS | 1 |
| Neuroendocrine | 1 |
| Pancreas | 1 |
| Parotide | 1 |
| Prostate | 1 |
| Other ST | 3 |
| Breast + skin (BCC) | 1 + 1 |
| Breast + skin (non-BCC) | 1 + 1 |
| Lung + skin (BCC) | 1 + 1 |
| Thyroid + bone sarcoma | 1 + 1 |
| CNS+ colorectal | 1 + 1 |
| Upper GI + CNS | 1 + 1 |
| Neuroendocrine + skin (BCC) | 1 + 1 |
| PTLD . | N = 145 . |
|---|---|
| Early PTLD | 130 |
| Late PTLD | 15 |
| AL/MDS | N = 23 |
| ALL | 3 |
| AML | 12 |
| Donor-derived AML | 3 |
| MDS | 5 |
| STs | N = 72∗ |
| Lung | 10 |
| Thyroid | 8 |
| Bone sarcoma | 1 |
| Soft tissue sarcoma | 6 |
| Oral cavity | 3 |
| Upper GI | 2 |
| Colorectal | 3 |
| Skin (BCC) | 4 |
| Skin (non-BCC) | 4 |
| Breast | 3 |
| Cervix | 2 |
| Kidney | 2 |
| Bladder | 2 |
| CNS | 1 |
| Neuroendocrine | 1 |
| Pancreas | 1 |
| Parotide | 1 |
| Prostate | 1 |
| Other ST | 3 |
| Breast + skin (BCC) | 1 + 1 |
| Breast + skin (non-BCC) | 1 + 1 |
| Lung + skin (BCC) | 1 + 1 |
| Thyroid + bone sarcoma | 1 + 1 |
| CNS+ colorectal | 1 + 1 |
| Upper GI + CNS | 1 + 1 |
| Neuroendocrine + skin (BCC) | 1 + 1 |
BCC, basal cell carcinoma; CNS, central nervous system.
Seven patients developed 2 STs, which corresponded to a total of 72 ST reported.
Donor-cell myeloid leukemia was observed in 3 patients (primary diagnosis: ALL [n = 2] and chronic myeloid leukemia [CML, n=1]) in a median of 20 months (range, 17-22) post-UCBT. Two patients had received MAC regimens, and all 3 had received fludarabine. ATG had been administered in 1, alemtuzumab in 1, and colony-stimulating factors in 2 of the 3 patients who developed donor-cell AML. The donor origin of the leukemia was confirmed by recipient/donor sex mismatch (n = 2) or molecular (n = 1) studies. A new allogeneic HCT (mismatched unrelated donor transplant) was performed in 1 patient. All 3 patients died in a median of 1.25 months (range, 0.5-8.4) owing to the progression of the donor-cell AML.
Solid tumors
Sixty-five patients developed 72 ST (7 patients with 2 ST) in a median of 4.5 years (range, 4 months-14 years; IQR, 2.5-7 years) after transplantation (Table 3). The median age was 50 years (range, 0.5-67) at UCBT. The most frequent tumors were lung (n = 11), bone and soft tissue (n = 8), thyroid (n = 9), GI (n = 7), skin (n = 7 basal cell carcinoma and n = 5 nonbasal cell carcinoma), oral cavity (n = 6), and breast (n = 5), in addition to other less common cancer sites (n = 14) (Table 5). Forty-eight (74%) patients had been heavily treated with at least 2 treatment lines before UCBT, including previous autologous or/and allogeneic transplants in 13 patients (20%) (Tables 3 and 4). Twenty-nine patients (45%) had received MAC regimens, 43 patients (66%) had received TBI (27 patients with doses ≤ 8 Gy, 16 patients with doses > 8 Gy), and 32 patients (49%) ATG or alemtuzumab. Alkylating agents had been administered to 62 patients (95%) and fludarabine to 50 patients (77%). Recipients and graft pairs had ≥2/6 HLA mismatches in 42% of cases. Acute GVHD had developed in 37% (n = 24) and cGVHD in 40% (n = 26) of the recipients. Two patients had received a new allogeneic transplant before the development of ST because of graft failure (n = 1) or relapse (n=1) of primary disease. The median length of follow-up since UCBT was 7.6 years (range, 1-24) for the patients who developed STs and 10 years (4.5-24) for those who survived with a ST. A total of 33 (51%) patients died with an ST diagnosis; 26 deaths were consequent to ST, 6 to relapse of primary disease, and 1 cause of death was missing. Overall, half of the ST cases (n = 32; 49%) were alive at the last follow-up. The 5-year probability of survival after the diagnosis of ST was 51% ± 6%. (Figure 1), with a median follow-up for survivors of 4.8 years (range,1-15) after diagnosis of ST.
The median length of follow-up since UCBT was 26 months (1-292) in our cohort of 233 patients. Overall, 68 patients (29%) were alive at last follow-up, with a median follow-up for survivors since UCBT of 104 (6.8-292) months (96 months for PTLD, 72 months for AL/MDS, and 125 months for ST). There was no difference in median length of follow-up of survivors after UCBT based on TBI exposure (107 months for TBI vs 103 months for no TBI), cGVHD (107 months for cGVH vs 103 months for no cGVH), or ATG administration (106 months for ATG vs 103 months for no ATG).
Discussion
Because of improved outcomes after UCBT over the last few decades and longer survival, late complications, including SNs, have been reported in UCB recipients with associated late posttransplant mortality. There is a paucity of studies addressing the risk of SNs after UCBT, and data related to SNs in long-term survivors after this procedure are scarce. Few observational studies6-8,14,15 reported the occurrence of SNs after UCBT but the sample sizes were too narrow to allow risk comparison with transplants using adult donor grafts.
In this large cohort of 10 358 patients who received UCBT in EBMT centers, 233 developed SNs; no major differences within tumor types were observed compared with those reported after transplants using other graft sources.7,8,16,17 PTLD was mainly diagnosed during the first year, AL/MDS after 1 to 2 years, and ST occurred later in the course of follow-up. Risk factors included previous exposure to alkylating agents, TBI (both low-doses ≤8 Gy and high-doses >8 Gy), ATG, and multiple lines of previous therapy (including HCT). The prolonged IS in the context of acute and chronic GVHD was also a critical risk factor. (Table 4) Outcomes were poor for all 3 SNs subtypes with an overall mortality rate of 71% (165/233). SN was the primary cause of death in 71% of the patients who died with a SN diagnosis. Similar results were published by the EBMT,7 the CIBMTR,16 and in other smaller series.8,17
PTLD was the most commonly reported SN in our study and was observed mainly in the first year after UCBT, likely owing to abnormal lymphoid proliferation of B cells in the absence of effective T cells or other immune regulation of B cells. The incidence of PTLD substantially declined 2 years after UCBT, and no cases were reported after the third posttransplant year. Late PTLD represents a distinct entity associated with GVHD,18,19 and was reported in 15 patients after the first transplant year, in the context of prolonged IS. One case of HL was observed 3 years after dUCBT in the context of prolonged, extensive GVHD. HL has been described as a type of PTLD characterized by late posttransplant onset (>2.5 years) with a relatively good prognosis if treated with appropriate chemotherapy, which is in agreement with the case observed in our study.20-22
PTLD is frequently attributed to EBV–induced B-cell proliferation of donor cells in the immune-compromised host.18,19,23 UCB grafts are characterized by EBV serological negativity, which may explain the higher susceptibility to posttransplant EBV reactivation/infection in UCBT recipients in the absence of anti-EBV immunity provided by the donor.6,8 Although EBV serology was not available in 34% of the recipients, 82 out of the 145 patients (57%) who developed PTLD had positive EBV serology and were at risk of posttransplant EBV reactivation.
In the literature, the incidence of PTLD after unrelated UCBT is 2.6% to 3.3% after MAC and 7% to 12.9% after reduced intensity conditioning (RIC) transplants,6,21 but higher rates (17%-21%) have been reported in UCBT with multiple risk factors, such as the association of ATG + RIC,6,7,24-27 and/or the use of dUCBT.24 In our cohort, the incidence of PTLD was as low as 1.4% (145/10 358) despite the increased use of RIC over the last decade. This low incidence compares favorably with the incidence reported after matched and mismatched sibling donors (1.16%-2.86%) or matched unrelated (3.97%) and mismatched unrelated donor HCT (11.24%).28,29
PTLD occurring after UCBT, Ballen et al8 reported 68% mortality in a small cohort of UCBT and a German group reported 82% mortality in the patients who developed PTLD.17 In our patients, we also observed poor response to rituximab therapy when administered and frequent relapse/progression even when chemotherapy was given. The mortality rate after diagnosis of PTLD was 80% (116/145); PTLD was the main cause of death in most (72%) patients, and many PTLD diagnoses were made in the perimortem period.
Overall, PTLD had a low occurrence rate in UCBT recipients but resulted in increased mortality, with a 5-year survival rate of 21%. This poor survival coincides with the results of Uhlin et al,30 who reported a 3-year survival rate of 20% in patients developing PTLD after allogeneic HCT, despite initial successful treatment with rituximab. However, new available and up-and-coming strategies (EBV-specific cytotoxic T lymphocytes,31 genetically engineered cytotoxic T lymphocytes,32 etc) might transform the therapeutic approach to PTLD in the near future with promising results and favorable toxicity profiles.
AL/MDS occurring after allogeneic HCT is usually of myeloid origin in 90% of cases. It represents a serious complication of cytotoxic therapy and a leading cause of nonrelapse posttransplant mortality. It occurs in heavily pretreated patients, it is more frequently described in the autologous setting,27,33,34 and is rather related to pretransplant factors than the transplant itself.35 The incidence rate of AL/MDS was 0.22% in our study, consistent with data reported after adult HCT using BM or peripheral blood (PB) grafts (<0.25%). AL/MDS developed between 1.5 and 4 years post-UCBT, in a median of 2 years. Potential risk factors were previous exposure to alkylating agents, fludarabine, radiotherapy, and previous autologous (n = 4) or allogeneic (n = 1) HCT in line with published series.36,37 The prognosis was poor, with a death rate of 69% (16/23), and a better chance to survive if a second allogeneic HCT was performed. Among the 7 patients who were alive at the last follow-up in our study, 6 had received a new allogeneic HCT after the diagnosis of AL/MDS.
Donor-cell–derived AL/MDS is a rare entity.38,39 In the context of our registry data, we observed an incidence of 0.03% (3/10358). This incidence was lower than that reported in other UCBT series8; it was also lower than the incidence (<0.25%) after adult donor HCT.9,40-45 A previous EBMT survey estimated the incidence to be 0.12% after HCT.46 However, the true incidence of donor-cell leukemia after UCB or adult donor transplantation is unknown and probably underreported because chimerism or cytogenetic studies are not routinely performed after HCT to search for donor origin to distinguish donor-cell AL/MDS from relapsed AML.8,47-49 In our cohort, all 3 patients with donor-cell leukemia died in a median of 1.2 months (range, 0.5-8.4) after diagnosis because of progression, despite salvage treatment with a new allogeneic HCT in 2 patients. Data were not available about the occurrence of leukemia in the UCB donors. The pathogenesis of secondary donor-cell leukemia remains unclear. The role of conditioning regimens is less important as donor cells are not exposed to chemotherapy. The possible presence of preleukemic clones in up to 5% of UCB units have been postulated,50 as well as the use of colony-stimulating factors that might foster preleukemic mutations, as described in patients with aplastic anemia.51-53
ST was observed in 65 patients who survived >2 years after UCBT. In our population of 10 358 UCBT recipients, 18 patients, 43 patients, and 58 patients developed ST at 5, 10, and 15 years after transplant, respectively, which corresponded to an incidence rate of subsequent STs of 0.17% at 5 years, 0.42% at 10 years, and 0.56% at 15 years, with increasing cumulative rates as per longer follow-up. Nevertheless, the incidence of STs after UCBT in our study was relatively low compared with that reported after BM or PB grafts in other registry-based studies.52-54 The CIBMTR reported a large cohort55 of allogeneic sibling BM recipients transplanted between 1964 and 1994, mostly after TBI-based MAC regimen (>70%). The CI of subsequent ST was 2.2% at 10 years and 6.7% at 15 years after transplantation. Later, Majhail et al56 analyzed the impact of non-TBI based MAC regimen after allogeneic HCT for AML/CML. The incidence of STs was 1.2% for AML and 2.4% for CML at 10 years. More recently, Atsuta et al57 reported 269 ST in 17 545 HCT recipients, with a CI of 1.7% at 10 years. In addition to registry data, most published studies58,59 reported incidences of subsequent STs ranging from 2.2% to 6.4% at 10 years after allogeneic BM or PB HCT. Ringden et al60 assessed the risk of subsequent STs in recipients of nonmyeloablative and RIC HCT and reported a CI of 3.35% in 10 years. Two studies55,61 reported CI of 3.3% and 3.8% at 20 years after HCT.
Although our study was not designed to evaluate risk factors, the ST sites observed were similar to those reported in other graft sources, with high numbers of lung, sarcoma (bone + soft tissue), oral, thyroid, and upper GI cancers.
The use of alkylating agents and TBI (to 96% and 66% of the patients who developed ST in our study population, respectively) has been associated with the development of ST, especially sarcoma.54 The exposure to TBI doses >8 Gy were associated with thyroid cancers, osteosarcomas, and central nervous system tumors.
ST was the primary cause of death in 78% (26/33) of the patients who died with an ST diagnosis in our study. Similar death rates attributed to ST were reported in the EBMT62,63 and the CIBMTR55,56 registries. In a recent EBMT cohort, Tichelli et al63 reported a 5-year OS after ST of 47%; ST was the primary cause of death in 74.8% of patients. In the CIBMTR55 cohort, ST was the reported cause of death in 63% of patients who died with an ST diagnosis. Finally, ST was the cause of late mortality in 86% in a large cohort of Japanese HCT recipients.57
The 5-year OS after ST was 51% in our cohort, with 34% patients surviving less than 1 year after the ST diagnosis. However, half of the patients with an ST diagnosis survived, highlighting the importance of strategies to promote lifelong surveillance of UCBT recipients.64
We acknowledge that our study has several limitations, mainly because of its retrospective nature and registry-based data reporting. In patients who developed PTLD, many data related to pretransplant treatment regimens and exposures, the EBV serological status of many recipients, the centers’ strategy for EBV screening (as recommended by current best practice), the EBV viral load at PTLD diagnosis, and rituximab administration were missing.
Finally, an underreported incidence of ST in this population might be speculated owing to a possible loss of contact between long-term survivors and the transplant centers, considering the long time period between UCBT and the time that might take for the development of a ST. We lacked detailed information specific to the ST, namely their stage and treatment. We also exclusively included in the study the patients who reported having developed SNs, therefore, making it impossible to assess risk or perform comparisons using advanced statistical (univariate and multivariate) analyses to identify variables that contribute to the development of SN.
Similarly, the incidences for the SNs reported in our cohort might be underestimated because of the assumption that recipients of UCBT with missing information did not develop SNs.
In conclusion, early- or late-onset SNs are extremely rare complications occurring after UCBT with very poor outcome and high mortality. Particular attention should be paid to subsequent STs because of their increasing rates with longer survival and their associated late mortality, highlighting the importance of life-long screening for prevention and early detection of solid malignancies to improve OS after UCBT.64
Acknowledgments
The authors thank all the principal investigators and the participating centers who provided the data: Jaime Sanz, Hospital Universitario La Fe, Spain; Regis Peffault De La Tour, Hopital St Louis, Paris, France; Gérard Michel, La Timone Children’s Hospital, Marseille, France; Albert Esquirol, Hospital de la Santa Creu i Sant Pau, IIB-Sant Pau, Spain; Patrice Chevallier, Hotel Dieu, CHU Nantes France; Marie-Therese Rubio, CHRU Nancy, France; Mauricette Michallet, Centre Leon Berard, Lyon, France; Jan J. Cornelissen, Erasmus MC-Daniel den Hoed Cancer Centre, The Netherlands; Petr Sedlacek, University Hospital Motol, Czech Republic; Henrik Sengeloev, National University Hospital Rigshospitalet, Denmark; Xavier Leleu, Hopital La Miletrie, France; Jose L Diez-Martin, Hospital Universitario Gregorio Maranon, Madrid, Spain; Rachel Protheroe, Bristol Royal Hospital for Children, United Kingdom; Johan Maertens, University Hospital Gasthuisberg, Belgium; Didier Blaise, Centre de Recherche en Cancérologie de Marseille, France; Eric Deconinck, Hopital Jean Minjoz, France; Noel Milpied, CHU Bordeaux, France; Ibrahim Yakoub-Agha, Hopital Claude Huriez, France; Pierre-Simon Rohrlich, Hôpital de L`Archet I, France; Roberto Foá, University ‘La Sapienza,’ Italy; Per Ljungman, Huddinge University Hospital, Sweden; N.H. Russell, Nottingham City Hospital, United Kingdom; Franca Fagioli, Regina Margherita Children's Hospital In Turin, Italy; Victoria Potter, King’s College Hospital NHS Foundation Trust, United Kingdom; Alessandro Rambaldi, Ospedale Bergamo, France; Nathalie Fegueux, CHU Lapeyronie Montpellier, France; Charlotte Jubert, Groupe Hospitalier Pellegrin-Enfants, CHU Bordeaux, France; Giuseppe Bandini, Bologna University, S.Orsola-Malpighi Hospital, Italy; Thierry Lamy, Hopital Pontchaillou, CHU Rennes, France; Joan Hendrik Veelken, Leiden University Hospital, The Netherlands; Eefke Petersen, University Medical Centre, The Netherlands; Dolores Caballero, Hospital Gregorio Marañón, Spain; Rafael Duarte, Hospital Universitario Puerta de Hierro, Spain; Jose Antonio Pérez-Simó, Hospital Universitario Virgen del Rocío, Spain; John Snowden, Sheffield Teaching Hospitals NHS Trust (Adult) BMT Programme, United Kingdom; Jean Yves Cahn, Hopital A. Michallon, Grenoble, France; Alberto Bosi, Ospedale di Careggi, Italy; Cristina Diaz de Heredia, Vall d`Hebron, Pediatrico, Spain; Michael Potter, Royal Marsden Hospital, United Kingdom; Peter J. Shaw, The Children’s Hospital at Westmead, Australia; Tracey O`Brien, Sydney Children`s Hospital, Australia; Olga Aleinikova, Belorussian Centre for Paediatric Oncology / Hematology, Belarus; L.A. Noens, University Hospital Gent, Belgium; Xavier Poiré, Cliniques Universitaires St Luc Belgium; Philippe Lewalle, Institut Jules Bordet, Belgium; Henrique Bittencourt, St Justine, Canada; Jean-Hugues Dalle, Hôpital Robert Debre, France; Sebastien Maury, Hôpital Henri Mondor, France; Bénédicte Bruno, Hôpital Jeane de Flandre, France; Schneider Pascale, Hôpital Charles Nicolle, CHU Rouen, France; Bruno Lioure, Hôpital de Hautepierre, France; Mathilde Hunault-Berger, CHRU Angers, France; Jacques-Olivier Bay, Service D`Hématologie Clinique Adulte et Pédiatrie, France; Véronique Leblond, Hopital de La Pitie Salpêtrière, France; Gandhi Damaj, Centre Hospitalier Universitaire, France; Benedicte Neven, Hôpital Necker, France; Gérard Michel, Hôpital Timone-Enfants, France; Ernst Holler, University Regensburg, Germany; Arndt Borkhardt, Universitaetsklinikum Zentrum für Kinderheilkunde, Düsseldorf, Germany; Mareike Verbeek, Klinkum Rechts der Isar, Germany; Johanna Tischer, Klinikum Grosshadern, Germany; Arnold Ganser, Hannover Medical University, Germany; Arnold Ganser, Hannover Medical University, Germany; Jerry Stein, Schneider Children’s Medical Center of Israel, Israel; Arnon Nagler, Chaim Sheba Medical Center, Israel; Franco Locatelli, IRRCS Ospedale Pediatrico Bambino Gesù, Italy; Marco Zecca, IRCCS Policlinico San Matteo, Italy; Luigi Rigacci, Ospedale S. Camillo-Forlanini, Italy; Attilio Olivieri, Azienda Ospedali Riuniti di Ancona, Italy; Fulvio Porta, Universitá degli Studi di Brescia, Spedali Civili, Italy; Renato Fanin, University Hospital, Italy; Nicola Mordini, Az. Ospedaliera S. Croce e Carle, Italy; Simona Sica, Universita Cattolica S. Cuore, Italy; Giuseppe Basso, Clinica di Oncoematologia Pediatrica, Italy; Emanuele Angelucci, Ospedale San Martino, Italy; Abdelghani Tbakhi, King Hussein Cancer Centre, Jordan; Jolanta Gozdzik, University Children`s Hospital in Krakow, Poland; Alexei Maschan, Russian`s Children`s Hospital, Russia; Antonio Perez Martinez, Hospital Universitario La Paz, Hospital Infantil, Spain; Arancha Bermúdez Rodríguez, Hospital U. Marqués de Valdecilla, Spain; Fernandez Navarro, Hospital Infantil La Fe, Spain; Maria Jesús Pascual, Hospital Regional de Málaga, Spain; Manuel Jurado Chacón, Hospital Universitario Virgen a de las Nieves, Spain; Carlos Solano, Hospital Clínico Universitario, Spain; Montserrat Rovira, Hospital Clinic, Spain; Jan-Erik Johansson, Sahlgrenska University Hospital, Sweden; Urs Schanz, University Hospital, Switzerland; Ellen Meijer, VU University Medical Center, The Netherlands; R.F. Wynn, Department of Paediatric Haematology, United Kingdom; Charles Craddock, Queen Elizabeth Hospital, United Kingdom; Brenda E. Gibson, Royal Hospital for Sick Children, United Kingdom; Keith M.O. Wilson, University of Wales, College of Medicine, United Kingdom; Matthew Collin, Royal Victoria Infirmary, United Kingdom; and Maria Anjum Khan, Yorkshire Blood & Marrow Transplant Programme, United Kingdom.
Authorship
Contribution: E.G., H.R., A.R., and V.R. designed the study; H.R., C.K., and F.V. prepared and analyzed data, H.R., F.V., and E.G. wrote the manuscript; H.R. and A.R. performed the statistical analysis; J.S., R.P.D.L.T., A.E., G.M., P.C., M.-T.R., J.J.C., and M.M. provided cases for the study; A.E., C.K., F.V., M.M.R.-F., B.C., and G.M.S. helped with data and manuscript preparation; and all authors edited and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hanadi Rafii, Eurocord, Hopital Saint-Louis, 1 Avenue Claude Vellefaux, Porte 5, 75010 Paris, France; e-mail: hanadi.rafii-elayoubi@aphp.fr.
References
Author notes
Data are available on request from the corresponding author, Hanadi Rafii (hanadi.rafii-elayoubi@aphp.fr).