TO THE EDITOR:
B-cell maturation antigen (BCMA) is a novel target for T-cell immunotherapy in multiple myeloma (MM) including bispecific antibody (bsAb), antibody drug conjugates, and chimeric antigen receptor T-cell therapy (CAR-T).1-3 BCMA signaling is critical for survival and proliferation of long-lived plasma cells.4 Impaired immune reconstitution, cytopenia, B-cell aplasia, and hypogammaglobinemia (HGG) can compound preexisting MM-induced immunosuppression.5 In addition, bsAb can redirect and activate regulatory T cells, thus theoretically increasing the risk of infections.6 Here, we describe the infectious complications observed across different BCMA-directed T-cell therapies (bsAb and CAR-T) in relapsed/refractory MM clinical trials at our center. Infections confirmed by clinical, imaging, microbiologic, or histopathologic evidence were captured from day 1 of the first cycle of bsAb and day 1 of lymphodepletion chemotherapy in autologous BCMA CAR-T therapies until disease progression or last follow-up. National Cancer Institute Common Terminology Criteria for Adverse Events, version 5, was used to describe the site and grade of infections.7 The serum immunoglobulin G concentration was evaluated before inception of treatment and approximately monthly thereafter. Antimicrobial prophylaxis was in accordance with institutional standards for CAR-T recipients (supplemental Table 1) and at physician’s discretion for patients on bsAb therapy. Descriptive statistics and comparisons were performed using 2-sample t test for continuous variables and χ2 goodness-of-fit test for categorical variables.
We identified 62 patients who received BCMA-directed T-cell therapies including bsAb (n = 36) and CAR-T (n = 26) between January 2019 and June 2021 (Table 1). The median age was 66.5 (range, 63-72) years with 44% females (27/65) and 14.5% of African American. The median time to bsAb and CAR-T trial from diagnosis were 6.6 (range, 0.83-15.5) and 2.6 (range, 0.35-14.4) years, respectively. The median lines of prior therapy were 5 (range, 4-6), with BCMA CAR-T recipients receiving fewer prior lines of therapy compared with bsAb (4 vs 6, P < .001). All patients receiving bsAb were triple class refractory and had progressive disease entering the study. Among patients receiving CART-T, 76.9% (n = 20) had progressive disease and 46% (n = 12) bridging therapy before CAR-T. Approximately 27% (n = 16) of patients had lymphopenia at study inception. Baseline HGG and severe HGG were present in 76% and 44% patients, respectively. Tocilizumab was used in 39% (bsAb 28.6% vs CAR-T 55.6%; P = .38) patients for cytokine release syndrome and was similar between the patients with and without infectious complications. IVIG was used in 23.7% of patients.
Baseline characteristics of patients
| Clinical variables . | bsAb (N = 36) . | CAR-T (N = 26) . | Total (N = 62) . | P value . |
|---|---|---|---|---|
| Median age (range) | 66.5 (63.7-73.2) | 65.5 (63-70) | 66 (63-72) | .40 |
| Sex | .22 | |||
| Female | 18 (50.0%) | 9 (34.6%) | 27 (43.5%) | |
| Male | 18 (50.0%) | 17 (65.4%) | 35 (56.5%) | |
| Ethnicity | .20 | |||
| African American | 7 (19.4%) | 2 (7.7%) | 9 (14.5%) | |
| Caucasian | 29 (80.6%) | 23 (88.5%) | 52 (83.9%) | |
| Others | 0 (0.0%) | 1 (3.8%) | 1 (1.6%) | |
| Immunochemical subtype | ||||
| IgG | 24 (66.7%) | 14 (53.8%) | 38 (61.3%) | |
| IgA | 10 (27.8%) | 4 (15.4%) | 14 (22.6%) | |
| IgD | 0 | 1 (3.8%) | 1 (1.6%) | |
| κ light chain | 2 (5.6%) | 3 (11.5%) | 5 (8%) | |
| Λ light chain | 0 | 4 (15.4%) | 4 (8%) | |
| Median prior lines of therapy (range) | 6 (5-7) | 3.5 (2-6) | 5 (4-6) | <.001 |
| Triple class refractory | 36 (100.0%) | 14 (53.8%) | 50 (80.6%) | <.001 |
| ISS stage | .844 | |||
| I | 6 (28.6%) | 4 (23.5%) | 10 (26.3%) | |
| II | 7 (33.3%) | 5 (29.4%) | 12 (31.6%) | |
| III | 8 (38.1%) | 8 (47.1%) | 16 (42.1%) | |
| R-ISS stage | .371 | |||
| I | 3 (37.5%) | 4 (26.7%) | 7 (30.4%) | |
| II | 5 (62.5%) | 7 (46.7%) | 12 (52.2%) | |
| III | 0 (0.0%) | 4 (26.7%) | 4 (17.4%) | |
| CRS grade* | .52 | |||
| 1 | 12 (33.3%) | 6 (23.1%) | 18 (29.0%) | |
| 2 | 2 (5.6%) | 3 (11.5%) | 5 (8.1%) | |
| Median time to therapy from diagnosis, y | 6.6 (0.83-15.5) | 2.6 (0.35-14.4) | 5.2 (0.3-15.5) | .04 |
| Use of tocilizumab | 4 (28.6%) | 5 (55.6%) | 9 (39.1%) | .38 |
| Median follow in months (range), mo | 8.5 (3.5-10.5) | 8.9 (3.1-18.1) | 9 (3.1-18) | .81 |
| Median IgG levels (mg/L) at start of therapy (range) | 889 (333-1931) | 605 (259-1315) | 673 (281-1826) | .13 |
| Baseline hypogammaglobinemia (≤700 mg/dL)† | 20 (80.0%) | 13 (72.2%) | 33 (76.7%) | .551 |
| Baseline severe hypogammaglobinemia (≤400 mg/dL)† | 11 (44.0%) | 8 (44.4%) | 19 (44.2%) | .97 |
| Baseline lymphopenia (≤300/mL) | 8 (23.5%) | 8 (30.8%) | 16 (26.7%) | .53 |
| IVIG supplementation | 8 (22.9%) | 6 (25%) | 14 (23.7%) | .84 |
| Cumulative infections | 25 | 5 | 30 | .01 |
| Number of bacterial infections (n) | 12 | 1 | 13 | .11 |
| Number of viral infections (n) | 8 | 2 | 10 | .69 |
| Aggregated hospitalizations for infection (n) | 15 | 3 | 18 | .03 |
| Average length of stay per patient, mean (SD) | 4.833 (3.460) | 9.000 (5.196) | 5.667 (4.030) | .11 |
| Best response | .75 | |||
| PD | 7 (25.9%) | 6 (30.0%) | 13 (27.7%) | |
| ≥PR | 20 (74.1%) | 14 (70.0%) | 34 (72.3%) |
| Clinical variables . | bsAb (N = 36) . | CAR-T (N = 26) . | Total (N = 62) . | P value . |
|---|---|---|---|---|
| Median age (range) | 66.5 (63.7-73.2) | 65.5 (63-70) | 66 (63-72) | .40 |
| Sex | .22 | |||
| Female | 18 (50.0%) | 9 (34.6%) | 27 (43.5%) | |
| Male | 18 (50.0%) | 17 (65.4%) | 35 (56.5%) | |
| Ethnicity | .20 | |||
| African American | 7 (19.4%) | 2 (7.7%) | 9 (14.5%) | |
| Caucasian | 29 (80.6%) | 23 (88.5%) | 52 (83.9%) | |
| Others | 0 (0.0%) | 1 (3.8%) | 1 (1.6%) | |
| Immunochemical subtype | ||||
| IgG | 24 (66.7%) | 14 (53.8%) | 38 (61.3%) | |
| IgA | 10 (27.8%) | 4 (15.4%) | 14 (22.6%) | |
| IgD | 0 | 1 (3.8%) | 1 (1.6%) | |
| κ light chain | 2 (5.6%) | 3 (11.5%) | 5 (8%) | |
| Λ light chain | 0 | 4 (15.4%) | 4 (8%) | |
| Median prior lines of therapy (range) | 6 (5-7) | 3.5 (2-6) | 5 (4-6) | <.001 |
| Triple class refractory | 36 (100.0%) | 14 (53.8%) | 50 (80.6%) | <.001 |
| ISS stage | .844 | |||
| I | 6 (28.6%) | 4 (23.5%) | 10 (26.3%) | |
| II | 7 (33.3%) | 5 (29.4%) | 12 (31.6%) | |
| III | 8 (38.1%) | 8 (47.1%) | 16 (42.1%) | |
| R-ISS stage | .371 | |||
| I | 3 (37.5%) | 4 (26.7%) | 7 (30.4%) | |
| II | 5 (62.5%) | 7 (46.7%) | 12 (52.2%) | |
| III | 0 (0.0%) | 4 (26.7%) | 4 (17.4%) | |
| CRS grade* | .52 | |||
| 1 | 12 (33.3%) | 6 (23.1%) | 18 (29.0%) | |
| 2 | 2 (5.6%) | 3 (11.5%) | 5 (8.1%) | |
| Median time to therapy from diagnosis, y | 6.6 (0.83-15.5) | 2.6 (0.35-14.4) | 5.2 (0.3-15.5) | .04 |
| Use of tocilizumab | 4 (28.6%) | 5 (55.6%) | 9 (39.1%) | .38 |
| Median follow in months (range), mo | 8.5 (3.5-10.5) | 8.9 (3.1-18.1) | 9 (3.1-18) | .81 |
| Median IgG levels (mg/L) at start of therapy (range) | 889 (333-1931) | 605 (259-1315) | 673 (281-1826) | .13 |
| Baseline hypogammaglobinemia (≤700 mg/dL)† | 20 (80.0%) | 13 (72.2%) | 33 (76.7%) | .551 |
| Baseline severe hypogammaglobinemia (≤400 mg/dL)† | 11 (44.0%) | 8 (44.4%) | 19 (44.2%) | .97 |
| Baseline lymphopenia (≤300/mL) | 8 (23.5%) | 8 (30.8%) | 16 (26.7%) | .53 |
| IVIG supplementation | 8 (22.9%) | 6 (25%) | 14 (23.7%) | .84 |
| Cumulative infections | 25 | 5 | 30 | .01 |
| Number of bacterial infections (n) | 12 | 1 | 13 | .11 |
| Number of viral infections (n) | 8 | 2 | 10 | .69 |
| Aggregated hospitalizations for infection (n) | 15 | 3 | 18 | .03 |
| Average length of stay per patient, mean (SD) | 4.833 (3.460) | 9.000 (5.196) | 5.667 (4.030) | .11 |
| Best response | .75 | |||
| PD | 7 (25.9%) | 6 (30.0%) | 13 (27.7%) | |
| ≥PR | 20 (74.1%) | 14 (70.0%) | 34 (72.3%) |
p value of less than .05 is statistically significant. PD, progressive disease; PR, partial response.
*The grading and management of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) was done in accordance with specific trial protocol.
†Accounted for functional hypogammaglobinemia in IgG isotype.
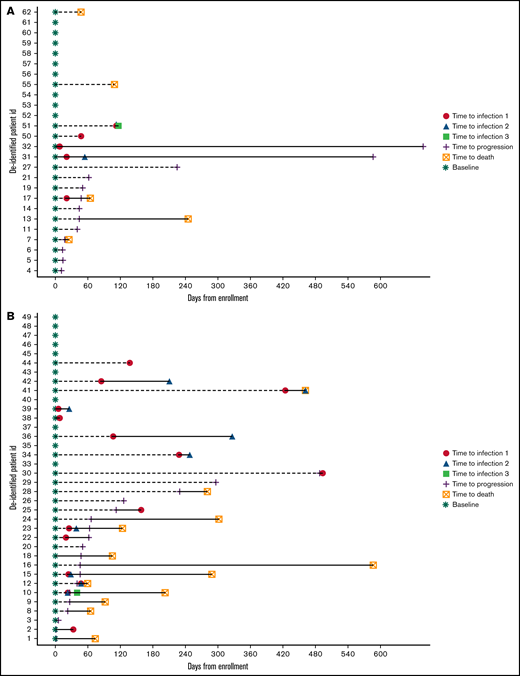
At a median follow-up of 9 (range, 3.1-18) months, cumulative incidence of infections in this cohort was 30, with 32% (n = 20) of all patients experiencing at least 1 episode of infection. The cumulative incidence of infection with bsAb and CAR-T was 25 and 5 (P = .012), respectively, with 41.2% of patients experiencing at least 1 episode of infection with bsAb and 23.1% with CAR-T (P = .141). Average infection density was higher with bsAb- compared with CAR-T-treated patients (23 vs 6 per 100 patients, P = .012) (supplemental Figure 1). The spectrum of infections was predominantly bacterial (n = 13). Although gram-negative infections (Escherichia coli and Klebsiella pneumoniae bacteremia and Proteus mirabilis and Pseudomonas aeruginosa urinary tract infections) were seen in 6 patients, skin infection including cellulitis occurred in 4 patients, including 1 case of necrotizing fasciitis requiring amputation of the involved limb in the recipient of bsAb therapy. We also observed bacteremia with rare opportunistic pathogens such Rhizobium radiobacter and Ochrobactrum anthropi. Ten cases of viral infections were seen in this cohort including rhinovirus (upper respiratory infection), cytomegalovirus reactivation with viremia, norovirus (diarrhea), parvovirus B19 reactivation, and SARS-CoV-2 (COVID-19). About 50% (n = 15) of infections were grade ≥3 infections with 2 grade 5 events (≥grade 3 events with bsAb = 12 vs CAR-T = 4). The 2 cases of grade 5 infections were severe COVID-19 (n = 1) and Pseudomonas spp sepsis (n = 1), with both patients having achieved MRD− complete response (10−5) on bsAb therapy. Although most infections occurred within the first 30 days of lymphodepletion chemotherapy in the CAR-T group, these occurred at a median 49 (range, 24-148.5) days from study enrollment with bsAb therapy (Figure 1). Most infections also occurred in the context of better than a very good partial response8 to therapy. The average length of inpatient hospital stay for infectious complications in this cohort was 5.6 (bsAb 4.8 vs CAR-T 9; P = .11) days.
Distribution of infections with (A) BCMA CAR T (1a) and (B) bsAb therapy.
This report informs us of the shifting spectrum of infections with this novel class of drugs in MM. In this cohort of 62 patients with MM treated with BCMA-targeting bsAb and CAR-T, one-third developed at least 1 infectious complication. Grade ≥3 infections were seen in 50% of cases, including 2 grade 5 events. Thus, we observed a high rate of infections and severity of infection (clinical significance and inpatient hospitalizations) in patients treated with these agents. Additionally, patients treated on BCMA CAR-T trials enrolled less heavily pretreated patients in earlier relapses (ie, with a shorter time from diagnosis to CAR-T), higher baseline lymphocyte count, and earlier in the natural history of the disease compared with patients on bsAb. Although this suggests a different risk for infections in the 2 groups, it is also plausible that bsAb is associated with a higher rate of infections than CAR-T because of continuous therapy, resulting in more ongoing profound B-cell aplasia and hypogammaglobinemia.
Morbidity and mortality from infectious complications are common in advanced and refractory MM where treatment options are also limited. In a phase 2 study of Selinexor, which is approved in the setting of pent-refractory MM, infections such as pneumonia (11%), sepsis (9%), and bacteremia (3.3%) were common serious adverse effects.9 In the Phase II, Open Label, Randomized, Two-Arm Study to Investigate the Efficacy and Safety of Two Doses of the Antibody Drug Conjugate GSK2857916 in Participants With Multiple Myeloma Who Had 3 or More Prior Lines of Treatment, Are Refractory to a Proteasome Inhibitor and an Immunomodulatory Agent and Have Failed an Anti-CD38 Antibody study of belantamab mafodotin, the first in anti-BCMA class antibody drug conjugate, ≥grade 3 pneumonia was reported in 6% of the study population.10 Of note, dose reductions were noted in 29% to 41% of study subjects resulting from toxicities and thus the full extent of infection risk is less clear. An inferior overall survival primarily due to patients succumbing to infections was also seen with venetoclax therapy.11 Salvage autologous cell transplantation, another acceptable treatment option in this population, is also associated with significant infectious complications.12,13 The only currently US Food and Drug Administration-approved BCMA CAR-T, idecabtagene vicleucel was associated with infections in 69% of study patients (relapsed/refractory MM), of which 22% were grade 3 or 4.2 Similar rates of infections have been observed with ciltacabtagene autoleucel.14 Infection rates range between 21% and 52% across various trials of BCMA-directed bsAb, with serious infections ranging from 8% to 30%.15-17
In addition to the limitation of examining a small cohort with a relatively short follow-up, the data presented here are limited to the phase 1/2 clinical trials of different products, some with dose escalation. Our findings highlight the need for the myeloma provider community to be aware of this risk and the need for guidelines on screening for infections before treatment, active surveillance for viral reactivation, antimicrobial and IVIG prophylaxis, and treatment of infections with support from infectious disease experts in this setting. The existing equivocal approach to IVIG replacement in HGG induced by MM therapy should also be revisited considering these newer therapies. This information also has major implications in the context of responses to COVID-19 vaccination.18 As the era of T-cell-directed immunotherapy dawns in MM care, we underscore the need for comprehensive infection management protocols, particularly in patients with deep durable remission on ongoing therapy.
Acknowledgments: The authors thank the patients and families for the opportunity to be involved in their care and their willingness to contribute to the advancement of the field.
Contribution: P.H., A.D., S.C., and M.M. conceived and designed the study; M.M. provided study materials and patients; M.M. collection and assembly of data; P.H., A.D., M.M., and S.N. provided data analysis and interpretation; M.M., P.H., and A.D. wrote the manuscript; and all authors provided final approval of manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Meera Mohan, Medical College of Wisconsin, 9200 W Wisconsin Ave, Milwaukee, WI 53226; e-mail: memohan@mcw.edu.
References
Author notes
Requests for data sharing may be submitted to Meera Mohan (memohan@mcw.edu).
The full-text version of this article contains a data supplement.