Key Points
Antiplatelet medication was not associated with an increased risk of ICH in patients with metastatic brain tumors.
Combined antiplatelet agents and anticoagulation was not associated with an increased risk of ICH compared with single-agent use.
Abstract
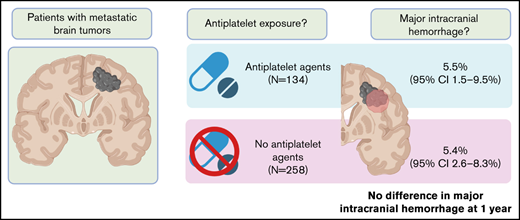
Although intracranial hemorrhage (ICH) is frequent in the setting of brain metastases, there are limited data on the influence of antiplatelet agents on the development of brain tumor–associated ICH. To evaluate whether the administration of antiplatelet agents increases the risk of ICH, we performed a matched cohort analysis of patients with metastatic brain tumors with blinded radiology review. The study population included 392 patients with metastatic brain tumors (134 received antiplatelet agents and 258 acted as controls). Non–small cell lung cancer was the most common malignancy in the cohort (74.0%), followed by small cell lung cancer (9.9%), melanoma (4.6%), and renal cell cancer (4.3%). Among those who received an antiplatelet agent, 86.6% received aspirin alone and 23.1% received therapeutic anticoagulation during the study period. The cumulative incidence of any ICH at 1 year was 19.3% (95% CI, 14.1-24.4) in patients not receiving antiplatelet agents compared with 22.5% (95% CI, 15.2-29.8; P = .22, Gray test) in those receiving antiplatelet agents. The cumulative incidence of major ICH was 5.4% (95% CI, 2.6-8.3) among controls compared with 5.5% (95% CI, 1.5-9.5; P = .80) in those exposed to antiplatelet agents. The combination of anticoagulation plus antiplatelet agents did not increase the risk of major ICH. The use of antiplatelet agents was not associated with an increase in the incidence, size, or severity of ICH in the setting of brain metastases.
Introduction
Brain metastases develop in 10% to 30% of all patients with cancer, and with the improved efficacy of systemic therapies and longer survival in advanced malignancy, the incidence of intracranial metastases is rising.1,2 Intracranial hemorrhage (ICH) is a frequent complication of metastatic brain disease.3 ICH carries high morbidity and is responsible for more than half of all cerebrovascular events in patients with cancer.4 ICH in the setting of brain tumors significantly increases health care costs and portends a poor prognosis, with a 30-day mortality in excess of 30%.5,6 In a nationwide study from 2016, there were 145 225 hospitalizations associated with brain metastases, of which 4145 had a concurrent diagnosis of ICH.6
In patients with metastatic brain tumors, systemic anticoagulation has not been associated with an increased risk of ICH.7-9 Agents that alter platelet activity, including aspirin and P2Y12 inhibitors, are commonly prescribed in the population with metastatic brain tumors because of the concomitant cardiac, peripheral vascular, and neurovascular disease.10-14 These agents have been associated with an increased risk of gastrointestinal bleeding,15,16 whereas data on the risk of ICH in the general population are mixed.17,18 Whether antiplatelet agents increase the risk of ICH in the setting of metastatic brain tumors is not known.
Methods
Study design
This study was a retrospective matched-cohort study conducted at Beth Israel Deaconess Medical Center (BIDMC). The study protocol was approved by the Institutional Review Board. Patients were identified through the BIDMC tumor registry between 2010 and 2020 based on the identification of metastatic brain disease at the time of primary tumor diagnosis. Patients with primary brain neoplasms were excluded. Only patients with ≥2 intracranial radiologic studies in the electronic medical record were included. Date of death or date of last known contact for patients who were discharged on hospice were recorded. Clinical variables, including medications received, comorbidities, and demographics, were extracted from the electronic medical record using an electronic query system and manual chart review. Details of cancer-directed treatment (systemic therapy, whole brain radiation, stereotactic brain radiation, and neurosurgery) were documented up to 6 months from cancer diagnosis (with concurrent brain metastasis). Exposure to anticoagulation agents included any documentation within 30 days prior to diagnosis and up to death, ICH, or censoring (last imaging study recorded).
Patients qualified for inclusion in the intervention group based on documented antiplatelet agent administration after diagnosis of metastatic brain tumor (eg, listed on a reconciled medication list or a discharge medication list). Patients with exposure to antiplatelet agents within 30 days prior to their metastatic brain tumor diagnosis, and any time afterward, were included in the intervention group. Antiplatelet agents included aspirin and P2Y12 inhibitors (clopidogrel, prasugrel, or ticagrelor). Each patient from the antiplatelet cohort was matched with a control not taking an antiplatelet agent using a “round-robin” scoring algorithm that ranked controls according to cancer type, exposure to anticoagulation, age, sex, and year of diagnosis.7,9 Each patient with an antiplatelet agent was matched with 1 control and, if available, a second.
The primary end point of the study was the cumulative incidence of ICH after tumor diagnosis. Potential bleeding events were identified on a manual chart review of all central nervous system (CNS) imaging reports, with radiologic images of potential bleeding events assessed by a neuro-oncologist blinded to cohort. ICH was classified as trace (<1 cm3), measurable (1-9.9 cm3), or major (symptomatic or ≥10 cm3).7,9 Bleed size was determined using the 1/2 ABC method for all types of bleeds, including subdural hematomas.19,20 If blood and tumor were mixed, tumor was included in the volume. Microbleeds were labeled as trace ICH, whereas hemosiderin residues were not. ICH was excluded if it was within 4 weeks of neurosurgery or if it was attributable to prior surgical intervention or stereotactic radiosurgery.
Statistical analysis
For the primary end point of the study, the cumulative incidence of bleeding was estimated by identifying death in the absence of bleeding as a competing risk.21 Statistical differences between treatment groups were assessed using the Gray test.21,22 The Fine-Gray method was used to construct time-to-event models and report the associated subdistribution function hazard ratios for covariables.21 A Wilcoxon rank-sum test was used to compare the distribution of volume of ICH between patients in treatment groups. Final date of analysis was death or censoring at date of last image.
A Kaplan-Meier analysis was used to estimate overall survival, with the log-rank test used for comparison of overall survival between the treatment and control groups. Overall survival was determined by the time from diagnosis of metastatic brain disease at the time of primary tumor diagnosis to the date of death, as defined above. Statistical significance was defined as P < .05 in all analyses.
Results
Demographics
The study population included 392 patients with metastatic brain tumors identified at the time of primary tumor diagnosis; 134 patients were exposed to antiplatelet agents, and 258 patients were not. Median age of patients at the time of diagnosis was 66.1 years, and 53.1% were female. As shown in Table 1, the most common primary malignancies represented were lung cancer (NSCLC; 74.0%), small cell lung cancer (9.9%), melanoma (4.6%), and renal cell carcinoma (4.3%). Antiplatelet agent exposure in the intervention group was aspirin alone for 116 patients (86.6%), clopidogrel alone for 4 patients (3.0%), and aspirin and clopidogrel for 12 patients (9.0%). One patient (0.7%) was exposed to aspirin and ticagrelor, whereas another patient (0.7%) was exposed to aspirin, clopidogrel, and prasugrel. In the study cohort, the median time between cancer diagnosis and initiation of antiplatelet agent was 20 days.
Patient demographics and characteristics
| Characteristic . | Antiplatelet, n = 134 . | Control, n = 258 . |
|---|---|---|
| Females | 68 (50.7) | 140 (54.3) |
| Age, median (interquartile range), y | 68.3 (62.1-74.8) | 65.9 (59.3-73.0) |
| Primary malignancy | ||
| NSCLC | 98 (73.1) | 192 (74.4) |
| Small cell lung cancer | 13 (9.7) | 26 (10.1) |
| Renal cell carcinoma | 7 (5.2) | 10 (3.9) |
| Melanoma | 6 (4.5) | 12 (4.7) |
| Gastrointestinal | 3 (2.2) | 6 (2.3) |
| Breast | 2 (1.5) | 4 (1.6) |
| Genitourinary | 2 (1.5) | 3 (1.2) |
| Miscellaneous | 3 (2.2) | 5 (1.9) |
| Comorbidities | ||
| Hypertension | 98 (73.1) | 135 (52.3) |
| Chronic kidney disease | 43 (32.1) | 72 (27.9) |
| Coronary artery disease/myocardial infarction | 20 (14.9) | 12 (4.7) |
| Hypercholesterolemia | 34 (25.4) | 39 (15.1) |
| Other arterial or peripheral vascular disease | 8 (6.0) | 8 (3.1) |
| Cerebrovascular disease | 6 (4.5) | 2 (0.8) |
| Atrial fibrillation or flutter | 6 (4.5) | 7 (2.7) |
| Venous thromboembolism | 5 (3.7) | 10 (3.9) |
| Prior ICH | 1 (0.7) | 2 (0.8) |
| Treatment of brain metastasis | ||
| Systemic therapy | 94 (70.1) | 167 (64.7) |
| Stereotactic brain radiation therapy | 70 (52.2) | 120 (46.5) |
| Whole brain radiation | 68 (50.7) | 111 (43.0) |
| Neurosurgery | 39 (29.1) | 90 (34.9) |
| Anticoagulation exposure | 31 (23.1) | 57 (22.1) |
| Enoxaparin | 27 (20.1) | 53 (20.5) |
| Apixaban | 3 (2.2) | 5 (1.9) |
| Rivaroxaban | 3 (2.2) | 1 (0.4) |
| Characteristic . | Antiplatelet, n = 134 . | Control, n = 258 . |
|---|---|---|
| Females | 68 (50.7) | 140 (54.3) |
| Age, median (interquartile range), y | 68.3 (62.1-74.8) | 65.9 (59.3-73.0) |
| Primary malignancy | ||
| NSCLC | 98 (73.1) | 192 (74.4) |
| Small cell lung cancer | 13 (9.7) | 26 (10.1) |
| Renal cell carcinoma | 7 (5.2) | 10 (3.9) |
| Melanoma | 6 (4.5) | 12 (4.7) |
| Gastrointestinal | 3 (2.2) | 6 (2.3) |
| Breast | 2 (1.5) | 4 (1.6) |
| Genitourinary | 2 (1.5) | 3 (1.2) |
| Miscellaneous | 3 (2.2) | 5 (1.9) |
| Comorbidities | ||
| Hypertension | 98 (73.1) | 135 (52.3) |
| Chronic kidney disease | 43 (32.1) | 72 (27.9) |
| Coronary artery disease/myocardial infarction | 20 (14.9) | 12 (4.7) |
| Hypercholesterolemia | 34 (25.4) | 39 (15.1) |
| Other arterial or peripheral vascular disease | 8 (6.0) | 8 (3.1) |
| Cerebrovascular disease | 6 (4.5) | 2 (0.8) |
| Atrial fibrillation or flutter | 6 (4.5) | 7 (2.7) |
| Venous thromboembolism | 5 (3.7) | 10 (3.9) |
| Prior ICH | 1 (0.7) | 2 (0.8) |
| Treatment of brain metastasis | ||
| Systemic therapy | 94 (70.1) | 167 (64.7) |
| Stereotactic brain radiation therapy | 70 (52.2) | 120 (46.5) |
| Whole brain radiation | 68 (50.7) | 111 (43.0) |
| Neurosurgery | 39 (29.1) | 90 (34.9) |
| Anticoagulation exposure | 31 (23.1) | 57 (22.1) |
| Enoxaparin | 27 (20.1) | 53 (20.5) |
| Apixaban | 3 (2.2) | 5 (1.9) |
| Rivaroxaban | 3 (2.2) | 1 (0.4) |
Unless otherwise noted, data are n (%).
A total of 88 patients (22.4%) in both cohorts received therapeutic anticoagulation within 30 days prior to or at any time after initial tumor diagnosis. Among all patients receiving anticoagulation, 91% (80) received enoxaparin, 9% (8) received apixaban, and 5% (4) received rivaroxaban. Four patients received multiple types of anticoagulation. The median number of CNS imaging studies was 6 for patients exposed to antiplatelet agents and 5 for the control group (P = .2).
Antiplatelet agents and development of ICH
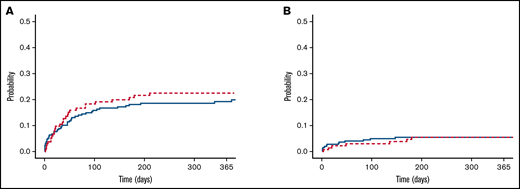
Among the cohort of patients not receiving antiplatelet agents, the cumulative incidence of any ICH at 1 year was 19.3% (95% confidence interval [CI], 14.1-24.4) compared with 22.5% (95% CI, 15.2-29.8) in those receiving antiplatelet agents (P = .22, Gray test; Figure 1A). Similarly, the cumulative incidence of major ICH at 1 year was 5.4% (95% CI, 2.6-8.3) among controls compared with 5.5% (95% CI, 1.5-9.5) in those receiving antiplatelet agents (P = .80, Gray test; Figure 1B). Among the subgroup taking aspirin, the cumulative incidence of major ICH at 1 year was 5.4% (95% CI, 1.2-9.6). All patients with major ICHs in the antiplatelet cohort had documented antiplatelet therapy within 30 days of the bleeding event.
Cumulative incidence of ICH in patients with metastatic brain tumors. No differences in the cumulative incidence of total (A) and major (B) ICHs were observed between the antiplatelet (red line) and control (blue line) cohorts (P > .5, Gray test).
Cumulative incidence of ICH in patients with metastatic brain tumors. No differences in the cumulative incidence of total (A) and major (B) ICHs were observed between the antiplatelet (red line) and control (blue line) cohorts (P > .5, Gray test).
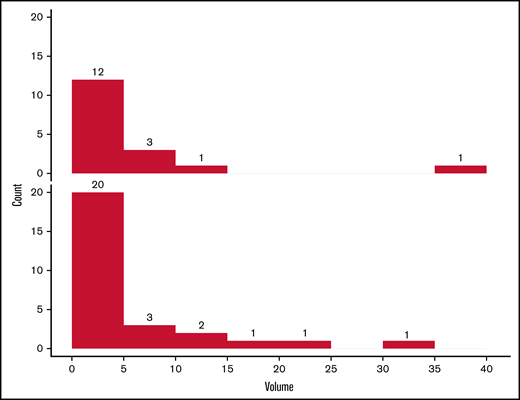
Among all patients with measurable or major ICHs, there was no statistically significant difference in their volumes between those exposed and not exposed to antiplatelet agents agents (P = .49; Figure 2). A summary of the characteristics of ICH events is included in Table 2.
Distribution of ICH size in patients with and without antiplatelet agent exposure. Number of ICHs in the cohort receiving antiplatelet agents (upper panel) and controls (lower panel), according to volume (cm3). The volume distribution was similar in the 2 groups (P = .49, Wilcoxon rank-sum test).
Distribution of ICH size in patients with and without antiplatelet agent exposure. Number of ICHs in the cohort receiving antiplatelet agents (upper panel) and controls (lower panel), according to volume (cm3). The volume distribution was similar in the 2 groups (P = .49, Wilcoxon rank-sum test).
Characteristics of ICH events
| . | All bleeds, n = 137 . | ICH with antiplatelet exposure, n = 54 . | ICH without antiplatelet exposure, n = 83 . |
|---|---|---|---|
| ICH category | |||
| Major | 47 (34.3) | 15 (27.7) | 32(38.5) |
| Measurable | 31 (22.6) | 11 (20.3) | 20 (24.0) |
| Trace | 59 (43.0) | 28 (51.8) | 31 (37.3) |
| Location of bleed | |||
| Intraparenchymal hemorrhage | 129 (94.1) | 51 (94.4) | 78 (93.9) |
| Subdural hemorrhage | 7 (5.1) | 2 (3.7) | 5 (6.0) |
| Intraventricular hemorrhage | 1 (0.7) | 1 (1.8) | 0 |
| Presence of symptoms | 29 (21.2) | 12 (22.2) | 17 (20.5) |
| ICH volume,*mean ± SD, cm3 | 8.6 ± 12.1 | 6.1 ± 9.1 | 9.9 ± 13.2 |
| Anticoagulation exposure | 31 (22.6) | 10 (18.5) | 21 (25.3) |
| . | All bleeds, n = 137 . | ICH with antiplatelet exposure, n = 54 . | ICH without antiplatelet exposure, n = 83 . |
|---|---|---|---|
| ICH category | |||
| Major | 47 (34.3) | 15 (27.7) | 32(38.5) |
| Measurable | 31 (22.6) | 11 (20.3) | 20 (24.0) |
| Trace | 59 (43.0) | 28 (51.8) | 31 (37.3) |
| Location of bleed | |||
| Intraparenchymal hemorrhage | 129 (94.1) | 51 (94.4) | 78 (93.9) |
| Subdural hemorrhage | 7 (5.1) | 2 (3.7) | 5 (6.0) |
| Intraventricular hemorrhage | 1 (0.7) | 1 (1.8) | 0 |
| Presence of symptoms | 29 (21.2) | 12 (22.2) | 17 (20.5) |
| ICH volume,*mean ± SD, cm3 | 8.6 ± 12.1 | 6.1 ± 9.1 | 9.9 ± 13.2 |
| Anticoagulation exposure | 31 (22.6) | 10 (18.5) | 21 (25.3) |
Unless otherwise noted, data are n (%).
Calculated for major and measurable bleeds only.
Combination of antiplatelet agents with anticoagulation
A total of 23.1% of the intervention cohort received anticoagulation along with antiplatelet agents (Table 1). Consistent with prior studies,7,23 the administration of anticoagulation did not increase the risk of major hemorrhage compared with the no antiplatelet/no anticoagulation group (hazard ratio [HR], 0.51; 95% CI, 0.11-2.25; P = .37). Similarly, the combined use of antiplatelet agents with anticoagulation did not impact the risk of major ICH compared with the use of antiplatelet agents alone (HR, 0.40; 95% CI, 0.05-3.25; P = .39) or no anticoagulation/no antiplatelet therapy (HR, 0.47; 95% CI, 0.06-3.65; P = .47).
Subgroup analysis of NSCLC
For patients with NSCLC, which represented the largest subset in the study, the cumulative incidence of any ICH at 1 year was 18.6% (95% CI, 12.7-24.5) in patients not receiving antiplatelet agents compared with 17.0% (95% CI, 9.3-24.6) in those receiving antiplatelet agents (P = .95, Gray test). The combined use of aspirin and anticoagulation did not increase the risk of ICH compared with no antiplatelet/no anticoagulation (HR, 1.06; 95% CI, 0.41-2.74; P = .91) or antiplatelet agent alone (HR, 0.90; 95% CI, 0.34-2.42; P = .84).
Overall survival
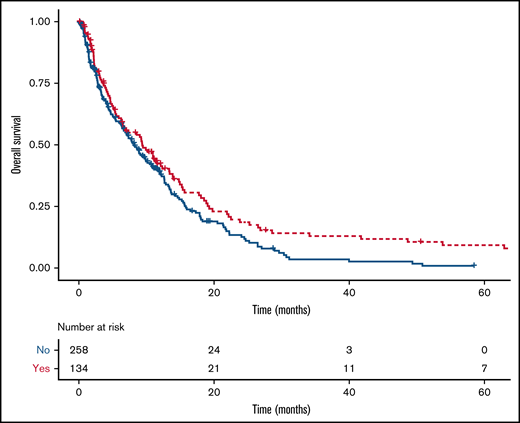
There was a statistically significant difference in the median survival of patients exposed (9.4 months; 95% CI, 6.5-13.4) or not exposed (8.2 months; 95% CI, 6.6-10.1; P = .03) to antiplatelet agents (Figure 3). Among patients with NSCLC as their primary cancer, median survival for those exposed to antiplatelet agents was 9.3 months (95% CI, 6.3-13.4) vs 8.3 months (95% CI, 6.3-11.5) in those without exposure (P = .07). The diagnosis of major ICH was not associated with a shorter overall survival (HR, 0.71; 95% CI, 0.48-1.03).
Kaplan-Meier curves for overall survival of intervention and control groups. The median survival time for patients exposed (red line) or not (blue line) to antiplatelet agents was 9.4 months (95% CI, 6.5-13.4) and 8.2 months (95% CI, 6.6-10.1; P = .03, log-rank test), respectively.
Kaplan-Meier curves for overall survival of intervention and control groups. The median survival time for patients exposed (red line) or not (blue line) to antiplatelet agents was 9.4 months (95% CI, 6.5-13.4) and 8.2 months (95% CI, 6.6-10.1; P = .03, log-rank test), respectively.
Discussion
The incidence of any ICH among patients with brain tumors is high but does not seem to be influenced by the administration of anticoagulants.4,7-9,24,25 Similar data on the safety of aspirin in patients with brain metastases are lacking. In this matched cohort study of nearly 400 patients with blinded radiology review of ICH, the administration of antiplatelet therapy was not associated with an increase in the incidence, size, or severity of ICH in the setting of brain metastases.
A small but definitive risk for ICH has been identified with the use of antiplatelet agents for the prevention and treatment of ischemic disease.17 A meta-analysis of >300 000 patients over 8 years estimated an ICH risk of 0.2 per 1000 patient-years.26 Aspirin use has been associated with larger ICH volume and worse outcomes.27,28 There is a paucity of data on the effect that antiplatelet agents have on bleeding in patients with cancer, specifically intracranial malignancy. In a series of patients with cancer and ICH over 7 years, 10% of the 208 patients received antiplatelet agents at the time of the bleed.5 In the current study, we did not detect a significant increase in the risk of ICH among patients with brain tumors who received aspirin, although this study was underpowered to detect the very small absolute differences described in cohorts of patients without cancer. Based on the point estimates of major ICH observed in the current cohort study, a study on a population scale would be required to conclude that there are not small differences in risk; however, such an analysis would be constrained by ICH assessment limitations, such as the inability to perform a central blinded radiologic review.
Although the administration of anticoagulants alone, such as low molecular weight heparin or direct oral anticoagulants, does not seem to increase the risk of ICH in patients with brain metastasis,7,8,24,25 we explored whether the combination of anticoagulant and antiplatelet agents significantly increased the risk of ICH. The coadministration of aspirin with anticoagulants is known to significantly increase the risk of major hemorrhage in cohorts without cancer.29-31 In a nationwide Norwegian registry study, the combination of oral antithrombotic and antiplatelet agents was associated with a significantly increased risk for ICH compared with antiplatelet agents or anticoagulants alone in the general population.17 The combination of aspirin with an anticoagulant significantly increased the risk of severe hemorrhage (including 9 ICHs) in a large prospective cohort of patients with myeloproliferative neoplasms.32 In the current study, the combined use of antiplatelet agents and anticoagulation did not seem to increase the risk of major ICH in intracranial metastasis compared with antiplatelet therapy alone. Although these data are the first to provide reassurance that dual antithrombotic therapy can be coadministered, considering the modest sample size of the combined-use cohort, a larger study would be needed to definitively conclude that aspirin, in combination with an anticoagulant, does not impact the risk of major ICH in this patient population.
Somewhat unexpectedly, we observed a survival advantage in the cohort of patients receiving antiplatelet agents. Platelets are implicated in enabling metastasis and shielding cancer cells from immune surveillance.33 Inhibition of prostanoid synthesis prevents metastatic progression of neoplasms in murine models.34-36 Aspirin has been linked to an improved survival in cancer.37-39 Specifically, in brain tumors, there are epidemiological data to suggest that regular aspirin use is associated with decreased glioma risk.40 Recognizing the limitations of retrospect analyses, there may be unaccounted confounders at play, such as selection bias of a population considered to have a prognosis sufficient to warrant cardiovascular risk modification. Conversely, cardiovascular risk factors typically are associated with shortened survival. Based on the provocative nature of these findings, we hope that future studies explore the observed survival signal in different brain tumor cohorts.
To the best of our knowledge, this is the first study specifically addressing the impact of antiplatelet drug use and ICH in patients with metastatic brain tumors. The cohorts were matched according to type of cancer, exposure to anticoagulation, year of diagnosis, age at diagnosis, and sex, but residual confounders are possible. Because assignment of the treatment groups relied on medication list accuracy in the electronic health record, only records from reconciled medication lists or discharge medication lists after a patient’s diagnosis with a metastatic intracranial malignancy during the study period were included. Because aspirin is an over-the-counter medication, it is possible that some patients in the non-aspirin cohort were taking this medication who not captured in the medical record. Similarly, we were unable to accurately extract stopping dates for the antiplatelet agents or precise dosing of aspirin, which limited our ability to perform more granular analyses. An additional limitation was the exclusion of patients with <2 intracranial images, because this could have excluded patients with poorer prognosis. Furthermore, this study only included patients with metastatic intracranial malignancy and does not provide insight into the safety of antiplatelet agents in patients with primary brain tumors. Finally, 74% of the patients in the study had NSCLC, potentially limiting the generalizability to patients with other primary cancers, especially those that are more prone to ICH, such as melanoma or renal cell carcinoma.
The findings of this study support the use of antiplatelet agents in patients with brain metastases when clinically indicated. The apparent safety associated with the combined use of antiplatelet and anticoagulant therapy is reassuring but warrants confirmatory investigations.
Acknowledgments
Assistance with data collection from the electronic medical record was provided by the BIDMC InSIGHT Core.
This work was supported by a grant from the American Society of Hematology as part of the Hematology Opportunities for the Next Generation of Research Scientists (HONORS) award (E.J.M.). Additional support was provided by the National Institutes of Health Heart, Lung, and Blood Institute CLOT Consortium (U01HL143365) (J.I.Z.) and a Dana Farber Harvard Cancer Center Core Grant (5P30 CA006516) (S.R. and D.N.).
The visual abstract was created using BioRender.
Authorship
Contribution: E.J.M., R.P., J.I.Z., and D.N. designed the study; E.J.M., R.P., H.S., P.E., G.M.W., and E.J.U. collected data; D.N. and S.R. analyzed data; E.J.M., R.P., and J.I.Z. wrote the manuscript; and all authors contributed to and reviewed the final version of the manuscript.
Conflict-of-interest disclosure: J.I.Z. has received research funding from Incyte and Quercegen; has acted as a consultant for Sanofi, CSL, and Parexel; and has received honoraria from and served as a member of the advisory board for Pfizer/Bristol Myers Squibb, Alexion, and Daiichi. The remaining authors declare no competing financial interests.
Correspondence: Jeffrey I. Zwicker, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215; e-mail: jzwicker@bidmc.harvard.edu.
References
Author notes
E.J.M. and R.P. contributed equally to this study.
Requests for data sharing may be submitted to Jeffrey I. Zwicker (jzwicker@bidmc.harvard.edu).