Key Points
Compared with IVIG, eltrombopag was noninferior and less costly for the perioperative management of adult patients with ITP.
Direct cost of the eltrombopag or IVIG was the main driver of overall cost for the perioperative management of ITP.
Abstract
Eltrombopag has been shown to be noninferior to intravenous immunoglobulin (IVIG) for improving perioperative platelet counts in patients with immune thrombocytopenia (ITP) in a randomized trial; thus, cost is an important factor for treatment and policy decisions. We used patient-level data from the trial to conduct a cost-effectiveness analysis comparing perioperative eltrombopag 50 mg daily starting dose, with IVIG 1 or 2 g/kg (according to local practice) from a Canadian public health care payer’s perspective over the observation period, from preoperative day 21 to postoperative day 28. Resource utilization data were obtained from the trial data (eltrombopag, n = 38; IVIG, n = 36), and unit costs were collected from the Ontario Schedule of Benefits, Ontario Drug Formulary, and secondary sources. All costs were adjusted to 2020 Canadian dollars. We calculated the incremental cost per patient for all patients randomized. Uncertainty was addressed using nonparametric bootstrapping. The use of perioperative eltrombopag for patients with ITP resulted in a cost-saving of $413 Canadian per patient. Compared with IVIG, the probability of eltrombopag being cost effective was 70% even with no willingness to pay. In a sensitivity analysis based on IVIG dose, we found that with the higher dose of IVIG (2 g/kg), eltrombopag saved $2,714 per patient, whereas with the lower dose of IVIG (1 g/kg), eltrombopag had a higher mean cost of $562 per patient. In summary, based on data from the randomized trial that demonstrated noninferiority, the use of eltrombopag for the management of ITP in the perioperative setting was less costly than IVIG.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune hematological disorder characterized by increased platelet destruction and impaired platelet production. Due to the resulting thrombocytopenia, individuals with ITP are at an increased risk of spontaneous bleeding events that range from minor bruising and petechiae to intracranial hemorrhage.1-4 In addition, the thrombocytopenia poses particular concerns for ITP patients who require surgery due to the risk of bleeding with invasive procedures. Previous research has found that adults with ITP who undergo surgery have a 75% higher risk of receiving a blood transfusion and a 90% higher risk of death at 30 days postoperatively after adjusting for preoperative risk factors compared with adults without ITP who undergo similar surgical procedures.5-7
Optimal treatment of patients with ITP around the time of surgery is controversial and may vary based on the patient's age, comorbidities, medications, type and urgency of the surgical procedure, and patient and provider preferences.8 In this setting, patients are commonly prescribed intravenous immunoglobulin (IVIG) infusion(s) in preparation for surgery; however, IVIG is resource-intensive, costly, and has been associated with side-effects including headache and allergic reactions. Furthermore, efforts to conserve IVIG use are needed, particularly during imminent shortages. Thrombopoietin receptor agonists (TPO-RAs) represent a class of engineered platelet growth factors that simulate the action of endogenous TPO on megakaryocytes and megakaryocytes precursors, boosting their growth and differentiation and increasing platelet production. Eltrombopag is an orally active, small-molecule nonpeptide TPO-RA that increases platelet production within 7 days of repeated dosing. It is approved for the treatment of thrombocytopenia in adults and children with chronic ITP.9
In a multicenter, parallel-arm, open-label, noninferiority trial (Bridging ITP trial; clinicaltrials.gov #NCT01621204), adult patients with primary or secondary ITP were randomized to receive oral daily eltrombopag from 21 days preoperatively to 7 days postoperatively or IVIG administered 7 days preoperatively and repeated within 7 days postoperatively if needed. In the trial, eltrombopag was noninferior to IVIG for improving perioperative platelet count levels.10 The aim of the current study was to assess the cost-effectiveness of eltrombopag compared with IVIG for achieving platelet count targets in adults with ITP undergoing elective surgical procedures.
Methods
Bridging ITP trial
The Bridging ITP trial was a multicenter, randomized, parallel-arm, open-label, noninferiority trial (clinicaltrials.gov #NCT01621204) that compared eltrombopag and IVIG for the primary outcome of treatment success, defined as the achievement of platelet count targets of 45 ×109/L or higher for minor surgery or 90 ×109/L or higher for major surgery from 1 day preoperatively until 7 days postoperatively without the use of rescue treatment. Rescue treatment was defined as any additional treatment administered during the perioperative period to increase the platelet count or prevent bleeding, such as prednisone, dexamethasone, methylprednisolone, IVIG, or platelet transfusions.11
Adult patients with primary or secondary ITP with platelet count <100 ×109/L before major surgery or <50 ×109/L before minor surgery were recruited across 8 academic hospitals in Canada. Patients were excluded if they had abnormal liver enzymes, thrombosis within the previous 12 months, known bone marrow reticulin or fibrosis, or active malignancy. Using a centralized, secure web-based system, patients were randomly assigned in a 1:1 ratio to receive oral eltrombopag 50 mg daily from 21 days preoperatively to 7 days postoperatively or IVIG 1 to 2 g/kg administered 7 days preoperatively (and repeated within 7 days postoperatively in case a significant drop in platelet count or excess bleeding risk was anticipated during the treatment period). Eltrombopag dose adjustments were done weekly based on platelet count levels. Patients were followed at weekly intervals from preoperative day 21 to postoperative day 28. Ethics approval for the trial was obtained from research ethics boards at each participating center. Written informed consent was obtained from all participants.
Cost-effectiveness analysis
We did a cost-effectiveness analysis using data from the Bridging ITP trial. We conducted the intention-to-treat analysis comparing direct cost and effect of eltrombopag vs IVIG from the Canadian public health care payer’s perspective over a time horizon of the 49-day observation period of the study (ie, preoperative day 21 to postoperative day 28). Direct cost is defined as the expense directly related to the delivery of health care services and excludes operational costs (eg, capital cost, maintenance, administrative salaries) and costs incurred by the patients and caregivers (eg, productivity loss, parking fees).12
The treatment success rate was used as the effect measure in the cost-effectiveness analysis. Health care resource utilization was collected prospectively using the study case report forms, which were completed weekly throughout the study period. All relevant resources used were considered including laboratory and diagnostic investigations, surgical procedure, procedure-related health care professionals, hospital stays, treatments of adverse events, medications (eltrombopag and others), blood product transfusion, and IVIG. The unit costs of surgery were obtained from the 2020 Schedule of Benefits for Physician Services from the Ontario (the province with the highest population in Canada) Ministry of Health and Long-Term Care,13 and the cost of medications was obtained from the Ontario Drug Benefit Formulary.14 The cost of medications that were not included in the Ontario Drug Benefit Formulary was obtained from the hospital or community pharmacy. The costs associated with blood-product transfusions and IVIG (including preparation and dispensing fees) were obtained from Canadian Blood Services.15 Where applicable, unit costs were adjusted to 2020 Canadian dollars using the inflation rate between the price base year and 202016 (supplemental Appendix 1). Costs were calculated by multiplying the natural unit by the corresponding unit cost for each health care use item. Ontario-based unit costs for all resource categories were applied across all patients and centers.
Base case and sensitivity analyses
The incremental cost-effectiveness ratio was computed by dividing the difference in the mean cost per patient between groups by the difference in the success rates, or incremental effect between groups. In the base case analyses, costs of medications that were not related to ITP were not included. In a sensitivity analysis, all medication costs were included. Further sensitivity analyses were conducted by excluding cost associated with surgery and adverse events and using a range of eltrombopag costs that have been reported in the literature.17-22 In the IVIG group, sensitivity analyses were done for patients who received 1 g/kg IVIG vs 2 g/kg IVIG. We also varied the cost per gram of IVIG by adding and subtracting $10 and $20 from the cost used in the base case analysis ($50.84 per gram of IVIG) because published costs for IVIG vary from $45 to $72 per gram.23-26 Lastly, we used a more conservative cost estimate of $51.95 per physician visit (ie, cost for repeat medical consult) instead of $85.80 (full medical consult) per visit used in base case analysis. The nonparametric bootstrapping method was used to analyze the uncertainty of the estimated incremental cost-effectiveness ratio based on 1000 bootstrap samples. A cost-effectiveness acceptability curve was used to show the probability of eltrombopag being cost-effective across a range of willingness-to-pay values per additional successfully treated patient compared with IVIG.
Role of the funding source
The main Bridging ITP trial was funded by Novartis and GlaxoSmithKline, and this economic analysis was funded by Novartis. Program funding for the McMaster Center for Transfusion Research was provided by Canadian Blood Services, the Federal Government of Canada (Health Canada), and provincial and territorial ministries of health. The funders had no role in the design of the study, the collection of data, or the interpretation of the results.
Results
In the clinical trial, 74 patients were randomized to receive either eltrombopag (n = 38, 51.4%; median age = 64 years, interquartile range [IQR]= 23) or IVIG (n = 36, 48.6%; median age = 63.5 years, IQR = 19) preoperatively (Table 1). Patients receiving concomitant ITP therapies were eligible for inclusion provided they had not started a new therapy or had any changes to an existing therapy for 2 weeks prior to randomization. Patients were excluded if they had received IVIG within 2 weeks or eltrombopag (or another medication in that drug class) within 4 weeks of randomization. One patient in each group had received eltrombopag prior to study participation, whereas 22 (58%) and 24 (67%) patients had previously received IVIG in the eltrombopag and IVIG groups, respectively. Of the 36 patients in the IVIG group, none received a second treatment of IVIG within 7 days postoperatively. By intention to treat, 30 (79%) patients in the eltrombopag group and 22 (61%) patients in the IVIG group achieved treatment success because they reached the perioperative platelet count targets. The clinical trial concluded that eltrombopag was noninferior to IVIG for perioperative treatment of ITP. The clinical findings of the Bridging ITP trial have been published elsewhere.10
Characteristics of the patients in the Bridging ITP trial
| . | Eltrombopag (n = 38) . | Intravenous immunoglobulin (n = 36) . |
|---|---|---|
| Recruitment site | ||
| Ontario | 32 (84.2%) | 31 (86.1%) |
| Alberta | 2 (5.3%) | 0 |
| Quebec | 4 (10.5%) | 5 (13.9%) |
| Females | 20 (53%) | 18 (50%) |
| Age, median (IQR) | 64 (50-73.3) | 63.5 (56-74.5) |
| Weight in kgs, median (IQR) | 84 (69.8-102.6) | 81.7 (68-93.6) |
| Immune thrombocytopenia | ||
| Secondary | 4 (11%) | 5 (14%) |
| Chronic | 29 (76%) | 29 (81%) |
| Duration in years | 8 (1.2-13.7) | 5.6 (1.8-15.1) |
| Surgery classification | ||
| Major | 17 (45%) | 14 (39%) |
| Minor | 21 (55%) | 22 (61%) |
| . | Eltrombopag (n = 38) . | Intravenous immunoglobulin (n = 36) . |
|---|---|---|
| Recruitment site | ||
| Ontario | 32 (84.2%) | 31 (86.1%) |
| Alberta | 2 (5.3%) | 0 |
| Quebec | 4 (10.5%) | 5 (13.9%) |
| Females | 20 (53%) | 18 (50%) |
| Age, median (IQR) | 64 (50-73.3) | 63.5 (56-74.5) |
| Weight in kgs, median (IQR) | 84 (69.8-102.6) | 81.7 (68-93.6) |
| Immune thrombocytopenia | ||
| Secondary | 4 (11%) | 5 (14%) |
| Chronic | 29 (76%) | 29 (81%) |
| Duration in years | 8 (1.2-13.7) | 5.6 (1.8-15.1) |
| Surgery classification | ||
| Major | 17 (45%) | 14 (39%) |
| Minor | 21 (55%) | 22 (61%) |
IQR, interquartile range.
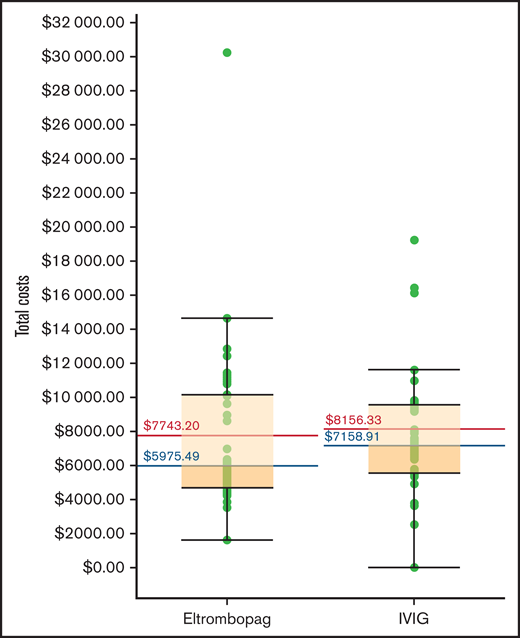
When all medication costs, including non-ITP medications, were included, eltrombopag was $1,101 Canadian less expensive per patient than IVIG (Table 2). The direct cost of eltrombopag (ie, cost of eltrombopag and dispensing fee) was $166 199, and the direct cost associated with IVIG (ie, cost of nursing time, infusion material, physician visit, immunoglobulin, preparation and dispensing fee, and pre-IVIG infusion medications) was $188 660 (supplemental Appendix 2). These were the largest cost drivers, accounting for 56% and 64% of the total costs in the 2 treatment groups, respectively. The eltrombopag group also incurred $30 474 for rescue IVIG infusions. The costs of blood product transfusions (non-IVIG) were $1,031 in the eltrombopag group and $6259 for the IVIG group; the higher costs in the IVIG group were associated with more red blood cell units (n = 4) and platelet transfusions (n = 7) administered to patients in the IVIG group compared with the eltrombopag group (red blood cell units, n = 1; platelet transfusions, n = 1). Surgery-related costs (ie, costs associated with the surgeon, anesthetist, surgical assistant, operating room nurse, and surgery-related hospitalization) were the second largest driver of overall costs, amounting to $86 147 (29.3% of total cost) in the eltrombopag group and $77 613 (26.1% of total cost) in the IVIG group. In the eltrombopag group, 1 patient had 1 emergency department visit and a 4-day hospitalization for suspected pulmonary embolism that was possibly related to the eltrombopag, and another patient had 1 emergency department visit and a 2-day hospitalization for vertigo that was unrelated to eltrombopag, amounting to a total of $5531 in adverse event-related costs. In the IVIG group, 3 patients had adverse events requiring a total of 1 emergency department visit and 17 hospitalization days due to surgery-related vulvar pain, air leak from chest tube, and pancreatitis, with a total cost of $14 553. All unplanned emergency department visits and hospital admissions in the IVIG group were unrelated to the IVIG. Overall, the mean plus or minus standard deviation total cost for treating 38 patients in the eltrombopag group was $294 242, or $7743 plus or minus $4897 per patient, and the total cost for treating 36 patients in the IVIG group was $293 628, or $8156 plus or minus $4255 per patient (Figure 1). In the base case, eltrombopag was less expensive than IVIG, saving $413 per patient.
Cost-effectiveness results for the base case and sensitivity analyses comparing eltrombopag and IVIG
| . | Total cost Mean ± SD . | Treatment success . | Incremental cost (CAD) . | Incremental effect* . |
|---|---|---|---|---|
| Base case | ||||
| Eltrombopag | 7743 ± 4897 | 30 (78.9%) | ||
| IVIG | 8156 ± 4355 | 22 (61.1%) | −413 | 0.18 |
| Sensitivity analyses | ||||
| All medication cost included (ITP and non-ITP) | ||||
| Eltrombopag | 8424 ± 5202 | 30 (78.9%) | ||
| IVIG | 9525 ± 6082 | 22 (61.1%) | −1101 | 0.18 |
| Surgery-related costs excluded† | ||||
| Eltrombopag | 5476 ± 3592 | 30 (78.9%) | ||
| IVIG | 6000 ± 3253 | 22 (61.1%) | −524 | 0.18 |
| Unplanned ER visits and hospitalization costs excluded | ||||
| Eltrombopag | 7597 ± 4804 | 30 (78.9%) | ||
| IVIG | 7752 ± 3898 | 22 (61.1%) | −155 | 0.18 |
| Lowest published cost for eltrombopag (CAD 1.87) | ||||
| Eltrombopag | 6518 ± 4481 | 30 (78.9%) | ||
| IVIG | 8156 ± 4255 | 22 (61.1%) | −1638 | 0.18 |
| Highest published cost for eltrombopag (CAD 7.03) | ||||
| Eltrombopag | 15 178 ± 8154 | 30 (78.9%) | ||
| IVIG | 8156 ± 4255 | 22 (61.1%) | 7022 | 0.18 |
| IVIG 1 g/kg only | ||||
| Eltrombopag | 7743 ± 4897 | 30 (78.9%) | ||
| IVIG: 1 g/kg (n = 24) | 7181 ± 4306 | 14 (58.3%) | 562 | 0.21 |
| IVIG 2 g/k only | ||||
| Eltrombopag | 7743 ± 4897 | 30 (78.9%) | ||
| IVIG: 2 g/kg (n=12) | 10 457 ± 4897 | 8 (66.6%) | −2714 | 0.12 |
| IVIG per-gram cost varied by −$10 (CAD 40.84) | ||||
| Eltrombopag | 7743 ± 4897 | 30 (78.9%) | ||
| IVIG | 7171 ± 3895 | 22 (61.1%) | 572 | 0.18 |
| IVIG per-gram cost varied by +$10 (CAD 60.84) | ||||
| Eltrombopag | 7743 ± 4897 | 30 (78.9%) | ||
| IVIG | 9142 ± 4624 | 22 (61.1%) | −1399 | 0.18 |
| IVIG per-gram cost varied by −$20 (CAD 30.84) | ||||
| Eltrombopag | 7743 ± 4897 | 30 (78.9%) | ||
| IVIG | 6185 ± 3570 | 22 (61.1%) | 1558 | 0.18 |
| IVIG per-gram cost varied by +20 (CAD 70.84) | ||||
| Eltrombopag | 7743 ± 4897 | 30 (78.9%) | ||
| IVIG | 10 128 ± 5017 | 22 (61.1%) | −2385 | 0.18 |
| Physician visit cost of $51.95 | ||||
| Eltrombopag | 7743 ± 4897 | 30 (78.9%) | ||
| IVIG | 7854 ± 3860 | 22 (61.1%) | −111 | 0.18 |
| . | Total cost Mean ± SD . | Treatment success . | Incremental cost (CAD) . | Incremental effect* . |
|---|---|---|---|---|
| Base case | ||||
| Eltrombopag | 7743 ± 4897 | 30 (78.9%) | ||
| IVIG | 8156 ± 4355 | 22 (61.1%) | −413 | 0.18 |
| Sensitivity analyses | ||||
| All medication cost included (ITP and non-ITP) | ||||
| Eltrombopag | 8424 ± 5202 | 30 (78.9%) | ||
| IVIG | 9525 ± 6082 | 22 (61.1%) | −1101 | 0.18 |
| Surgery-related costs excluded† | ||||
| Eltrombopag | 5476 ± 3592 | 30 (78.9%) | ||
| IVIG | 6000 ± 3253 | 22 (61.1%) | −524 | 0.18 |
| Unplanned ER visits and hospitalization costs excluded | ||||
| Eltrombopag | 7597 ± 4804 | 30 (78.9%) | ||
| IVIG | 7752 ± 3898 | 22 (61.1%) | −155 | 0.18 |
| Lowest published cost for eltrombopag (CAD 1.87) | ||||
| Eltrombopag | 6518 ± 4481 | 30 (78.9%) | ||
| IVIG | 8156 ± 4255 | 22 (61.1%) | −1638 | 0.18 |
| Highest published cost for eltrombopag (CAD 7.03) | ||||
| Eltrombopag | 15 178 ± 8154 | 30 (78.9%) | ||
| IVIG | 8156 ± 4255 | 22 (61.1%) | 7022 | 0.18 |
| IVIG 1 g/kg only | ||||
| Eltrombopag | 7743 ± 4897 | 30 (78.9%) | ||
| IVIG: 1 g/kg (n = 24) | 7181 ± 4306 | 14 (58.3%) | 562 | 0.21 |
| IVIG 2 g/k only | ||||
| Eltrombopag | 7743 ± 4897 | 30 (78.9%) | ||
| IVIG: 2 g/kg (n=12) | 10 457 ± 4897 | 8 (66.6%) | −2714 | 0.12 |
| IVIG per-gram cost varied by −$10 (CAD 40.84) | ||||
| Eltrombopag | 7743 ± 4897 | 30 (78.9%) | ||
| IVIG | 7171 ± 3895 | 22 (61.1%) | 572 | 0.18 |
| IVIG per-gram cost varied by +$10 (CAD 60.84) | ||||
| Eltrombopag | 7743 ± 4897 | 30 (78.9%) | ||
| IVIG | 9142 ± 4624 | 22 (61.1%) | −1399 | 0.18 |
| IVIG per-gram cost varied by −$20 (CAD 30.84) | ||||
| Eltrombopag | 7743 ± 4897 | 30 (78.9%) | ||
| IVIG | 6185 ± 3570 | 22 (61.1%) | 1558 | 0.18 |
| IVIG per-gram cost varied by +20 (CAD 70.84) | ||||
| Eltrombopag | 7743 ± 4897 | 30 (78.9%) | ||
| IVIG | 10 128 ± 5017 | 22 (61.1%) | −2385 | 0.18 |
| Physician visit cost of $51.95 | ||||
| Eltrombopag | 7743 ± 4897 | 30 (78.9%) | ||
| IVIG | 7854 ± 3860 | 22 (61.1%) | −111 | 0.18 |
All numbers are rounded to the nearest whole number.
CAD, Canadian dollar; ITP, immune thrombocytopenia; IVIG, intravenous immunoglobulin; SD, standard deviation.
Incremental effect is the difference in the rate of achieving platelet count without rescue therapy in eltrombopag and IVIG.
Includes costs associated with transfusion of blood product during surgery and during postoperative hospitalization.
Total cost associated with perioperative eltrombopag and intravenous immunoglobulin (IVIG) for patients with ITP.
Total cost associated with perioperative eltrombopag and intravenous immunoglobulin (IVIG) for patients with ITP.
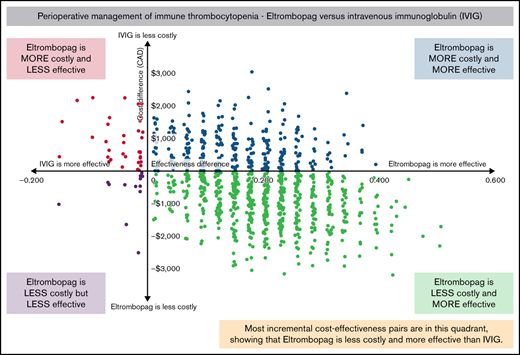
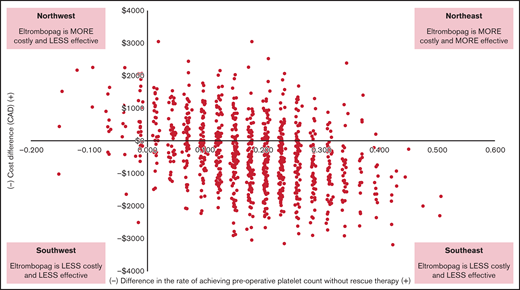
In the eltrombopag group, the success rate was 78.9% (30/38), and in the IVIG group, the success rate was 61.1% (22/36). The cost-effectiveness plane showing the results of 1000 bootstrap cost-effect pairs demonstrates that the majority of the cost-effect pairs appeared in the “southeast quadrant” of the plane, indicating that eltrombopag was both more effective and less costly (Figure 2). Eltrombopag had a 70% probability of being cost-effective if the willingness-to-pay was $0 per additional successfully treated patient and an 88.9% probability if the willingness-to-pay increased to $10 000 compared with IVIG (Figure 3).
Incremental cost-effectiveness plane comparing eltrombopag with intravenous immunoglobulin.
Incremental cost-effectiveness plane comparing eltrombopag with intravenous immunoglobulin.
Cost-effectiveness acceptability curve showing the probability that eltrombopag is cost-effective compared with intravenous immunoglobulin over a range of willingness-to-pay values per rescue treatment averted.
Cost-effectiveness acceptability curve showing the probability that eltrombopag is cost-effective compared with intravenous immunoglobulin over a range of willingness-to-pay values per rescue treatment averted.
Sensitivity analyses
When surgery costs were excluded, eltrombopag was $524 less expensive per patient than IVIG (Table 2). When the lowest published cost of eltrombopag ($1.87 per milligram) was used, eltrombopag was $1638 less expensive per patient; however, when the highest published cost of eltrombopag was used ($7.03 per milligram), the cost in the eltrombopag group was $7022 higher per patient than IVIG. Patients on the trial received IVIG 1 g/kg (n = 24) or IVIG 2 g/kg (n = 12). We found that when 1 g/kg of IVIG was used, 58% of patients in the IVIG group achieved preoperative platelet count targets, and eltrombopag had a higher mean cost of $562 per patient, whereas when 2 g/kg of IVIG was used, 66% patients in the IVIG group achieved preoperative platelet count targets and eltrombopag saved $2714 per patient. When the IVIG per-gram cost were reduced by $10 and $20 from the cost used in base case analysis ($50.84), IVIG group had cost savings of $572 and $1558, respectively. However, when the costs were increased by $10 and $20, the eltrombopag was found to save $1399 and $2385, respectively. When a conservative estimate of the cost of physician visit for IVIG ($51.95) was used, eltrombopag remained superior in terms of cost-effectiveness and was associated with cost saving of $111.
Discussion
We evaluated the cost-effectiveness of eltrombopag and IVIG in the perioperative setting from a Canadian public health care payer perspective. Compared with IVIG, eltrombopag was both noninferior and less costly at achieving perioperative platelet count targets during the time horizon of the study. The cost of the treatment itself and postoperative hospitalizations were associated with the highest cost burden in our analysis, followed by the cost of investigating or managing adverse events (ie, emergency department visits and unplanned hospital admissions). A higher proportion of patients in the IVIG group received blood product transfusion (including red blood cells, platelets, and plasma) or had hospitalizations that were unrelated to the treatments compared with the eltrombopag group. When the cost of unrelated hospitalization and emergency department visits were excluded, eltrombopag was still associated with lower cost. In a sensitivity analysis of IVIG dosing, eltrombopag was $562 more costly per patient when 1 g/kg IVIG was used, whereas eltrombopag was −$2714 less costly when 2 g/kg of IVIG was used.
The majority of the randomly drawn incremental cost-effect pairs fell in the “southeast quadrant” of the cost-effectiveness plane, where eltrombopag was both more effective and less costly than IVIG. This indicates low uncertainty in the cost-effectiveness profile of eltrombopag in comparison with IVIG as only 2% of the pairs fell into the “northwest quadrant,” where IVIG was more effective and less costly, which was likely caused by a few resource-intensive cases and the small sample size. The high resource utilization in few patients was primarily due to the type of surgical procedure, days spent in the hospital, or concomitant medications, none of which was directly related to the intervention. The wide confidence intervals on the costs reflect skewed cost data distribution and the small sample size; nevertheless, the differences in the mean per-patient cost and the total cost between groups can inform policy for determining overall budget impact associated with alternative treatments.
This study used patient-level data to estimate the cost-effectiveness of eltrombopag and IVIG from randomized controlled trial data in the perioperative setting. Previous studies have examined the cost of eltrombopag either alone or in comparison with the watch-and-rescue approach,17 other TPO-RAs drugs,17-21 and rituximab19-22 for chronic ITP or patients with hepatitis C virus infection. A substantial variation is noted in the lifetime costs for eltrombopag for chronic ITP patients based on modeling studies ranging from $440 000 US to $1.5 million US,17,18,20,21 including costs for managing bleeding or other adverse events. Five17-20,22 of the 6 studies17-22 concluded that eltrombopag was cost-effective compared with romiplostim, another TPO-RA medication, over 6 months and lifetime due to lower cost of acquiring and administering the drug and lower bleeding episode rates.
Eltrombopag was the dominant intervention in our study (ie, both clinically noninferior and cost saving) even after the costs of surgery were excluded from the analysis. With regard to the cost of eltrombopag itself, the cost in this study was $2.6 per mg, which is within the range of values reported in the literature and by the Canadian Agency for Drugs and Technologies in Health.27 Based on the published literature, the costs for eltrombopag were lowest in Europe and United Kingdom (approximately $1.87 to $2.27 per mg)20-22 and highest in the United States ($5.12 to $7.03 per mg).18 When the highest reported eltrombopag cost ($7.03) was used, eltrombopag was more costly than IVIG. If the cost of the eltrombopag was adjusted to approximately $2.83 per gram (from $2.60 per gram), the cost would be very similar between the 2 treatments (mean cost eltrombopag, $8129, and mean cost IVIG, $8,156). An important but expected trend was that as the cost of IVIG increased, the cost savings associated with eltrombopag also increased. Recent data suggests that immunoglobulin costs have increased exponentially in the past decade, making it the third highest drug category for private payers in the United States and fourth under Medicare.28 With the rising costs of immunoglobulin, it is imperative that cost-effective alternatives are carefully considered for those who require it.
Finally, we found that the dose of IVIG impacted the cost-analysis: 1 g/kg IVIG was associated with cost-savings with IVIG, whereas 2 g/kg IVIG was associated with cost-savings with eltrombopag. When results from randomized trials and this cost-effectiveness are triangulated, it suggests that clinicians who prescribe 1 g/kg IVIG routinely may continue to favor IVIG without any health care resource or patient outcome-related implications. Clinicians who prescribe 2 g/kg IVIG could consider 1 g/kg IVIG or eltrombopag. However, due to the small sample size, this finding is hypothesis-generating and should be explored in future studies.
The use of clinical data from a randomized, controlled trial and comprehensive data on cost are strengths of this study. The results are timely as many health care providers struggle with IVIG shortages for the treatment of ITP, and, at the same time, limited access to TPO receptor agonist medications. The comprehensive reporting of unit costs and resources used in this study will be useful to compare groups across different settings and countries. The small sample size (n = 74) limits the certainty around the cost-effectiveness estimates; thus, although the cost associated with the use of (non-IVIG) blood products was higher in the IVIG group, the small number of outcome events limited inferences about the effects of the intervention on blood product transfusions. This study did not include patient-reported outcomes such as quality of life, fatigue, or functional assessments, which may have significant impact on patients with ITP.29-33 In addition, other factors besides cost contribute to treatment decisions, including confidence in the diagnosis of ITP and previous response to therapies, which were not directly captured in this study. Another limitation was the omission of overhead and indirect costs incurred by patients or their caregivers. We reasoned that including these costs would have made an even stronger case for eltrombopag because patients who receive IVIG spend more time in the outpatient hospital or medical day unit, which results in added costs to the health care system and hours lost from work. Most patients on the trial (∼85%) were from Ontario, Canada; therefore, Ontario-based costs were applied to all patients. Results of this study should be interpreted in the context of a high resource setting in a publicly insured health system.
Cost-effectiveness analyses are especially important for interventions that are clinically noninferior, as is the case for eltrombopag and IVIG for the perioperative management of ITP. Our study demonstrates that eltrombopag was less costly than IVIG from a public health care payer’s perspective in Canada. These data could help inform policies on drug accessibility.
Acknowledgments
M.N.K. is supported by the Canadian Institutes of Health Research Fellowship Award (2020-23). The views herein do not necessarily reflect the views of Canadian Blood services or the federal, provincial, or territorial governments of Canada.
Funding support for this article was provided by the GlaxoSmithKline and Novartis. Programme funding for the McMaster Center for Transfusion Research was provided by Canadian Blood Services, the Federal Government of Canada (Health Canada), and provincial and territorial ministries of health.
Authorship
Contribution: M.N.K., D.M.A., and F.X. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; D.M.A., F.X., M.N.K., E.J., N.L., Y. Liu, and J.C. are responsible for the concept and design of the study, acquiring and interpreting the data; D.M.A., C.H., M.B., M.S., Y. Lin, J.K., L.L., N.M.H., R.J.C., and A.T. acquired and/or analyzed primary source data from the Bridging ITP trial for this economic analysis; M.N.K. and F.X. are responsible for drafting the manuscript and performed statistical analysis; D.M.A. and F.X. supervised the work; and all authors critically revised the manuscript for important intellectual content.
Conflict-of-interest disclosure: D.M.A. reports grants from GlaxoSmithKline and Novartis during the conduct of the study; grants and personal fees from Novartis and Amgen outside the submitted work; grants from Bristol Myers Squibb outside the submitted work; and personal fees from UCB, Principia, and Rigel outside the submitted work. C.H. reports personal fees from Novartis, Amgen, and GlaxoSmithKline outside the submitted work. E.J. reports personal fees from Novartis outside the submitted work. M.S. reports personal fees from Novartis outside the submitted work. Y. Lin and Y. Liu reports grants from Novartis during the conduction of the study and consulting and advisory board fees from Pfizer outside the submitted work. L.L. reports personal fees from Novartis outside the submitted work. The remaining authors declare no competing interests.
Correspondence: Feng Xie, Health Research Methods, Evidence, and Impact, Faculty of Health Sciences, McMaster University, Hamilton, ON, Canada; e-mail fengxie@mcmaster.ca; and Donald M. Arnold, Medicine, Hematology & Thromboembolism, Health Sciences Center, McMaster University, 3V-50, 1280 Main St West, Hamilton, ON, Canada; e-mail: arnold@mcmaster.ca.
References
Author notes
Deidentified individual patient-level clinical data that underlie the reported results are available from arnold@mcmaster.ca, and economic data are available from fengxie@mcmaster.ca.
The full-text version of this article contains a data supplement.