Key Points
ABCB6 is expressed on the cell surface and by multiple organelles, but transport specificity is incompletely understood.
In all types of porphyria, ABCB6 polymorphisms are not overrepresented when compared with the overall population.
Abstract
The Mendelian inheritance pattern of acute intermittent porphyria, hereditary coproporphyria, and variegate porphyria is autosomal dominant, but the clinical phenotype is heterogeneous. Within the general population, penetrance is low, but among first-degree relatives of a symptomatic proband, penetrance is higher. These observations suggest that genetic factors, in addition to mutation of the specific enzyme of the biosynthetic pathway of heme, contribute to the clinical phenotype. Recent studies by others suggested that the genotype of the transporter protein ABCB6 contribute to the porphyria phenotype. Identifying the molecule(s) that are transported by ABCB6 has been problematic and has led to uncertainty with respect to how or if variants/mutants contribute to phenotypic heterogeneity. Knockout mouse models of Abcb6 have not provided a direction for investigation as homozygous knockout animals do not have a discrete phenotype. To address the proposed link between ABC6 genotype and porphyria phenotype, a large cohort of patients with acute hepatic porphyria and erythropoietic protoporphyria was analyzed. Our studies showed that ABCB6 genotype did not correlate with disease severity. Therefore, genotyping of ABCB6 in patients with acute hepatic porphyria and erythropoietic protoporphyria is not warranted.
Introduction
The following disease entities comprise the acute hepatic porphyrias (AHPs); acute intermittent porphyria (AIP); hereditary coproporphyria (HCP); variegate porphyria (VP). These are rare diseases of heme biosynthesis that are inherited in an autosomal dominant fashion with low penetrance.1 For example, AIP, the most common of the AHPs, arises as a consequence of mutations affecting the enzyme hydroxymethylbilane synthase (HMBS), which is required to convert porphobilinogen to hydroxymethylbilane (the third of eight steps in the biosynthetic pathway of heme). In an international study of genomic/exomic databases, Chen and colleagues2 identified 12 pathogenic sequence variants among ∼92 000 alleles, a frequency of 0.00056. The estimated prevalence of symptomatic patients with heterozygous mutant HMBS is ∼0.000005.3 Based on these data, the estimated penetrance of pathogenic HMBS mutants is ∼1%.4 However, among first-degree relatives of affected patients, the penetrance is ∼20%.3 Together, these data suggest that genetic factors, in addition to mutant HMBS, determine the clinical phenotype of AIP. The basis of the low penetrance observed in AHPs is an area of active investigation, as discovery of genes that modify the disease phenotype would provide both relevant clinical information and a more a detailed understanding of heme metabolism.
Steps in the heme biosynthetic pathway take place in both the mitochondria and the cytosol. Therefore, genes that encode proteins that transport heme metabolites between cellular components are logical targets to investigate for sequence variants that contribute to the disease heterogeneity characteristic of porphyria. Krishnamurthy and colleagues reported that ATP-binding cassette B6 (ABCB6) is a mammalian mitochondrial porphyrin transporter.5 In unrelated studies, ABCB6 was shown to specify the Langereis (Lan) blood group system.6 Fukuda et al hypothesized that sequence variants affecting ABCB6 modulate the clinical phenotype of porphyria by altering the transport of toxic metabolic intermediates.7 Those investigators reported data suggesting that sequence variants in ABCB6 were causally related to disease severity both in patients with AHP and in patients with the cutaneous porphyria, erythropoietic protoporphyria (EPP).7 The cohort of patients examined by Fukuda et al, however, was small (total of 36 patients). To investigate further the relationship between ABCB6 and the severity of the AHPs and EPP, we studied the ABCB6 gene sequence in 562 patients (1124 alleles). Our findings demonstrate that with two exceptions, ABCB6 variants are present at the same frequency in patients with EPP and each type of AHP as in the general population, and when EPP and AHPs are analyzed together, there is no correlation between ABCB6 genotype and porphyria phenotype.
Materials
A retrospective study was performed on DNA samples obtained from patients with AIP, HCP, VP, EPP, and X-linked protoporphyria (XLP). The samples were collected under studies approved by the local Institutional Review Boards of the 6 academic medical centers of the Porphyrias Consortium or by the French Porphyria Group. All research was performed in accordance with the World Medical Association Declaration of Helsinki ethical principles for medical research involving human subjects and its subsequent amendments (R162-16-7 and 145-15-4 French ethical agreement). All family members provided written informed consent to participate in genetics studies. The European Union Institutional Review Board at INSERM approved the study protocols.
Methods
Genomic DNA (gDNA) was isolated from peripheral blood mononuclear cells using an automated magnetic bead-based system (PerkinElmer Chemagic 360). Briefly, whole blood was transferred to 50-mL conical tubes containing 50 µL of protease and 900 µL of magnetic beads that bind DNA. Lysis buffer, binding buffer, and wash buffers were sequentially dispensed by the machine during the extraction run. Samples of DNA were removed from beads with 1 mL of elution buffer (10 mM Tris HCL, pH = 8.0), and the sample was incubated at 65°C for 1 hour. DNA was quantified using a Nanodrop One spectrophotometer (Thermo Fisher Scientific, Carlsbad, CA), and the concentration was adjusted to 200 ng/µL. Based on agarose gel electrophoresis, samples used for sequencing consisted of high molecular weight DNA.
Sequencing of the French cohort for ABCB6 was performed using primers shown in supplemental Table 1. All patient sequences were compared with the NCBI Reference Sequence, NG_032110.1, based on the ABCB6 sequence that was initially reported by Helias and colleagues.6 Allele typing of the American cohort was performed using an ABCB6 single nucleotide polymorphism (SNP) detection protocol developed by the Molecular Diagnostic Laboratory at the Icahn School of Medicine at Mount Sinai. ABCB6 exons were amplified in 3 groups: Ex1-4 (3445 bp); Ex 5-13 (3426 bp); and Ex13-19 (3435 bp). All polymerase chain reaction primers and sequencing primers (supplemental Table 2) were designed in-house using the MacVector software and checked for common SNPs by the SNP-check program (http://www/ngrl.org.uk/Manchester/projects/snpcheck). Polymerase chain reaction amplification was performed with the Takara PrimeStar GXL DNA Polymerase kit from Clontech Laboratories (TaKaRa, San Jose, CA) using the Bio-Rad C1000 Touch Thermal Cycler (Bio-Rad, Hercules, CA). ABCB6 exons were sequenced bi-directionally. Sequencher software (Gene Codes Corporation, Ann Arbor, MI) was used for alignment and analysis of traces and SNP identification.
The ExAC database of 3 January 2019, was the source of allele frequency data. Fisher’s Exact Test was used to compare allele frequencies among groups.8 Differences were considered significant if the P value was ≤.05. In cases in which all 11 ABCB6 polymorphisms were examined across the genome, the significance of the result is compounded by the number of tests performed. Therefore, the Bonferroni correction method was applied to the data (0.05/11), resulting in a significant P value of ≤.0045.
Results
Sequencing of ABCB6 allele variants
We sequenced the gene encoding ABCB6 in 557 subjects with various forms of porphyria (1124 alleles analyzed). Subjects with a confirmed molecular diagnosis were divided into 2 groups: those with severe disease (216 patients) and those with mild/asymptomatic disease (341 patients) (Table 1). Subjects with AIP (allele variant in HMBS) were divided into those with severe disease (67 patients experiencing >4 acute attacks per year) and those with mild/asymptomatic disease (155 patients experiencing <2 acute attacks per year). The mild/asymptomatic cohort included 105 individuals identified by sequencing HMBS in first-degree relatives of probands.
Cohort of 562 patients with porphyria from the United States (American Porphyria Consortium) and Europe (French Porphyria Center)
| . | Cohort composition . | AIP . | HCP . | VP . | EPP . | XLP . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Total (USA/France) . | USA . | FPC . | USA . | FPC . | USA . | FPC . | USA . | FPC . | USA . | FPC . |
| All | 1124 | ||||||||||
| (n alleles) | (390/734) | 250 | 194 | 0 | 98 | 4 | 246 | 130 | 196 | 6 | 0 |
| Severe | 432 | ||||||||||
| (n alleles) | (90/342) | 22 | 112 | 0 | 30 | 2 | 90 | 60 | 110 | 6 | 0 |
| Mild/ Asymptom. | 472 | ||||||||||
| (n alleles) | (80/392) | 18 | 82 | 0 | 68 | 2 | 156 | 60 | 86 | 0 | 0 |
| Unknown severity* | 220 | ||||||||||
| (n alleles) | (220/0) | 210 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| . | Cohort composition . | AIP . | HCP . | VP . | EPP . | XLP . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Total (USA/France) . | USA . | FPC . | USA . | FPC . | USA . | FPC . | USA . | FPC . | USA . | FPC . |
| All | 1124 | ||||||||||
| (n alleles) | (390/734) | 250 | 194 | 0 | 98 | 4 | 246 | 130 | 196 | 6 | 0 |
| Severe | 432 | ||||||||||
| (n alleles) | (90/342) | 22 | 112 | 0 | 30 | 2 | 90 | 60 | 110 | 6 | 0 |
| Mild/ Asymptom. | 472 | ||||||||||
| (n alleles) | (80/392) | 18 | 82 | 0 | 68 | 2 | 156 | 60 | 86 | 0 | 0 |
| Unknown severity* | 220 | ||||||||||
| (n alleles) | (220/0) | 210 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
AIP, acute intermittent porphyria; FPC, French Porphyria Center; HCP, hereditary coproporphyria; EPP, classical erythropoietic protoporphyria; USA, United States; VP, variegate porphyria; XLP, X-linked protoporphyria + EPP variant form (CLPX, others).
Known allele carriers from family study with no documentation of clinical history.
The study group included 49 subjects with HCP, 15 presenting with a severe clinical phenotype and 34 presenting with mild/moderate symptoms (Table 1). The study group also included 125 subjects with VP: 46 with severe disease and 79 with mild/moderate symptoms (Table 1).
There were 30 subjects with EPP, and of those, half were classified as having mild disease based on the frequency and severity of symptoms (Table 1). Five subjects were included in the study that had XLP, the X-linked form of EPP (Table 1). XLP is the result of gain of function mutations in the X-linked gene ALAS2, which increase enzymatic activity by 2- to 3-fold,9 leading to increased erythrocyte protoporphyrin IX (PPIX) and Zn-PPIX. Also included in the analysis were 5 subjects who had biochemically confirmed increases of PPIX and Zn-PPIX consistent with EPP but no sequence variants involving genes known to cause EPP (Table 2).
Allele frequency of ABCB6 polymorphisms by porphyria subtype comparing severity
| . | AIP . | HCP . | VP . | EPP . | EPP* . | XLP . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | S† . | M‡ . | S . | M . | S . | M . | S . | M . | . | . | Total . | MAF§ . |
| Patients | 67 | 155 | 15 | 34 | 46 | 79 | 85 | 73 | 5 | 3 | 562 | |
| Alleles∥ | 134 | 310 | 30 | 68 | 92 | 158 | 170 | 146 | 10 | 6 | 1124 | |
| R192Q | 2 | 5 | 1 | 3 | 1 | 12 | 0.00342 | |||||
| R192W | 2 | 1 | 1 | 2 | 1 | 2 | 3 | 2 | 14 | 0.00183 | ||
| R247C | 1 | 1 | 2 | 0.00646 | ||||||||
| R276W | 1 | 9 | 1 | 1 | 1 | 2 | 1 | 16 | 0.01895 | |||
| R343Q | 1 | 1 | 2 | 0.01911 | ||||||||
| A492T | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 10 | 0.00716 | |||
| T521S | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 11 | 0.00385 | ||
| G588S | 2 | 7 | 2 | 1 | 3 | 2 | 1 | 18 | 0.00467 | |||
| A681T | 0 | 0.00027 | ||||||||||
| G729S | 1 | 1 | 0.00005 | |||||||||
| R589R | 1 | 1 | 0 | |||||||||
| Total VUS∥ | 12 | 29 | 3 | 6 | 5 | 9 | 14 | 7 | 1 | 1 | 87 | |
| . | AIP . | HCP . | VP . | EPP . | EPP* . | XLP . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | S† . | M‡ . | S . | M . | S . | M . | S . | M . | . | . | Total . | MAF§ . |
| Patients | 67 | 155 | 15 | 34 | 46 | 79 | 85 | 73 | 5 | 3 | 562 | |
| Alleles∥ | 134 | 310 | 30 | 68 | 92 | 158 | 170 | 146 | 10 | 6 | 1124 | |
| R192Q | 2 | 5 | 1 | 3 | 1 | 12 | 0.00342 | |||||
| R192W | 2 | 1 | 1 | 2 | 1 | 2 | 3 | 2 | 14 | 0.00183 | ||
| R247C | 1 | 1 | 2 | 0.00646 | ||||||||
| R276W | 1 | 9 | 1 | 1 | 1 | 2 | 1 | 16 | 0.01895 | |||
| R343Q | 1 | 1 | 2 | 0.01911 | ||||||||
| A492T | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 10 | 0.00716 | |||
| T521S | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 11 | 0.00385 | ||
| G588S | 2 | 7 | 2 | 1 | 3 | 2 | 1 | 18 | 0.00467 | |||
| A681T | 0 | 0.00027 | ||||||||||
| G729S | 1 | 1 | 0.00005 | |||||||||
| R589R | 1 | 1 | 0 | |||||||||
| Total VUS∥ | 12 | 29 | 3 | 6 | 5 | 9 | 14 | 7 | 1 | 1 | 87 | |
VUS, variant of unknown significance.
Protoporphyrin accumulation without mutation in FECH, ALAS2, CLPX. A681T was not identified in this population.
S, severe phenotype.
M, mild/asymptomatic phenotype.
MAF, minor allele frequency.
All allele variants have been deposited with Single Nucleotide Polymorphism database and accession are listed in supplemental Table 4.
Eleven sequence variants were identified in ABCB6 in the 1124 alleles examined: 10 were classified as missense mutations, and one was a synonymous variant. This SNP led to a synonymous change in the codon used for arginine (R589R, c.1767 T>C) and was identified in the heterozygous state in a patient with AIP. Of the 1124 alleles sequenced, 86 missense variants were identified in the 10 known ABCB6 polymorphisms (Table 2).
ABCB6 variants in acute intermittent porphyria
There were 222 subjects with a diagnosis of AIP; 67 were classified as severe and 155 were classified as mild (Table 1). In the severe AIP group, 134 alleles were sequenced, and 11 ABCB6 variants were identified in 6 of the 10 missense alleles (Table 2). In the mild AIP group, the 310 alleles sequenced had 29 ABCB6 variants in 9 of the missense alleles. None of the identified ABCB6 variants were associated with disease severity (range of P values = .21-1.00).
Two of the known ABC6 missense variants, one involving the codon for arginine at position 192 (R192W) and the second involving the glycine 588 codon (G588S), were significantly overrepresented in the total of subjects with AIP (the severe and mild/moderate groups combined) when compared with the allele frequencies reported in the ExAC database (P = .0038, P = .0042, respectively) (Table 2). The presence of missense variants did not correlate with the urine concentration of ALA and/or PBG, and the frequency of these missense variants was not significantly different compared with patients with mild vs severe disease. Missense alleles were identified in 9 other positions in ABCB6; however, none were overrepresented when compared with the ∼120 000 ABCB6 alleles in the ExAC database (Table 2).
ABCB6 variants in HCP and VP
In the HCP population, 49 subjects were evaluated, with 15 classified as severe and 34 classified as mild (Table 2). A total of 9 ABCB6 allele variants were identified in this group. In the VP population, DNA from 125 patients was evaluated; 46 patients had a severe classification and 79 had a mild classification. A total of 14 allele variants in ABCB6 was identified in the VP population. When the frequency of ABCB6 variant alleles was compared between the severe and mild groups, for HCP and VP independently, variants were not associated with disease severity in either type of porphyria (Table 2). There was no increase in variants in ABCB6 when compared with the ExAC database.
Allele frequency in EPP
The study included 166 subjects with EPP, 85 with the severe phenotype. Three had XLP. There were 78 subjects with biochemically confirmed EPP that presented with mild symptoms (less severe sun sensitivity) with 73 having genetic validation of EPP (mutations in FECH, ALAS2, CLPX). ABCB6 missense alleles were identified in 23 of the 332 alleles sequenced, with no variant overrepresented compared with the allele frequencies in the ExAC database.
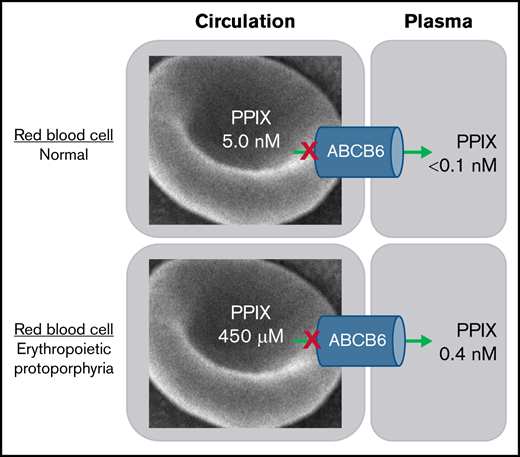
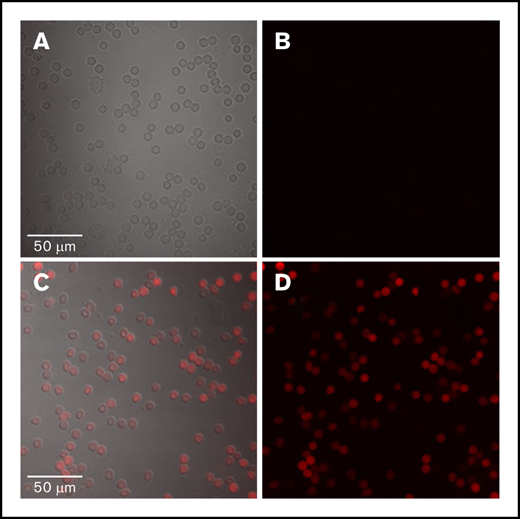
Red blood cells (RBCs) from patients with EPP and XLP have a marked increase in fluoresce due to increased PPIX content (Figure 1). These cells are referred to as fluorocytes and have PPIX concentrations in the ∼450 µM range when analyzed by HPLC. In comparison, the level of PPIX in RBCs from normal individuals is in the low nanometer range. The concentration of PPIX in the plasma of patients with EPP is approximately 0.350 nM (±73 nM) and 0.423 nM (±149 nM) in patients with XLP, with a normal range of 0 to 10 nM.10 Most patients with EPP are wild-type at both ABCB6 loci, and none are homozygous or compound heterozygous for any of the variants in ABCB6 (Table 2). In the EPP/XLP cohort, the cellular concentration of PPIX is approximately 0.5 mM and the plasma PPIX is 0.5 nM.
Peripheral blood film. The blood film from a control subject (A and B) and a patient with erythropoietic protoporphyria (EPP) (C and D) all are wild-type at ABCB6. (A and C) The differential interference contrast (DIC) and fluorescence image are shown as an overlay. (B and D) Only fluorescence is shown. Fluorescent protoporphyrin is visualized by excitation at 405 nm with emission peak at 600 to 650 nm. Protoporphyrin is present in RBCs but not in lymphocytes. PPIX accumulates in EPP erythrocytes, suggesting that ABCB6 does not act as a PPIX exporter.
Peripheral blood film. The blood film from a control subject (A and B) and a patient with erythropoietic protoporphyria (EPP) (C and D) all are wild-type at ABCB6. (A and C) The differential interference contrast (DIC) and fluorescence image are shown as an overlay. (B and D) Only fluorescence is shown. Fluorescent protoporphyrin is visualized by excitation at 405 nm with emission peak at 600 to 650 nm. Protoporphyrin is present in RBCs but not in lymphocytes. PPIX accumulates in EPP erythrocytes, suggesting that ABCB6 does not act as a PPIX exporter.
Erythrocytes from a patient with EPP and wild-type at ABCB6 retain PPIX, as evidenced by autofluorescence observed by exciting a peripheral blood sample at 405 nm. Erythrocyte PPIX was identified based on fluorescence emitted at 600 to 650 nm (Figure 1).
Discussion
Our data show that the frequency of sequence variants that produce missense mutations in ABCB6 is not different among patients with severe porphyria compared with those with mild and asymptomatic disease. With two exceptions (R192W and G588S in patients with AIP), the frequency of each sequence variant among patients with porphyria types was not different from that of the general population (Tables 1 and 2). We hypothesize that neither R192W nor G588S is clinically relevant because the prevalence of neither genotype is statistically different when ABCB6 genotypes from all patients with porphyria are compared with the general population, and these ABCB6 genotypes do not correlate with clinical severity of EPP (Table 2). ABCB6 specifies the LAN blood group. Therefore, if ABCB6 genotype were a clinically relevant porphyrin transporter, EPP phenotype would likely be influenced by sequence variants that decrease expression or function of the transporter.7 In our study of 158 patients with classic EPP, none of the sequence variants reported by Fukuda and colleagues7 impacted disease severity. Our observations suggest that in patients with AHP and EPP, the ABCB6 genotype is clinically irrelevant and therefore does not warrant analysis in patients with porphyria.
The unique pathology of each porphyria type is due to the particular pattern of accumulation of heme intermediates when one of the enzymes in the heme biosynthetic pathway is mutated. In AIP, an autosomal dominant disease, acute attacks are characterized by increased levels of ALA and PBG in the urine. ALA is produced in the mitochondria and then transported into the cytosol to form PBG. During an acute attack, levels of PBG are generally in the range of 20 to 300 mg/g creatinine (normal = 0-2 mg/g creatinine), with ALA approximately half the value of PBG. Therefore, values during an attack are 10 to 150 times above normal.11 The levels decrease between attacks; however, values often remain elevated above normal for months to years.12 During an acute attack, there can also be a modest increase in urine uroporphyrin in the range of 20 to 200 µg/g creatinine (normal <10 µg/g creatinine). Upon successful treatment of the acute attack, uroporphyrin values typically return to normal, and levels of urine uroporphyrin in asymptomatic carriers are generally in the normal range.13
HCP is characterized by significant increases in fecal coproporphyrin III. Urinary increases in coproporphyin III are also present. The clinical presentation of patients with HCP is similar to that of patients with AIP; however, an associated cutaneous photosensitivity may be observed in patients with HCP due to an increase in circulating plasma porphyrins.1 In VP, PPIX accumulates in the plasma, and acute attacks are similar to those of AIP. Accumulation of protoporphyrin may result in photosensitivity that is similar to HCP.1
EPP is a consequence of accumulation of PPIX in RBCs that is released into the plasma. On exposure of skin to sunlight, the increased plasma PPIX produces a painful, nonblistering photosensitivity response.14,15 Symptoms gradually decrease in 48 to 72 hours after elimination of sun exposure.11 Multiple genetic causes have been linked to PPIX accumulation, including mutations in the gene encoding ferrochelatase (FECH), the erythroid form of ALA-synthase (ALAS2), and the mitochondrial protein CLPX, an AAA-ATPase family member that is needed to insert the pyridoxal phosphate cofactor into ALAS and then ultimately degrade the ALAS protein.16 Mutations in FECH account for 90% to 95% of all cases of EPP. The X-linked form of EPP, XLP, accounts for 5% to 10% of cases, and in the remaining 1% to 2% of cases, the genetic basis is unknown.17
When FECH is rate-limiting due to mutation as in EPP, PPIX accumulates in erythrocytes because enzyme activity is insufficient to meet the demand for insertion of ferrous iron into available PPIX to form heme. In XLP, nonsense mutations in ALAS2 result in truncation of the last 18 to 25 residues of the ALAS2 protein.9 These are gain-of-function mutations that lead to an approximate 2- to 3-fold increase in enzymatic activity.18,19 The RBCs of these subjects accumulate PPIX because FECH becomes rate-limiting as a consequence of increased flux of intermediates through the heme biosynthetic pathway. In XLP, erythrocyte Zn-PPIX concentration is higher compared with that of patients with EPP because FECH activity is preserved.
The compound that is transported by ABCB6 has not been identified. The location of the protein varies in different cell types, and loss of ABCB6 in mouse and human models have minimal phenotypic consequences.6 Specific identification of a defect in cells or animals lacking ABCB6 suggests that the compound(s) transported are essential. As such, other transporters accommodate the transport need in situations in which ABC6 function is lost or in which the ABCB6 transporter is needed for a specific metabolite that is nonessential for viability in cells or organisms.20,21 Once a true biologic substrate has been identified, it may be possible to define a stress situation that uncovers the physiological relevance of ABCB6. Several studies link ABCB6 to heme biosynthesis; however, ABCCB6 has not been consistently localized to the mitochondria.5,20 , –24 Identifying the cellular location of ABCB6 is important because the initial step and the final 3 steps in heme biosynthesis take place in the mitochondria, with enzymatic steps 2 to 5 being catalyzed in the cytosol.25 Many of the enzymes in heme biosynthesis are reported to be expressed with the same temporal pattern as ABCB6.7 Recent studies show that heme biosynthesis requires a large shift in metabolic patterns within the developing erythron. Feeding appropriate abundant intermediates, such as α-ketoglutarate, into the tricarboxylic acid cycle for the formation of succinyl-CoA, a required metabolic building block of δ-aminolevulinic acid, has been shown.26 This metabolic shift requires that a different set of host proteins be produced. Conceivably, ABCB6 is required for amino acid balancing rather than for transporting intermediates of heme biosynthesis.26 More recent studies have shown that ABCB6 is necessary for proper melanin production.24 These studies, however, did not identify the ABCB6 substrate. ABCB6 was localized to the endosomal compartment in this system, suggesting that ABCB6 is not involved in heme biosynthesis because none of the 8 reactions of the heme pathway take places in the endosomal compartment.
ABCB6 is the gene encoding the Langereis (Lan) blood group antigen.6 Lan-negative individuals have no clinical phenotype, suggesting that ABCB6 is not essential for erythropoiesis. Further, the absence of signs and symptoms of porphyria (particularly an erythropoietic type of porphyria such as EPP or congenital erythropoietic porphyria) in Lan-negative individuals argues against ABCB6 as an essential transporter of heme metabolites. This interpretation is supported by our data showing that the presence of wild-type ABCB6 does not prevent accumulation of PPIX in the erythrocytes of a patient with EPP (Figure 1).
Murine studies have demonstrated that the localization pattern and quantity of PPIX accumulation in the liver varies among strains, indicating that genetic factors influence PPIX homeostasis.27 Intercrossing mice with different PPIX hepatic deposition phenotypes did not identify allelic segregation patterns in the F1 or F2 hybrid generations that mapped to the Abcb6 locus in these progeny (data not shown). However, several genetic loci were highly ranked, suggesting that hepatic uptake and processing of PPIX from the plasma into the biliary system is modulated by genetic factors.
Fukuda et al showed that some of the ABCB6 polymorphisms that they identified affect the stability of the protein. However, without knowing the transported substrate, the pathophysiological consequences of the polymorphisms that affect stability of ABCB6 are speculative.7 Our study and the study by Fukuda et al do not account for the contribution of familial patterns to overrepresentation of any single polymorphism (supplemental Table 3).7 Although the role of ABCB6 in erythrocyte function has not been delineated, it does not appear to involve transport of intermediates of the heme biosynthetic pathway. This conclusion is further supported by the phenotypic characterization of the Abcb6−/− mouse that showed no defect in RBC development or tetrapyrrole homeostasis.28
Acknowledgments
This work was supported by the Laboratory of Excellence Gr-Ex (Labex Gr-Ex) and the Institut National de la Santé et de la Recherche Medicale (INSERM) (H.P., L.G., G.N., and Z.K.). The Labex GR-Ex is funded by the program “Investissements d’avenir” of the French National Research Agency (reference ANR-11-IDEX-0005-02). This work was also supported in part by the Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai and National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (U54 DK083909) and the American Porphyria Foundation (APF) (R.D. and B.C.) and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (U54 DK118058 and U54 DK083909) and the APF (C.P.F., C.J.P., and J.D.P.).
Authorship
Contribution: C.P.F., G.N., J.L., Z.K., and D.T. performed experiments; C.P.F., G.N., L.G., B.C., H.P., and J.D.P. analyzed data; C.P.F., G.N., R.D., C.J.P., B.C., H.P., and J.D.P. prepared the manuscript; and L.G., H.P., and J.D.P. designed the experiment.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John D. Phillips, Division of Hematology, 30 North 1900 E, 5C330 SOM, Salt Lake City, UT 84132; e-mail: john.phillips@hsc.utah.edu.
References
Author notes
C.P.F. and G.N. contributed equally to this study.
Alleles of ABCB6 identified in the porphyria population in this study have been curated with the Single Nucleotide Polymorphism database. Entries can be found in supplemental Table 4.
Requests for data sharing may be submitted to John D. Phillips (john.phillips@hsc.utah.edu).
The full-text version of this article contains a data supplement.