TO THE EDITOR:
Thrombotic microangiopathy (TMA) is a life-threatening clinical syndrome characterized by microangiopathic hemolysis, thrombocytopenia, and organ damage.1 TMA can be a primary disorder, such as thrombotic thrombocytopenic purpura and complement-mediated atypical hemolytic uremic syndrome (aHUS), or secondary in the setting of infection, drugs, malignancy, or hematopoietic stem cell transplantation (HSCT).1 TMA occurs in up to 15% of patients with cancer2 and may be attributed directly to the effects of malignancy, or more commonly, anticancer therapies, including cytotoxic drugs, such as gemcitabine, anti-VEGF,3 and other targeted therapies.2-8 Cancer/chemotherapy–associated TMA responds poorly to conservative measures or plasma exchange,9 is associated with significant morbidity and mortality, and limits the use of otherwise effective anticancer therapies. The mechanisms underlying cancer and antineoplastic therapy–associated TMA are heterogenous7; however, given clinical similarities with aHUS, complement inhibition has been used to treat it with reports of efficacy.10 Here, we report on 8 cases of cancer/chemotherapy–associated TMA, which responded to eculizumab therapy and were able to resume anticancer therapy without TMA recurrence.
Consecutive patients with cancer and/or chemotherapy–associated TMA treated with eculizumab enrolled in the Hopkins Complement Associated Disorders Registry were included.11,12 TMA was diagnosed as (1) platelet count <150 × 109/L or at least 30% lower than baseline; (2) serum creatinine >2.25 mg/dL; (3) schistocytes on peripheral blood smear and acute TMA on renal biopsy, if performed; (4) ADAMTS13 activity >10%; and (5) Shiga toxin negative. Criteria numbers 1 and 3 to 5 were required for diagnosis. Criteria 2 (serum creatinine >2.25 mg/dL) was considered supportive of the diagnosis but was not required, and patients with a lower serum creatinine could be included if otherwise consistent with TMA/aHUS. Eculizumab doses of 900 mg weekly for 4 doses followed by maintenance dosing at 1200 mg every 2 weeks was used as recommended for aHUS. All patients received vaccinations for Neisseria meningitidis (quadrivalent meningococcal vaccine and Meningococcal group B vaccine) and prophylactic antibiotics. Next-generation sequencing using a panel of 15 genes with known function related to complement activation and regulation as previously described.12 Complete methods are provided in the supplemental data. The Institutional Review Board at Johns Hopkins University approved this study, which was performed in accordance with the Declaration of Helsinki.
Patient 1
A 61-year-old male developed acute renal insufficiency (peak serum creatinine, 2 mg/dL), pulmonary edema, and fatigue 21 months after initiation of gemcitabine and paclitaxel for pancreatic cancer. Laboratory workup revealed thrombocytopenia (platelet count nadir, 6000/μL), anemia (hemoglobin nadir, 5.9 g/dL), and proteinuria. Following platelet and red cell transfusions, he received eculizumab and restarted gemcitabine and paclitaxel. He then developed chemotherapy-induced anemia and thrombocytopenia (without microangiopathic hemolysis) requiring a 4-month break in therapy, which was subsequently resumed with concomitant eculizumab and without recurrent TMA.
Patient 2
A 73-year-old woman with pancreatic adenocarcinoma receiving gemcitabine monotherapy for 1 year presented with worsening shortness of breath, anemia (hemoglobin nadir, 7.7 g/dL), thrombocytopenia (platelet count nadir, 21 000/μL), and acute renal failure (serum creatinine, 2.5 mg/dL). She was treated with eculizumab with rapid resolution of thrombocytopenia. Serum creatinine stabilized at 1.2 to 1.4 mg/dL. She stopped eculizumab after 13 weeks without TMA recurrence.
Patient 3
A 55-year-old male with locally advanced nasal squamous cell carcinoma presented with renal failure (serum creatinine, 4.5 mg/dL), thrombocytopenia (platelet count nadir, 29 000/μL), and anemia (hemoglobin nadir, 6.3 g/dL) 17 months after initiating chemoradiotherapy. He started dialysis and received 2 doses of eculizumab with modest improvement in platelet count. However, he had a cardiac arrest several days after admission, followed by respiratory failure. He continued hemodialysis until he died ∼6 months after TMA diagnosis.
Patient 4
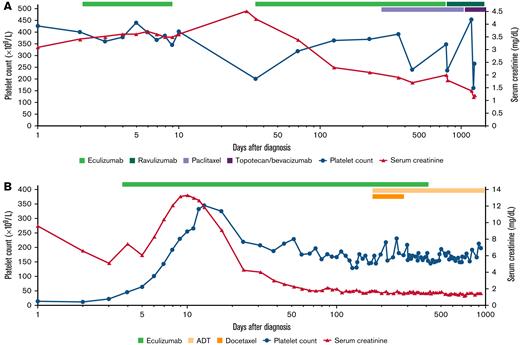
A 67-year-old woman with recurrent, platinum-resistant ovarian/fallopian tube cancer presented with anuric renal failure (serum creatinine, 4.2 mg/dL) and anemia (hemoglobin nadir, 5.9 g/dL) with microangiopathic hemolysis 8 months after starting gemcitabine treatment. Renal biopsy confirmed TMA. She was treated with eculizumab with rapid improvement in renal output and hematological parameters (Figure 1A). She missed 2 doses of eculizumab after discharge and was readmitted with recurrent TMA with acute kidney injury, which resolved on restarting eculizumab. After 7 months, she started weekly paclitaxel and continued receiving eculizumab infusions. She switched to ravulizumab for dosing convenience 18 months later.
Time course of platelet count and serum creatinine levels for 2 patients with cancer/chemotherapy–associated TMA treated with terminal complement inhibition. Time (x-axis) is plotted on a logarithmic scale. (A) The course of patient 4 who developed recurrent TMA owing to nonadherence with therapy but was successfully salvaged to continue chemotherapy. Patient 4 presented with renal failure, microangiopathic hemolytic anemia, and a platelet count that was in the high normal range at diagnosis but 50% lower than the patient’s baseline platelet count. On starting eculizumab, platelet count increased within 1 week, and serum creatinine level stabilized. The patient was discharged to continue eculizumab as an outpatient but missed a month of treatment leading to a TMA relapse with thrombocytopenia and worsening renal function, which resolved rapidly with restarting eculizumab. She remained on anti-C5 therapy (eculizumab and later ravulizumab) while receiving taxol and later topotecan and bevacizumab on disease progression without TMA recurrence. (B) The course of patient 6 who responded to eculizumab and was able to discontinue therapy. He presented with renal failure and severe thrombocytopenia with microangiopathic hemolytic anemia. Platelet count started to increase within days of starting eculizumab; urine output increased within 1 week of starting therapy, and serum creatinine decreased within 2 to 3 weeks. He remained on eculizumab while starting androgen deprivation therapy (ADT) (and docetaxel) for prostate cancer. He stopped eculizumab after 12 months of therapy when the prostate cancer was stable on ADT and remains free of TMA recurrence.
Time course of platelet count and serum creatinine levels for 2 patients with cancer/chemotherapy–associated TMA treated with terminal complement inhibition. Time (x-axis) is plotted on a logarithmic scale. (A) The course of patient 4 who developed recurrent TMA owing to nonadherence with therapy but was successfully salvaged to continue chemotherapy. Patient 4 presented with renal failure, microangiopathic hemolytic anemia, and a platelet count that was in the high normal range at diagnosis but 50% lower than the patient’s baseline platelet count. On starting eculizumab, platelet count increased within 1 week, and serum creatinine level stabilized. The patient was discharged to continue eculizumab as an outpatient but missed a month of treatment leading to a TMA relapse with thrombocytopenia and worsening renal function, which resolved rapidly with restarting eculizumab. She remained on anti-C5 therapy (eculizumab and later ravulizumab) while receiving taxol and later topotecan and bevacizumab on disease progression without TMA recurrence. (B) The course of patient 6 who responded to eculizumab and was able to discontinue therapy. He presented with renal failure and severe thrombocytopenia with microangiopathic hemolytic anemia. Platelet count started to increase within days of starting eculizumab; urine output increased within 1 week of starting therapy, and serum creatinine decreased within 2 to 3 weeks. He remained on eculizumab while starting androgen deprivation therapy (ADT) (and docetaxel) for prostate cancer. He stopped eculizumab after 12 months of therapy when the prostate cancer was stable on ADT and remains free of TMA recurrence.
Patient 5
A 53-year-old woman with a germ cell tumor treated with bleomycin, etoposide, and cisplatin presented 6 days after starting treatment with thrombocytopenia (platelet count nadir, 43 000/μL), anemia (hemoglobin nadir, 6.2 g/dL), and renal failure (serum creatinine, 4.8 mg/dL; lactate dehydrogenase [LDH], 3459 U/L). Chemotherapy was held, and she was treated with eculizumab, rescuing renal function, and improvement in hematological parameters. She subsequently underwent pelvic exenteration surgery and restarted chemotherapy while continuing eculizumab.
Patient 6
A 54-year-old male was admitted for severe thrombocytopenia (platelet count nadir, 11 000/μL) and oliguric acute kidney injury (serum creatinine, 13.5 mg/dL; LDH, 5280 U/L). The patient was found to have markedly elevated prostate-specific antigen levels (380.5 ng/mL) and was diagnosed with prostate cancer on prostate biopsy with bone metastases to the spine. Because of initial concern for thrombotic thrombocytopenic purpura [TTP], he underwent urgent plasmapheresis and was started on hemodialysis. However, plasma exchange was stopped after 24 hours when ADAMSTS13 activity resulted at >100%. Eculizumab was started with improvement in TMA and renal function (Figure 1B). He later started androgen deprivation therapy, which he continues. After 12 months on therapy, eculizumab was discontinued, and he was monitored according to our published protocol.11 He has been monitored for another 20 months without recurrence of TMA.
Patient 7
A 69-year-old man with a relapse of diffuse large B cell presented with renal injury (serum creatinine, 2.3 mg/dL) and TMA (platelet count, 17 000/μL; hemoglobin nadir, 6.5 g/dL with schistocytes). Rapid clinical deterioration and altered mental status led to intubation. ADAMTS13 was 15%, and he was initially treated with plasmapheresis without improvement. He then received eculizumab with resolution of TMA and clinical improvement (renal function recovery and extubation). Brentuximab vedotin was initiated, and eculizumab was continued until completion of therapy and stabilization of renal function (serum creatinine, 1.4-1.7 mg/dL).
In this series, 7 patients with cancer/chemotherapy–associated TMA were successfully treated with eculizumab. Five patients continued cancer-directed therapy without recurrent TMA while on continued anti-C5 therapy (Table 1).
Characteristics and outcomes of patients with cancer and chemotherapy associated TMA
| Patient . | Age/ sex . | Type of cancer . | Chemotherapy duration (mo) . | ADAMTS 13 activity (%) . | Platelet count (G/L) . | Hemoglobin (g/dL) . | LDH . | Serum creatinine . | Hematological response . | Renal response . | Serum creatinine at end of follow-up . | Treated with eculizumab (duration) . | Eculizumab continued/ stopped . | Chemotherapy restarted . | Status (duration of follow-up) . | Genetics . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 61/M | Pancreatic | 21 | 87 | 6 | 5.9 | 547 | 2.0 | Y | N | 2.2 | Y/13 mo | Continued | Y | Alive/24 mo | No variants |
| 2 | 73/F | Pancreatic | 12 | 96 | 21 | 7.7 | 742 | 2.5 | Y | Y | 1.4 | Y/13 wk | Stopped | Unknown | Alive/3 mo | No variants |
| 3 | 55/M | SCC of Nasal Cavity | 17 | 56 | 29 | 6.8 | 695 | 4.5 | Y | N | 4 | Y/2 wk | Stopped | N | Deceased/6 mo | No variants |
| 4 | 67/F | Ovarian | 8 | 79 | 199 | 5.9 | 797 | 4.2 | Y | Y | 2.1 | Y/41 mo | Continued | Y | Alive/41 mo | N/A |
| 5 | 53/F | Germ cell tumor | <1 | 42 | 43 | 6.3 | 3459 | 4.8 | Y | Y | 3 | Y/Unknown | Continued | Y | Unknown | No rare pathogenic variant∗ |
| 6 | 54/M | Prostate | N/A | 100 | 11 | 7.4 | 5280 | 13.5 | Y | Y | 1.33 | Y/12 mo | Stopped | Y | Alive/32 mo | No variants |
| 7 | 69/M | DLBCL | N/A | 15 | 17 | 6.5 | 676 | 2.3 | Y | Y | 1.4 | Y/6 wk | Continued | Y | Deceased/65 mo | No variants |
| Patient . | Age/ sex . | Type of cancer . | Chemotherapy duration (mo) . | ADAMTS 13 activity (%) . | Platelet count (G/L) . | Hemoglobin (g/dL) . | LDH . | Serum creatinine . | Hematological response . | Renal response . | Serum creatinine at end of follow-up . | Treated with eculizumab (duration) . | Eculizumab continued/ stopped . | Chemotherapy restarted . | Status (duration of follow-up) . | Genetics . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 61/M | Pancreatic | 21 | 87 | 6 | 5.9 | 547 | 2.0 | Y | N | 2.2 | Y/13 mo | Continued | Y | Alive/24 mo | No variants |
| 2 | 73/F | Pancreatic | 12 | 96 | 21 | 7.7 | 742 | 2.5 | Y | Y | 1.4 | Y/13 wk | Stopped | Unknown | Alive/3 mo | No variants |
| 3 | 55/M | SCC of Nasal Cavity | 17 | 56 | 29 | 6.8 | 695 | 4.5 | Y | N | 4 | Y/2 wk | Stopped | N | Deceased/6 mo | No variants |
| 4 | 67/F | Ovarian | 8 | 79 | 199 | 5.9 | 797 | 4.2 | Y | Y | 2.1 | Y/41 mo | Continued | Y | Alive/41 mo | N/A |
| 5 | 53/F | Germ cell tumor | <1 | 42 | 43 | 6.3 | 3459 | 4.8 | Y | Y | 3 | Y/Unknown | Continued | Y | Unknown | No rare pathogenic variant∗ |
| 6 | 54/M | Prostate | N/A | 100 | 11 | 7.4 | 5280 | 13.5 | Y | Y | 1.33 | Y/12 mo | Stopped | Y | Alive/32 mo | No variants |
| 7 | 69/M | DLBCL | N/A | 15 | 17 | 6.5 | 676 | 2.3 | Y | Y | 1.4 | Y/6 wk | Continued | Y | Deceased/65 mo | No variants |
F, female; M, male; N, no; N/A, not applicable; Y, yes.
Patient 4 had a variant of uncertain significance in CFI (c.1246A>C, p.Ile416Leu, heterozygous) reported on clinical sequencing completed at Cincinnati Children’s Hospital. This was also detected on research sequencing but not considered positive for a rate variant in our analysis because the minor allege frequency was not <0.005 in any ethnic population, our threshold for calling a rare germline variant.
Similar to aHUS, patients presented with microangiopathic hemolytic anemia, acute kidney injury, and either absolute thrombocytopenia or an at least 50% decline in platelet count (for patients who had a normal platelet count at presentation), and ADAMTS13 activity >10%. Approximately 50% of patients with aHUS are found to harbor a predisposing germline variant in a complement regulatory gene.13,14 Patients with HSCT-associated TMA are also likely to carry some combination TA-TMA–associated variants in complement regulatory genes and Complement Factor H [CFH] autoantibodies.15-17 However, targeted sequencing of 5 patients with available samples failed to reveal any rare variants (minor allele frequency <0.005). The response to complement inhibition suggests that complement activation, possibly owing to direct endothelial toxicity and thromboinflammation, is a driver of chemotherapy-associated TMA and does not require the presence of a predisposing genetic lesion.7 None of the patients in our series had bevacizumab-associated TMA, so we cannot comment on whether anti-VEGF TMA responds to complement inhibition. In addition, it is likely that some of these cases may have responded to holding the offending drug but would have precluded retreatment.
In summary, terminal complement inhibition is effective in treating chemotherapy-associated TMA and allows patients to remain on effective, and sometimes curative, therapies without the morbidity and mortality associated with recurrent TMA.
Acknowledgment: This work was supported by National Institutes of Health, Heart, Lung and Blood Institute grant K99HL150594 (S.C.).
Contribution: H.S. collected data, wrote, edited, and reviewed the manuscript; E.M.B. designed the study; E.M.B. and H.C. performed sequencing and analyzed sequencing data; S.C. designed the study and wrote part of the first draft of the manuscript; X.-Z.B., A.M., and R.A.B. edited and critically reviewed the manuscript; and all authors read and approved the final draft of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shruti Chaturvedi, Division of Hematology, Johns Hopkins University School of Medicine, 720 Rutland Ave, Ross Research Building, Rm 1025, Baltimore, MD 21205; e-mail: schatur3@jhmi.edu.
References
Author notes
Additional data and materials can be obtained by e-mailing the corresponding author, Shruti Chaturvedi, at schatur3@jhmi.edu.
The full-text version of this article contains a data supplement.