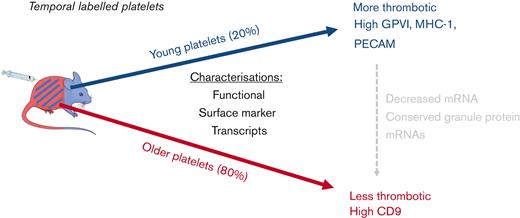
Key Points
Age-specific analysis of young platelets confirm higher thrombotic potential but independent of platelet size.
Apparent refined control of individual mRNAs with those encoding granule proteins are greatly conserved across platelet lifespan.
Abstract
The proportion of young platelets, also known as newly formed or reticulated, within the overall platelet population has been clinically correlated with adverse cardiovascular outcomes. However, our understanding of this is incomplete because of limitations in the technical approaches available to study platelets of different ages. In this study, we have developed and validated an in vivo temporal labeling approach using injectable fluorescent antiplatelet antibodies to subdivide platelets by age and assess differences in functional and molecular characteristics. With this approach, we found that young platelets (<24 hours old) in comparison with older platelets respond to stimuli with greater calcium flux and degranulation and contribute more to the formation of thrombi in vitro and in vivo. Sequential sampling confirmed this altered functionality to be independent of platelet size, with distribution of sizes of tracked platelets commensurate with the global platelet population throughout their 5-day lifespan in the circulation. The age-associated decrease in thrombotic function was accompanied by significant decreases in the surface expression of GPVI and CD31 (PECAM-1) and an increase in CD9. Platelet messenger RNA (mRNA) content also decreased with age but at different rates for individual mRNAs indicating apparent conservation of those encoding granule proteins. Our pulse-chase–type approach to define circulating platelet age has allowed timely reexamination of commonly held beliefs regarding size and reactivity of young platelets while providing novel insights into the temporal regulation of receptor and protein expression. Overall, future application of this validated tool will inform age-based platelet heterogeneity in physiology and disease.
Introduction
Understanding the fundamental changes that occur during a platelet’s lifespan are central to many avenues of current research in both cardiovascular and hematologic disease. However, research has been severely limited because of a lack of methodologies to identify circulating platelets of different ages. Platelets inherit a finite complement of messenger RNA (mRNA) from the parent megakaryocyte,1 which is rapidly lost once they are released into the circulation. As platelets are anucleate, they are unable to replenish these levels2 and so nucleic acid dyes such as thiazole orange or SYTO13 have been widely used to differentiate young platelets based on their higher mRNA content (sometimes inferred to reflect an immature platelet fraction).3-8
Increased platelet turnover in a number of diseases, including diabetes mellitus, chronic kidney disease, and essential thrombocythemia9,10 and the consequent increases in the immature platelet fraction have been associated with a higher incidence of acute coronary syndromes11 and a reduced effectiveness of antiplatelet drugs.12,13 Similarly, several groups have used nucleic acid dyes and cell sorting to establish that young platelets defined in this way are hyperreactive and of increased thrombotic potential.4-6 We have previously demonstrated that young platelets identified by higher mRNA content localize to the core of aggregates and drive aggregate formation even in the presence of standard antiplatelet therapies, potentially reducing drug effectiveness in high platelet turnover conditions.14
Although useful as basic methodologies, there are limitations with mRNA dye–based approaches to fractionate platelets. First, staining levels do not accurately advise on platelet age as fluorescence has not been temporally benchmarked. Second, following the rapid early loss of mRNA from young platelets, the fluorescent signal can no longer discriminate platelets by age. Moreover, confounding factors including platelet size and nucleotide-rich granule content can cause undue bias or interference with counts or estimated fractions of immature young platelets.15,16
As a result of these shortcomings, there continues to be a search for alternative techniques to differentiate the newly formed platelet fraction. A common strategy in this regard has been to cause rapid thrombocytopenia in mice by injection of antiplatelet antibodies.17,18 This acute depletion is followed by a burst of platelet production such that the entire circulating platelet population is taken as being reticulated or newly formed. However, such an approach involves a pathological insult to drive rapid platelet production and makes the questionable assumption that platelets released under such conditions are representative of platelets newly formed under normal circumstances.
We therefore sought to develop an alternative in vivo labeling approach in mice using antiplatelet fluorescent antibodies to provide a tool for the age-related tracking and isolation of platelet subpopulations. Here, we demonstrate the application of this novel approach in revisiting widely held ideas regarding the phenotype of young platelets, identifying new age-related alterations in surface marker expression and revealing preferential retentions of mRNAs.
Methods
Ethical statement
Animal procedures were conducted in accordance with the United Kingdom Home Office approval (Project Licenses 70–7013 and PP1576048) under the United Kingdom Animals (Scientific Procedures) Act Amendment 2013 and were subject to local approval from the Queen Mary University of London (London, United Kingdom) and Imperial College London (London, United Kingdom) ethical review panels.
Temporal labeling of platelets in vivo
In vivo grade fluorescently conjugated anti-CD42c antibodies (DyLight-x488 or x649, Emfret) were administered (0.1 μg/g, IV) to the tail vein of C57BL/6 mice at specific time points as per supplier guidance. In establishing the model, labeling efficacy was confirmed by comparative in vitro platelet labeling using anti–CD41-BV421 (clone MWReg30, BioLegend). Labeled platelets were identified and tracked using flow cytometry (Novocyte, Agilent Technologies) after microsampling from the tail vein.
Blood collection and isolation of platelets
Mice were anesthetized with intraperitoneal ketamine (Narketan, 100 mg/kg; Vetoquinol) and xylazine (Rompun, 10 mg/kg; Bayer), and blood was collected from the inferior vena cava into lepirudin (Refludan, 25 μg/mL; Celgene, Windsor) or trisodium citrate (3.2%; Sigma). Platelet-rich plasma (PRP) was isolated as previously published.19 Briefly, whole blood was diluted 1:1 with the modified Tyrode N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) buffer (134 mmol/L sodium chloride, 2.9 mM potassium chloride, 0.34 mmol/L disodium phosphate, 12 mmol/L sodium bicarbonate, 20 mmol/L HEPES, and 1 mmol/L magnesium chloride; pH 7.4; Sigma) and centrifuged at 100 × g for 8 minutes.
Immunofluorescence of temporally labeled platelets
Platelets fixed in paraformaldehyde (4%; VWR) were centrifuged onto poly-L-lysine coverslips (VWR; 600 × g, 5 minutes). Samples were mounted with Prolong Diamond antifade mount (Thermo Fisher Scientific). Confocal microscopy was performed using an inverted Zeiss LSM880 with Airyscan confocal microscope; 63× lens objective, 1.4 Oil DICII (Zeiss), collecting 10 individual fields of view per mouse. Analysis was conducted using Zen Software (2.3 SP1, Zeiss) and ImageJ (National Institutes of Health).
Quantification of P-selectin expression and active confirmation of GPIIb/IIIa
PRP was diluted in phosphate-buffered saline (PBS; 1:40; Sigma) and incubated with the protease-activated receptor-4–activating peptide, AYPGKF amide (PAR4-amide; 200 μM; Bachem) or PBS for 20 minutes at 37°C in presence of anti–CD62P-BV421 (clone RB40.34; BD Bioscience) or anti-CD41/61-PE (clone JON/A, Emfret). Samples were subsequently fixed with 1% formalin (Sigma) and samples acquired and analyzed by flow cytometry (Novocyte) using NovoExpress software (Agilent Technologies).
Quantification of calcium dynamics
Platelets were isolated from mice 24 hours after injection with anti–CD42c-DyLight-x649 and were subsequently incubated with Fluo-4 AM (5 μM; Thermo Fisher Scientific) for 30 minutes at 37°C, followed by counterstaining with anti–CD41-BV421 (1:100; BD Biosciences) for 15 minutes and supplementation with calcium chloride (2 mM; Sigma). Baseline Fluo-4 fluorescence was recorded for 30 seconds, followed by challenge with PAR4-amide (25 μM), a collagen-related peptide (CRP-XL, 1 μg/mL; CAMBCOL), the stable thromboxane A2 mimetic U46619 (10 μM; Enzo), or ionomycin (10 μM; Invitrogen) and subsequent recording for 2 minutes. Samples were acquired on a BD LSRII (BD Bioscience) using FACSDiva acquisition software and analyzed using FlowJo software, version 10.
Ex vivo whole-blood aggregation
Platelet aggregation was performed in whole blood as previously described.19 Briefly, in half-area 96-well microtiter plates (Greiner Bio-One), whole blood anticoagulated with lepirudin was incubated with PBS, arachidonic acid (0.05-0.5 mM; Sigma), the Horm collagen (0.1-10 μg/mL; Takeda), PAR4-amide, (50-100 μM), or U46619 (0.1-10 μM) under mixing conditions (1200 revolutions per minute, Bioshake iQ, Qinstruments). Samples were subsequently diluted (1:20 acid citrate dextrose) and analyzed by flow cytometry (Novocyte) to identify and determine proportions of the single-platelet population.
PE injury model
Pulmonary embolism (PE) was induced as previously described.20 Briefly, the Horm collagen (50 μg/kg, Takeda) and U46619 (210 μg/kg, Enzo) were administered IV. Blood was microsampled before and after (4 minutes) administration of platelet activators for determination of circulating labeled platelets.
Surface marker expression
PRP obtained from temporally labeled mice was diluted 1:40 in modified Tyrode HEPES buffer and incubated for 20 minutes at room temperature with one of the following PE-conjugated antibodies: anti-CD9 (clone MZ3), anti-CD49b (clone DX5), anti-CD31 (clone 390), anti-H2 (clone 1/42; all BioLegend), or anti-GPVI (clone 784808, R&D systems). PE-conjugated isotype antibodies of Rat immunoglobulin M (IgM) (clone RTK2118), IgG1 (clone 2071), or IgG2a (clone RTK2758; all BioLegend) were used as corresponding nonspecific-binding controls and used to correct data values for both tracked and global populations before comparison. After staining, samples were fixed with 1% formalin, acquired on Novocyte flow cytometry, and analyzed using FlowJo software, version 10.
RNA extraction, complementary DNA synthesis, and quantitative real-time polymerase chain reaction of sorted, temporally labeled platelets
Full details of mRNA analysis of platelet populations are available in supplemental Methods. RNA was extracted from fluorescence-activated cell-sorted platelets (2.5 million per population) using the miRNeasy mini kit (Qiagen, 217004), following the manufacturer’s instructions. Samples were spiked with Cel-miR-39-3p (Qiagen, 219600) and MS2 carrier RNA (Roche, 10165948001) after the first step of RNA isolation (ie, addition of Qiazol to sample). Complementary DNA was generated using VILO RT Superscript (Thermo Fisher, 11755-250) from 8 μL of RNA, as per the manufacturer’s instructions. Transcripts were quantified using SYBR Select Master Mix (Applied Biosystems, 4472908) with custom-designed primers (Integrated DNA Technologies), in a ViiA 7 real-time polymerase chain reaction system (Applied Biosystems) using ViiA 7 software (Applied Biosystems).
Statistics and data analysis
Data are presented as mean ± standard error of the mean (SEM) and data sets analyzed, including principal component analysis, using Prism, version 9 (GraphPad software). As required, the single-platelet population was gated based on forward scatter and antiplatelet immunoreactivity (fluorescence intensity). In addition, global platelet populations were defined as all platelets present in the sample, incorporating all platelet ages, and used to provide a baseline comparison for temporally labeled populations. Statistical significance was determined by paired t test unless otherwise stated, and data sets were considered different if P < .05. Each n value represents a data point from a separate animal.
Results
Temporal fluorescence labeling of platelets in vivo to identify age-defined populations
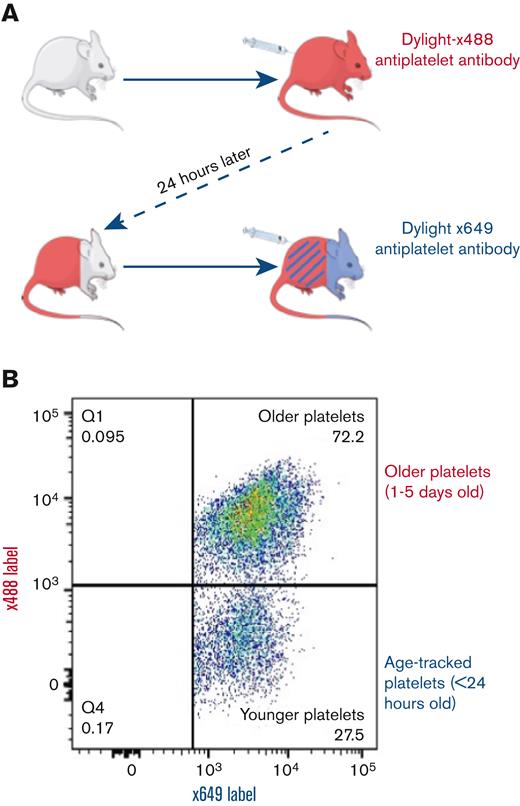
IV injections of short-lived fluorescence-labeled anti-CD42c antibody (DyLight-x488) produced ∼95% labeling of circulating platelets as determined after in vitro counterstaining with anti–CD41-BV421 (supplemental Figure 1). Platelets remained fluorescently tagged for the duration of their life. After 24 hours, an unlabeled platelet subpopulation, representing ∼20% of the entire population, was seen because of normal platelet removal and replacement. At this time, an injection of the same anti-CD42c antibody conjugated to an alternative fluorophore (DyLight-x649) created 2 differentially fluorescently labeled platelet subpopulations (Figure 1A). A minority single-labeled (x649+/x488−) population corresponding to platelets <24 hours old and a majority dual-labeled (x649+/x488+; Figure 1B) population corresponding to platelets >24 hours old.
Methodological diagram of in vivo temporal labeling of platelets in mice using fluorescently tagged antibodies. (A) Mice are injected with Dylight-x488–labeled anti-CD42c to achieve near universal labeling (>95%), followed by a second injection after 24 hours with Dylight-x649–labeled anti-CD42c results in a dual-labeled platelet population and a minority x649 single-label population corresponding to platelets generated in intervening 24-hour period since the first injection. (B) Representative pseudocolor dot plot of differentially labeled platelets from blood sample as detected by flow cytometry.
Methodological diagram of in vivo temporal labeling of platelets in mice using fluorescently tagged antibodies. (A) Mice are injected with Dylight-x488–labeled anti-CD42c to achieve near universal labeling (>95%), followed by a second injection after 24 hours with Dylight-x649–labeled anti-CD42c results in a dual-labeled platelet population and a minority x649 single-label population corresponding to platelets generated in intervening 24-hour period since the first injection. (B) Representative pseudocolor dot plot of differentially labeled platelets from blood sample as detected by flow cytometry.
Younger platelets demonstrate increased reactivity, contributing disproportionately to thrombotic response
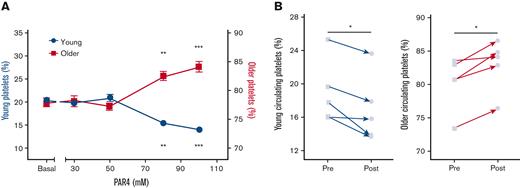
No significant differences were observed in basal P-selectin expression or JON-A binding between platelets <24 hours old and >24 hours old (Figure 2A-B). After incubation with a PAR-4 peptide agonist, the younger platelets demonstrated higher P-selectin expression (83 147 ± 858 mean fluorescence index [MFI]) than older platelets (80 880 ± 1257 MFI; P < .005; Figure 2A) but lower JON-A binding (864 ± 18 vs 884 ± 17; P < .01; Figure 2B). Younger platelets also displayed significantly stronger increases in intracellular calcium levels after exposure to the PAR4 peptide (Figure 2C), ionomycin (Figure 2D), and CRP-XL or U46619 (supplemental Figure 2).
Differential degranulation, integrin activation, and calcium flux of young platelets compared with older platelets. Younger (<24 hours old) platelets express higher levels of P-selectin (A) but lower levels of active GPIIb/IIIa (B) in response to PAR4-specific stimulation compared with corresponding older platelets in the same sample. Younger platelets have a greater calcium flux (measured as area under the curve [AUC]) in response to PAR4-specific stimulation (C) and ionomycin (D) (n = 5). (C) Data are individual replicates or mean ± SEM. ∗∗P < .01, ∗∗∗P < .001 by paired t test as indicated. ns, not significant.
Differential degranulation, integrin activation, and calcium flux of young platelets compared with older platelets. Younger (<24 hours old) platelets express higher levels of P-selectin (A) but lower levels of active GPIIb/IIIa (B) in response to PAR4-specific stimulation compared with corresponding older platelets in the same sample. Younger platelets have a greater calcium flux (measured as area under the curve [AUC]) in response to PAR4-specific stimulation (C) and ionomycin (D) (n = 5). (C) Data are individual replicates or mean ± SEM. ∗∗P < .01, ∗∗∗P < .001 by paired t test as indicated. ns, not significant.
Next, to assess the relative aggregatory potential of young and older platelets, the proportions of each subpopulation were determined in whole-blood samples after stimulation. In such experiments, equal aggregatory potential and contribution would result in no differences between the proportions found in the unaggregated single-platelet population before and after stimulation. However, in response to multiple agonists, the proportions diverged significantly. In response to the PAR4 peptide, the proportion of younger platelets remaining unaggregated significantly decreased (24.7% ± 0.3% to 18.6% ± 0.6%; P < .05), whereas the older population significantly increased (75.3% ± 0.3% to 81.4% ± 0.6%; P < .05; Figure 3A). Similar patterns of response were seen when aggregation was stimulated by CRP-XL or U46619 (supplemental Figure 3). To confirm these in vitro observations in vivo, we used a murine model of PE and assessed circulating proportions of the labeled platelet populations before and after embolism. Compared with before injury, a significant decrease in the circulating proportion of young platelets (19.0% ± 1.7% to 17.0% ± 1.8%; P < .05) and a corresponding significant increase in the proportion of older platelets remaining unaggregated (80.2% ± 1.8% to 82.9% ± 1.7%; P < .05; Figure 3B) was observed.
Younger platelets disproportionately contribute to thrombotic response. (A) Population tracking in anticoagulated whole blood after stimulation with a range of concentrations (30-100 μM) of PAR4-specific stimulation to drive ex vivo aggregation resulted in decreasing proportions of younger platelets and corresponding increased proportions of older platelets remaining in the nonaggregated single-platelet portion (n = 4). Data are mean ± SEM or individual replicates. (B) Circulating proportions of labeled younger platelets in vivo decreased following PE challenge with corresponding increase in proportions of circulating older platelets. ∗P < .05, ∗∗P < .01 by 2-way analysis of variance with the Dunnett multiple comparison to their respective basal level or paired t test as appropriate.
Younger platelets disproportionately contribute to thrombotic response. (A) Population tracking in anticoagulated whole blood after stimulation with a range of concentrations (30-100 μM) of PAR4-specific stimulation to drive ex vivo aggregation resulted in decreasing proportions of younger platelets and corresponding increased proportions of older platelets remaining in the nonaggregated single-platelet portion (n = 4). Data are mean ± SEM or individual replicates. (B) Circulating proportions of labeled younger platelets in vivo decreased following PE challenge with corresponding increase in proportions of circulating older platelets. ∗P < .05, ∗∗P < .01 by 2-way analysis of variance with the Dunnett multiple comparison to their respective basal level or paired t test as appropriate.
Newly formed platelets are heterogenous in size and do not change size distribution with age
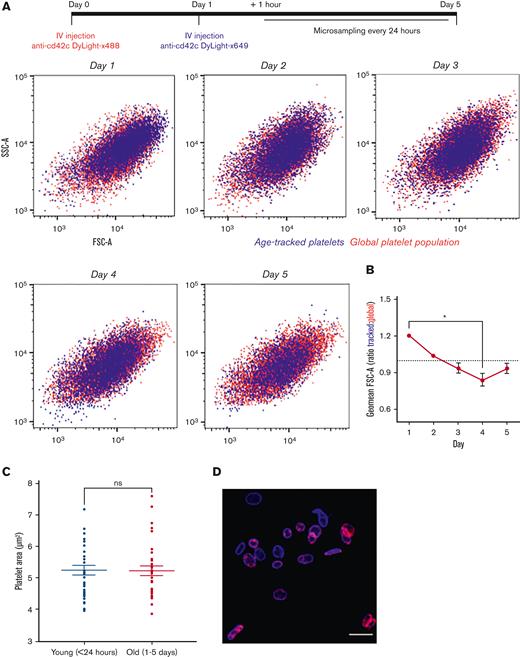
Platelets identified as <24 hours old were heterogenous in size with no discernable difference in size distribution to the global platelet population as determined using cytometry-derived forward scatter (FSC) and side scatter. The same tracked population remained heterogenous in size and commensurate relative to the global population in samples obtained from the same mice across days 2 to 5 (Figure 4A; supplemental Figure 4). Comparison of the calculated geometric mean of FSC values relative to the global population at each time point, indicates that the tracked population decreases in size with age, (ratio 1.2:1 at day 1 vs 0.9:1 at day 4; P < .05; Figure 4B; supplemental Figure 4). However, immunofluorescence performed on fixed samples did not discern a difference in platelet cross-sectional area between young (<24 hours) and older (1-5 days) platelets (5.28 ± 0.13 μm vs 5.24 ± 0.25 μm, respectively; P > .05; Figure 4C-D).
Platelets are heterogenous in size when formed and do not change size with age in the circulation. Following temporal labeling, serial blood samples were obtained each day for 5 days. (A) Representative data of age-tracked platelets (blue) in an individual mouse. Tracked platelets were detectable in circulation for the full 5 days, and distribution of size was comparable with the counterstained global platelet population (red) as determined by flow cytometric FSC and side scatter (SSC) properties. (B) Decrease in ratio of geometric mean FSC of tracked platelet relative to global populations across circulating lifespan (n = 4). (C) Confocal microscopy of fixed platelets in PRP revealed no difference in area of younger and older platelets. (D) Representative image of younger (blue) and older (blue and red) present in analyzed platelet samples isolated from labeled mouse. Data are individual replicates from 4 mice with overlaid mean ± SEM with ∗P < .05 as analyzed by Friedman test with the Dunn multiple comparison test or mean ± SEM with paired t test.
Platelets are heterogenous in size when formed and do not change size with age in the circulation. Following temporal labeling, serial blood samples were obtained each day for 5 days. (A) Representative data of age-tracked platelets (blue) in an individual mouse. Tracked platelets were detectable in circulation for the full 5 days, and distribution of size was comparable with the counterstained global platelet population (red) as determined by flow cytometric FSC and side scatter (SSC) properties. (B) Decrease in ratio of geometric mean FSC of tracked platelet relative to global populations across circulating lifespan (n = 4). (C) Confocal microscopy of fixed platelets in PRP revealed no difference in area of younger and older platelets. (D) Representative image of younger (blue) and older (blue and red) present in analyzed platelet samples isolated from labeled mouse. Data are individual replicates from 4 mice with overlaid mean ± SEM with ∗P < .05 as analyzed by Friedman test with the Dunn multiple comparison test or mean ± SEM with paired t test.
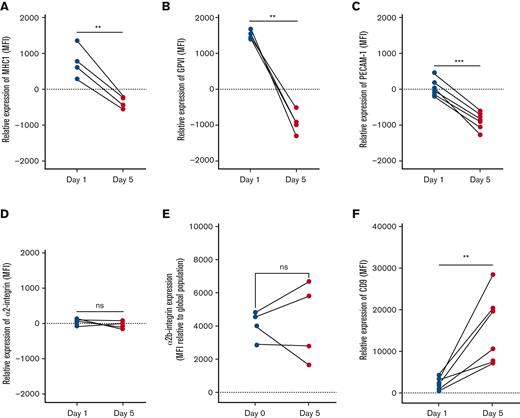
MHC-I expression decreases with platelet age
The expression of major histocompatibility class I (MHC-I) on platelets aged <24 hours was higher than the global median and decreased significantly with age (Figure 5A), equating to a loss of 30% ± 6% across the platelet lifespan.
Differential surface receptor expressions of aging platelets in circulation. Isotype control–corrected expression of receptors on age-tracked platelets, relative to global population expression (represented as 0 on y-axis for normalization), as measured by flow cytometry in samples obtained from individual mice on day 1 (green) and 5 (cyan) after labeling. Between days 1 and 5 of age, significant decreases in expression of MHC-I (A), GPVI (B), and CD31/PECAM-1 (C), but no difference in expression of CD49a/integrin α2 (D) or CD41/integrin α2b (E) was detected, whereas significant increase in CD9 expression (F) was detected. ∗∗P < .01, ∗∗∗P < .001 as analyzed by paired t test.
Differential surface receptor expressions of aging platelets in circulation. Isotype control–corrected expression of receptors on age-tracked platelets, relative to global population expression (represented as 0 on y-axis for normalization), as measured by flow cytometry in samples obtained from individual mice on day 1 (green) and 5 (cyan) after labeling. Between days 1 and 5 of age, significant decreases in expression of MHC-I (A), GPVI (B), and CD31/PECAM-1 (C), but no difference in expression of CD49a/integrin α2 (D) or CD41/integrin α2b (E) was detected, whereas significant increase in CD9 expression (F) was detected. ∗∗P < .01, ∗∗∗P < .001 as analyzed by paired t test.
Platelets lose GPVI and PECAM-1 but increase expression of CD9 with age
GPVI expression was significantly higher on platelets <24 hours old and significantly lower on day 5 when compared with the global medians, a total loss equating to 39% ± 2% (P < .01; Figure 5B).
Expression of PECAM-1 (CD31) significantly decreased between days 1 and 5 (20% ± 3% loss; P < .001; Figure 5C). By contrast, CD9 expression was significantly higher than the global median on day 5 (P < .01; Figure 5F) equating to an increase of 13% ± 4% relative to day 1. No significant changes in expression of integrins α2 (CD49b) or α2b (CD41) were found (Figure 5D-E).
Platelets favor retention of granule protein encoding mRNA
Full data set of mRNA measures are reported in supplemental Table 1 and subject to the following analysis. Principal component analysis of mRNA content demonstrated 3 analyzed populations, younger (<24 hours), older (1-5 days old), and global, clustered individually (Figure 6A). The loading plot of individual mRNAs demonstrated a unidirectional pattern for PC1 indicating that a decreasing abundance of mRNAs likely predominates this algorithm. Interestingly, however, mRNAs also appeared to cluster separately according to PC2, indicating potential for individual patterns of decreased abundance (Figure 6B). Further investigation grouping mRNAs by function, namely signaling, structural, receptors, and granules, suggested a reduction in abundance associated with age, with platelets <24 hours old having the highest and platelets 1 to 5 days old having the lowest amounts of each mRNA (Figure 6C). However, the amount of loss varied by specific mRNA (median Δ Cq range, −2.31 to −5.85). These changes were not contingent on starting levels (Figure 6C), but there was some association with functional categorization. In particular, mRNAs classified as associated with signaling proteins demonstrated the greatest reduction in abundance, whereas mRNAs associated with granule proteins demonstrated the greatest retention (median Cq change, −4.87 vs −2.99, respectively).
Platelet mRNA content decreases with age but differential loss according to function of encoded protein. Younger, older, and total platelets (2.5 million per population) were isolated by fluorescence-activated cell sorting and mRNA extracted for comparative analysis of 25 highly abundant transcripts. (A) Principal component analysis of samples resulted in individual clustering of samples according to age. (B) Loading plot analysis depict unidirectional relationship for principal component 1 (PC1) but differential PC2 relationship. (C) Grouping of measured mRNAs by downstream function and mRNA level (quantification cycle [Cq]) analysis illustrates variable content of individual mRNAs (green) but with universal loss from highest levels in younger (blue) to lowest levels in older (red) platelets. (D) Calculated change (Δ Cq) from younger to older populations reveals differential scales of loss within groupings with mRNAs associated with encoding granule proteins experiencing greatest retention. Data are mean ± SEM (n = 4).
Platelet mRNA content decreases with age but differential loss according to function of encoded protein. Younger, older, and total platelets (2.5 million per population) were isolated by fluorescence-activated cell sorting and mRNA extracted for comparative analysis of 25 highly abundant transcripts. (A) Principal component analysis of samples resulted in individual clustering of samples according to age. (B) Loading plot analysis depict unidirectional relationship for principal component 1 (PC1) but differential PC2 relationship. (C) Grouping of measured mRNAs by downstream function and mRNA level (quantification cycle [Cq]) analysis illustrates variable content of individual mRNAs (green) but with universal loss from highest levels in younger (blue) to lowest levels in older (red) platelets. (D) Calculated change (Δ Cq) from younger to older populations reveals differential scales of loss within groupings with mRNAs associated with encoding granule proteins experiencing greatest retention. Data are mean ± SEM (n = 4).
Discussion
We have developed an in vivo labeling approach, which allows for age-related subpopulations of platelets to be identified and tracked. This technique can be coupled with a range of functional and molecular analyses to provide unique insights into platelet age–associated physiology. Here, we have used it to address the 2 fundamental assumptions that newly formed platelets are larger and more reactive. Furthermore, we have used this new approach to gain insights into changes in the expression of platelet receptors and the retention of mRNA during aging that may underpin alterations in their functionality.
The nature of the relationship among platelet age, size, and reactivity has remained contentious for many years. Perhaps the point of least contention is that mRNA-rich “young” platelets are more reactive, with a raft of literature over the years using in vitro, ex vivo, and in vivo studies supporting this hypothesis.14,21 Indeed, our recent studies identifying platelet age through RNA labeling of healthy human platelets are consistent with a decline in hemostatic function as platelets age. We demonstrated that young platelets express higher levels of P-selectin and have an enhanced calcium signaling capacity.4,6 Supporting our work in healthy human blood, our temporal labeling approach confirmed that the youngest platelets have enhanced degranulation and calcium signaling in response to a range of agonists. Conversely, after stimulation, older platelets have increased levels of the activated GPIIb/IIIa receptor, indicating a higher propensity for fibrinogen binding; however, the functional significance of this remains to be determined. Despite this, ex vivo aggregation studies and an in vivo PE thrombosis model demonstrate that under healthy turnover conditions, young platelets contribute highly and disproportionately to forming thrombi.
A factor that continues to confound our understanding of why young platelets are more reactive or have higher thrombotic potential is the enduring dogma that young platelets are larger. Originally proposed in the 1960s,22 this hypothesis posits that younger platelets have greater volume resulting in greater granule content and greater surface area resulting in greater surface receptor number. In support of this hypothesis, in vivo platelet studies in a range of species after pathological insult such as platelet depletion have observed that the subsequently produced platelets are larger in size.23 Similarly, associations have been found between mean platelet volume and immature platelet fraction measurements in patient populations. However, a series of reports in the mid-1980s reported no correlation between platelet age and size.24-26 Similarly, in vivo biotinylation studies in healthy rabbits have shown that younger platelets are no larger than older platelets.27 We therefore coupled our temporal labeling technique to flow cytometry and immunofluorescence to assess the sizes of platelets of different ages. Our data clearly show that in healthy mice, younger and older platelets exist in the same range of sizes throughout their 5-day lifespan in the circulation and therefore cannot be differentiated on the basis of size alone. Notably, the aforementioned in vivo studies that associate younger platelets with larger size are predicated on a pathological insult and the subsequent acute response to restore platelet number. It seems reasonable to conclude that the processes that underpin such an acute response differ from those supporting normal platelet production and release and could produce a different population of platelets. Interestingly, comparison of the average sizes of our tracked and global populations indicates, as measured by flow cytometry, that platelets may decrease to a degree in size during their aging process in the circulation. In our recent studies of human platelets, we observed an association between platelet age and decreased cytoskeletal protein content that may be consistent with small alterations in the FSC measures detected in this study.6 However, such potential alterations in size will require further investigation by other modalities to validate this observation.
Similar research has also been applied to identifying other markers of age. The observation that platelet size does not change substantially with age, coupled with our recent observation of loss of protein content in aging human platelets,6 might support proposals that platelet density is a good marker of platelet age.28 However, the calculation of platelet density is technically challenging and laborious, whereas the measurement of surface molecular markers or receptor expression is much more easily accomplished. Angenieux et al29 recently identified that across the platelet population, young platelets, identified by thiazole orange staining, had the highest levels of MHC-I/HLA-1. In our study using defined temporal labeling, we confirmed this observation and found novel differential expression patterns according to platelet age for several other receptors associated with collagen responsiveness. We identified higher surface expression levels of GPVI and PECAM-1 in younger compared with older platelets, whereas, conversely, the levels of CD9 increased as platelets age in the circulation. The origin of this increase in surface CD9 is unclear, but de novo production or accumulation from exosomes30 are 2 possibilities. Despite its functional role remaining unclear,31 CD9 expression is generally high on platelets32 and it has been shown to localize with GPVI33 and ADAM1034 within tetraspanin-enriched microdomains. Both GPVI and PECAM-1 undergo proteolytic cleavage mediated by ADAM-10 and MMP2, respectively, under physiological and pathological conditions,35-37 and therefore, constitutive or triggered activity may contribute to their decline in surface expression as platelets age. With relevance to the decline in platelet responsiveness associated with aging, a decrease in GPVI expression would correlate with the impaired CRP-XL–induced activation observed in our functional studies.
In addition to changes in receptor expression, we sought to examine the mRNA content of platelets. Young platelets are well accepted as having the highest levels of mRNA and that this mRNA content decreases with time.38 Indeed, the results of our measurement, from the selection of mRNA, would support this view. Platelets have been reported as containing ∼10 000 different mRNA transcripts39 and that this mRNA has a half-life of ∼6 hours.38,40 We wished to examine whether this loss of mRNA was universal using our temporal labeling approach. Unbiased principal component analysis confirmed that young platelets had higher overall levels of mRNA but also revealed a differential reduction in specific platelet mRNAs. Further analysis revealed the rate of loss for mRNAs to vary greatly, with those encoding granule proteins experiencing the least reduction. This variation in rates of loss strongly suggests that previous reports of 6 hours as the decay half-life of platelet mRNA38,40 is a general number, whereas the decay of particular mRNAs is under more refined control. The molecular nature and purpose of this apparent conservation will be important to elucidate, but research by Mills et al40 has indicated that ribosomes protect mRNA through binding, which may enhance specific translation. Following our observation that mRNAs that encode granule proteins are particularly conserved, it is possible to hypothesize that this will support their specific protein synthesis. Having been reported for over 50 years,41 de novo protein synthesis in platelets, particularly in response to activation,42-44 is not a new concept. However, owing to the technically challenging nature of these studies, much interrogation of this process has occurred in an ex vivo setting and questions remain over the quantity of proteins produced45 and their physiological and/or pathophysiological significance. Our labeling approach will help to unravel whether such de novo synthesis is part of a maturation step of platelets in vivo or following reports of selective protein packaging and release of granular contents and extracellular vesicles,46-48 whether selective retention of granular mRNA for protein synthesis prolongs their period of reactivity.
In summary, we present a novel approach for discovering age-related alterations in platelet form and function. Our approach has discovered changes in platelet GPVI, PECAM-1, and CD9 expression as platelets age in the circulation and provides insights into differential regulation and retention of platelet mRNAs. These changes may well underpin alterations in platelet functionality during the aging process. Therefore, this study reports novel insights into fundamental assumptions of platelet size and reactivity as they age in the circulation and provides an approach that will act as an important tool for future research.
Acknowledgments
This work was supported by the British Heart Foundation (RG/19/8/34500) (T.D.W. and P.C.A.), (FS/18/60/34181) (C.G.), and (FS/16/31699) (N.S.K.) and the Wellcome Trust (101604/Z/13/Z).
Authorship
Contribution: P.C.A. designed the research, performed the assays and collected data, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; H.E.A. performed assays, analyzed and interpreted the data, performed statistical analysis, and revised the manuscript; C.G. and A.J. performed analysis and collected and analyzed data; M.C. assisted with the assays and collecting data; and N.S.K., J.A.M., M.M., and T.D.W. analyzed and interpreted the data and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul C. Armstrong, Centre for Immunobiology, Blizard Institute, Faculty of Medicine and Dentistry, Queen Mary University of London, 4 Newark St, London E1 2AT, United Kingdom; e-mail: p.c.armstrong@qmul.ac.uk.
References
Author notes
Data are available on request from the corresponding author, Paul C. Armstrong (p.c.armstrong@qmul.ac.uk).
The full-text version of this article contains a data supplement.

![Differential degranulation, integrin activation, and calcium flux of young platelets compared with older platelets. Younger (<24 hours old) platelets express higher levels of P-selectin (A) but lower levels of active GPIIb/IIIa (B) in response to PAR4-specific stimulation compared with corresponding older platelets in the same sample. Younger platelets have a greater calcium flux (measured as area under the curve [AUC]) in response to PAR4-specific stimulation (C) and ionomycin (D) (n = 5). (C) Data are individual replicates or mean ± SEM. ∗∗P < .01, ∗∗∗P < .001 by paired t test as indicated. ns, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/6/23/10.1182_bloodadvances.2022007099/5/m_blooda_adv-2022-007099-gr2.jpeg?Expires=1769875680&Signature=paCAewTBnjdxGsDeEagvFWALFgj4Kx1sBXPyzBUZls8cbDf5I0je8DEWXzbx4b8W5qkhlou9vJd~Ek1ozLWtk2FOWoWVNgcyQXVrt4qTyfHUi0L9HwjLd9ZJ9vhnblVKXIbsmMnnkVMxDugoXdkjWCF3mTJJB9tOYjX0drBtecegwpzH9X3DjDlE~GaqnlCZXpBQLbVOYVUAi-wqZ3QmW1gK5webmJ0ovKuv~JnhLbdiQlD1nC~Dp286C9asTMMWVrzNueJKkBlVrqABGU009ZxjYw5DWUncXNBsGu9ukMnRk-4A-tbsGyCFh0OXwu-JkP-c8k6b1C5afiHJ0ztnSw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Platelet mRNA content decreases with age but differential loss according to function of encoded protein. Younger, older, and total platelets (2.5 million per population) were isolated by fluorescence-activated cell sorting and mRNA extracted for comparative analysis of 25 highly abundant transcripts. (A) Principal component analysis of samples resulted in individual clustering of samples according to age. (B) Loading plot analysis depict unidirectional relationship for principal component 1 (PC1) but differential PC2 relationship. (C) Grouping of measured mRNAs by downstream function and mRNA level (quantification cycle [Cq]) analysis illustrates variable content of individual mRNAs (green) but with universal loss from highest levels in younger (blue) to lowest levels in older (red) platelets. (D) Calculated change (Δ Cq) from younger to older populations reveals differential scales of loss within groupings with mRNAs associated with encoding granule proteins experiencing greatest retention. Data are mean ± SEM (n = 4).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/6/23/10.1182_bloodadvances.2022007099/5/m_blooda_adv-2022-007099-gr6.jpeg?Expires=1769875680&Signature=ZgeTWbLK239xTnlTdP9t7jI92fNkBR4Do4gAFGw~fFkNRwa8WO~XbgsET8sN9nAyl~2MM41RtDrV3os09fn~VIm8R6veNcyuhLkqlirMAkjUaW9iq53N9J-gYU3iwJbKUI4q3FOUf6IHLaGJu72NIVTIsSxWy753o~vJGtu~mBtAoQy6n-HnA-eEEBFgYWyAn9TUh0~~hP3UirEpaTpy8p~BV9YrbfYyTNsxhD6Rxd2KqfXCzuELak-VDCwAwMNyGNnJ~EdGabSsZET8wU-iBbyO0UwdNXmxUmxpuFAyBHNGr8K464Bs-YL~AY6ij89U9USMQmjg5InUKCMmXqM0Kg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)