Key Points
Pembrolizumab improved health-related quality of life over brentuximab vedotin in patients with relapsed/refractory cHL
Pembrolizumab should be considered the preferred treatment option for relapsed/refractory cHL post-ASCT or in patients ineligible for ASCT
Abstract
KEYNOTE-204 (NCT02684292) demonstrated a progression-free survival advantage for pembrolizumab over brentuximab vedotin (BV) in patients who had relapsed or refractory classical Hodgkin lymphoma (R/R cHL) following, or who were ineligible for, autologous stem cell transplantation (ASCT). Health-related quality of life (HRQoL), measured by patient-reported outcomes (PROs) from KEYNOTE-204, are reported from patients who received ≥1 dose of study treatment and completed ≥1 PRO assessment. The EORTC QoL Questionnaire Core 30 (QLQ-C30) and EuroQoL EQ-5D were administered at baseline, every 6 weeks until week 24, and every 12 weeks thereafter. Prespecified end points included least squares mean (LSM) changes from baseline to week 24 and time to true deterioration (TTD; ≥10-point decline from baseline). Comparisons were evaluated using 2-sided P values uncontrolled for multiplicity. High compliance at baseline (>90%) and through week 24 (>80%) was demonstrated across treatment groups (PRO analysis set: pembrolizumab, n = 146; BV, n = 150). The EORTC QLQ-C30 global health status (GHS)/quality of life (QoL) score improved from baseline to week 24 on pembrolizumab and worsened on BV and demonstrated significant LSM differences at 24 weeks (GHS/QoL: 8.60 [95% confidence interval, 3.89-13.31]; P = .0004). Significant improvements were observed in each QLQ-C30 domain except emotional and cognitive functioning. Compared with BV, pembrolizumab prolonged TTD for GHS/QoL (hazard ratio, 0.40 [95% CI, 0.22-0.74]; P = .003) and each QLQ-C30 domain except cognitive functioning. In conclusion, pembrolizumab demonstrated overall improvements in PROs of HRQoL measures over BV in the KEYNOTE-204 study. These data and previously reported efficacy results support pembrolizumab as the preferred treatment option for patients with R/R cHL who are ineligible for or experience relapse after ASCT.
Introduction
Prognosis is poor in patients with relapsed or refractory classical Hodgkin lymphoma (R/R cHL), particularly those who have failed or are ineligible for autologous stem cell transplantation (ASCT).1-3 Health-related quality of life (HRQoL) for patients requiring additional therapy can be adversely affected by treatments; for example, after first-line treatment failure, subsequent rounds of chemotherapy for R/R cHL are associated with reduced quality of life (QoL) according to patient-reported outcomes (PROs).4 The antitumor activity and tolerable safety profiles demonstrated by immunotherapy options represent promising earlier-line treatment options in the R/R disease setting, and PROs may be used to assess risk-benefit profiles among emerging treatment strategies.
PROs include subjective measures of HRQoL independent of clinical evaluation and can be used to weigh burden of disease and determine overall treatment effects (ie, tolerability and disease control) that impact daily life.5 PROs provide patient-based assessments that balance a patient's experience of treatment-related adverse events (safety) and relief from disease-associated processes (efficacy). The resultant data can inform patients, caregivers, providers, regulatory authorities, and payers alike of the value of a treatment. Reports of PROs in cHL, however, are lacking, particularly those taken prospectively in clinical trials during the active treatment phase.6
Brentuximab vedotin (BV), an antibody–drug conjugate targeting CD30, has become an established standard as second-line therapy, either alone or with additional immunotherapy or chemotherapy, following ASCT or as subsequent-line therapy following ≥2 prior chemotherapy regimens in those ineligible for ASCT, despite not yet being approved by the FDA for this indication.7,8 In the pivotal phase 3 AETHERA study, patients receiving consolidation BV treatment following ASCT achieved a sustained progression-free survival (PFS) rate of 59% (vs 41% on placebo) with 5 years of follow-up.9 However, prespecified PROs in these BV-treated patients demonstrated a decline in QoL measures during treatment based on 2 years of follow-up.10 Thus, there is a need for effective treatment that does not impair QoL for patients with R/R cHL.
Several cancer types can evade immune system detection by upregulating expression of programmed cell death 1 (PD-1) or its ligand (PD-L1), and cHL is characterized by alterations in chromosome 9p24.1 that cause overexpression of these proteins.11,12 Two anti–PD-1 monoclonal antibody therapies have been approved to treat cHL: nivolumab and pembrolizumab.13,14 After a median follow-up of 18 months in the single-arm CheckMate-205 study, nivolumab demonstrated an objective response rate (ORR) of 69%, with a complete response rate of 16%.15 Similarly, in the single-arm KEYNOTE-087 study, the ORR to pembrolizumab after a median follow-up of 27.6 months was 71.9%, with a complete response rate of 27.6%.16 Recently, pembrolizumab showed greater antitumor activity than BV in an international, randomized, open-label, phase 3 study for patients with R/R cHL (KEYNOTE-204; NCT02684292), significantly reducing risk of progression/death by 35% and demonstrating clinically meaningful improvements in ORR and duration of response.17 Pembrolizumab was also associated with fewer treatment-related adverse events (AEs) overall, grade 3 to 5 treatment-related AEs, and discontinuations due to AEs (including drug-related) than BV. Additionally, in the KEYNOTE-087 study, patients treated with pembrolizumab experienced overall improvement and/or maintenance in their health status, function, and symptoms over time as determined by the EuroQoL EQ-5D-3L (EQ-5D) and the European Organization for Research and Treatment of Cancer (EORTC) QoL Questionnaire Core 30 (QLQ-C30).18 Taken together with the positive efficacy and safety outcomes from KEYNOTE-204,17 we predict that PROs would reflect an overall improvement in HRQoL with pembrolizumab compared with BV.
Methods
Study design
KEYNOTE-204 was an international, randomized, open-label, phase 3 study that compared the efficacy and safety of pembrolizumab with that of BV in cHL. Key eligibility criteria and study treatments are described in the supplemental Appendix.17
All patients provided written informed consent. The study was approved by the independent institutional review board at each study site and conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki.
PRO assessments
PROs of health-related QoL measures were assessed using 2 PRO questionnaires: the EQ-5D and the EORTC QLQ-C30 (supplemental Appendix). Questionnaires were administered electronically at week 1, every 6 weeks up to week 24, every 12 weeks up to 1 year during treatment (or until disease progression), and again at discontinuation and at the 30-day safety follow-up. Questionnaires were administered before drug administration, AE evaluation, and disease status notification in the following order: EQ-5D then QLQ-C30.
Outcomes and statistical analyses
The PRO analysis population included all randomly assigned patients who received at least 1 dose of study treatment and completed at least 1 PRO assessment. Patients were considered to have completed at least 1 PRO assessment if they completed at least 1 item on a PRO instrument according to the missing item rules outlined in the QLQ-C30 and EQ-5D manuals. Completion rate was defined as the proportion of participants who completed at least 1 assessment over the number of participants in the PRO analysis population. Compliance rate was defined as the proportion of participants who completed at least 1 assessment over the number of eligible participants who were expected to complete the PRO assessment, excluding the participants missing by design (eg, because of death, discontinuation, unavailablity of translation services, or no scheduled visit).
Key PRO end points were the mean score changes from baseline to a prespecified week 24 in QLQ-C30 global health status (GHS)/QoL and functioning scale scores as well as EQ-5D visual analog scale (VAS) and utility scores. These were analyzed using a constrained longitudinal data analysis model, with PRO scores as the response variable and treatment by study visit interaction and stratification factors at randomization as covariates. The constrained longitudinal data analysis model implicitly treats missing data as missing at random.
Supportive PRO end points were also analyzed. This included the proportions of patients with deteriorated, stable, or improved scores at week 24 from baseline on the QLQ-C30 GHS/QoL and functional scales: ≥10-point decreases indicated deteriorated scores, change of <10 points indicated stable scores, and ≥10-point increases indicated improved scores, in alignment with the magnitudes of clinically meaningful changes based on earlier observations using these metrics.19,20 These end points were summarized with the use of least squares mean (LSM) for the primary analysis as prespecified in the statistical analysis plan. In this study with expected missing data, particularly for patients experiencing disease progression or decline in patient-reported outcomes because of drug-related AEs, the use of LSM was preferred over standard arithmetic mean. Missing data were based on missing at random imputation to obtain valid statistical inference. The between-group difference in improvement rates in QLQ-C30 GHS/QoL and physical functioning scales was evaluated using the stratified Miettinen and Nurminen method. Time to true deterioration (TTD) was defined as the time to first onset of a ≥10-point decrease from baseline on the QLQ-C30 GHS/QoL and functioning scales, with confirmation using the right-censoring rule. The between-group difference was estimated using a stratified log-rank test, with the hazard ratio (HR) determined using a stratified Cox model with treatment as a covariate.
All PRO analyses were exploratory and not adjusted for multiplicity. Nominal P values presented were 2-sided. The database cutoff date for these analyses was 16 January 2020.
Results
Completion and compliance rates of PRO questionnaires
A total of 304 patients were randomly assigned (pembrolizumab, n = 151; BV, n = 153) and 296 patients (pembrolizumab, n = 146; BV, n = 150) were included in the PRO analysis population (received at least 1 treatment and completed at least 1 PRO assessment). Baseline characteristics and patient disposition from the total patient population had been described previously.17
Both the pembrolizumab and the BV treatment groups had high (>90%) completion rates at baseline for QLQ-30 and EQ-5D questionnaires (Table 1). Compliance rates for QLQ-C30 and EQ-5D were comparable and high at baseline (>90%) and at week 24 (≥80%) for both treatment groups (Table 1). Completion rates decreased at each time point as participants discontinued treatment, primarily because of disease progression.
Compliance and completion rates for the EORTC QLQ-C30 and EuroQoL EQ-5D
| . | QLQ-C30 . | EQ-5D . | ||
|---|---|---|---|---|
| Pembrolizumab n = 146 . | BV n = 150 . | Pembrolizumab n = 146 . | BV n = 150 . | |
| Baseline | ||||
| Completion | 134 (91.8) | 138 (92.0) | 135 (92.5) | 140 (93.3) |
| Compliance | 134/146 (91.8) | 138/150 (92.0) | 135/146 (92.5) | 140/150 (93.3) |
| Week 6 | ||||
| Completion | 139 (95.2) | 138 (92.0) | 139 (95.2) | 138 (92.0) |
| Compliance | 139/146 (95.2) | 138/149 (92.6) | 139/146 (95.2) | 138/149 (92.6) |
| Week 12 | ||||
| Completion | 132 (90.4) | 126 (84.0) | 133 (91.1) | 126 (84.0) |
| Compliance | 132/143 (92.3) | 126/142 (88.7) | 133/143 (93.0) | 126/142 (88.7) |
| Week 18 | ||||
| Completion | 115 (78.8) | 90 (60.0) | 115 (78.8) | 90 (60.0) |
| Compliance | 115/129 (89.1) | 90/111 (81.1) | 115/129 (89.1) | 90/111 (81.1) |
| Week 24 | ||||
| Completion | 103 (70.5) | 68 (45.3) | 103 (70.5) | 69 (46.0) |
| Compliance | 103/120 (85.8) | 68/85 (80.0) | 103/120 (85.8) | 69/85 (81.2) |
| Week 36 | ||||
| Completion | 91 (62.3) | 45 (30.0) | 91 (62.3) | 45 (30.0) |
| Compliance | 91/106 (85.8) | 45/58 (77.6) | 91/106 (85.8) | 45/58 (77.6) |
| Week 48 | ||||
| Completion | 74 (50.7) | 27 (18.0) | 75 (51.4) | 27 (18.0) |
| Compliance | 74/85 (87.1) | 27/35 (77.1) | 75/85 (88.2) | 27/35 (77.1) |
| . | QLQ-C30 . | EQ-5D . | ||
|---|---|---|---|---|
| Pembrolizumab n = 146 . | BV n = 150 . | Pembrolizumab n = 146 . | BV n = 150 . | |
| Baseline | ||||
| Completion | 134 (91.8) | 138 (92.0) | 135 (92.5) | 140 (93.3) |
| Compliance | 134/146 (91.8) | 138/150 (92.0) | 135/146 (92.5) | 140/150 (93.3) |
| Week 6 | ||||
| Completion | 139 (95.2) | 138 (92.0) | 139 (95.2) | 138 (92.0) |
| Compliance | 139/146 (95.2) | 138/149 (92.6) | 139/146 (95.2) | 138/149 (92.6) |
| Week 12 | ||||
| Completion | 132 (90.4) | 126 (84.0) | 133 (91.1) | 126 (84.0) |
| Compliance | 132/143 (92.3) | 126/142 (88.7) | 133/143 (93.0) | 126/142 (88.7) |
| Week 18 | ||||
| Completion | 115 (78.8) | 90 (60.0) | 115 (78.8) | 90 (60.0) |
| Compliance | 115/129 (89.1) | 90/111 (81.1) | 115/129 (89.1) | 90/111 (81.1) |
| Week 24 | ||||
| Completion | 103 (70.5) | 68 (45.3) | 103 (70.5) | 69 (46.0) |
| Compliance | 103/120 (85.8) | 68/85 (80.0) | 103/120 (85.8) | 69/85 (81.2) |
| Week 36 | ||||
| Completion | 91 (62.3) | 45 (30.0) | 91 (62.3) | 45 (30.0) |
| Compliance | 91/106 (85.8) | 45/58 (77.6) | 91/106 (85.8) | 45/58 (77.6) |
| Week 48 | ||||
| Completion | 74 (50.7) | 27 (18.0) | 75 (51.4) | 27 (18.0) |
| Compliance | 74/85 (87.1) | 27/35 (77.1) | 75/85 (88.2) | 27/35 (77.1) |
Data are n (%) unless otherwise specified.
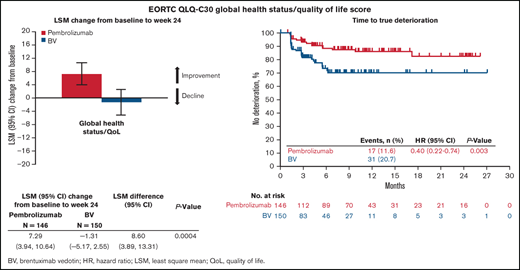
Change from baseline in PROs
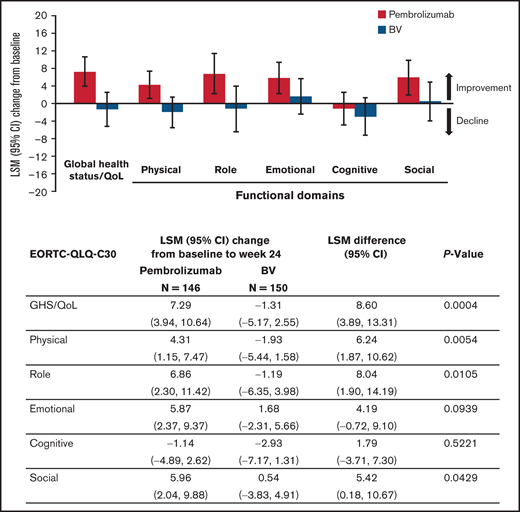
QLQ-C30 GHS/QoL scores at baseline were similar between the treatment groups (Table 2). At week 24, GHS/QoL scores had improved from baseline for patients receiving pembrolizumab (LSM 7.29 [95% confidence interval [CI], 3.94-10.64]) compared with worsening for those receiving BV (−1.31 [95% CI, −5.17 to 2.55]). A statistically significant difference of 8.60 points in LSMs between pembrolizumab and BV at week 24 (95% CI, 3.89-13.31; 2-sided nominal P = .0004 not controlled for multiplicity) was observed (Table 2). Pembrolizumab was associated with improvements at week 24 in each domain of the QLQ-C30 except cognitive functioning, which did not change substantially from baseline (Figure 1). In contrast, BV showed worsening in all QLQ-C30 domains except social and emotional functioning. Significant improvements in domains were observed with the exception of emotional and cogitative (Figure 1). Notably, pembrolizumab demonstrated mean improvement in QLQ-C30 GHS/QoL from baseline to week 24 compared with BV regardless of patient response to treatment, with a difference that did not appear to be statistically significant for those with response (5.10 [−2.53 to 12.73]; 2-sided nominal P = .187), and significant improvement in nonresponders (11.76 [5.66-17.86]; 2-sided nominal P = .0002). Furthermore, pembrolizumab significantly improved utility and VAS scores compared with BV (Table 2).
Change from baseline to week 24 in QLQ-C30 GHS/QoL and EQ-5D VAS and utility scores
| . | QLQ-C30 GHS/QoL score . | EQ-5D VAS score . | EQ-5D utility score . | |||
|---|---|---|---|---|---|---|
| Pembrolizumab n = 146 . | BV n = 150 . | Pembrolizumab n = 146 . | BV n = 150 . | Pembrolizumab n = 146 . | BV n = 150 . | |
| Baseline score, mean (SD) | 68.2 (18.1) | 67.0 (20.2) | 71.3 (17.9) | 71.2 (17.9) | 0.79 (0.19) | 0.76 (0.20) |
| Week 24, mean score (SD) | 76.5 (16.9) | 69.1 (17.1) | 80.5 (15.1) | 76.9 (15.5) | 0.83 (0.17) | 0.76 (0.18) |
| Change from baseline | ||||||
| LSM change from baseline (95% CI) | 7.29 (3.94-10.64) | −1.31 (−5.17 to 2.55) | 8.53 (5.42-11.64) | 2.41 (−1.05 to 5.87) | 0.04 (0.00-0.08) | −0.05 (−0.09 to −0.01) |
| LSM difference (95% CI) | 8.60 (3.89-13.31); P = .0004 | 6.12 (1.91-10.34); P = .0046 | 0.09 (0.04-0.14); P = .0004 | |||
| . | QLQ-C30 GHS/QoL score . | EQ-5D VAS score . | EQ-5D utility score . | |||
|---|---|---|---|---|---|---|
| Pembrolizumab n = 146 . | BV n = 150 . | Pembrolizumab n = 146 . | BV n = 150 . | Pembrolizumab n = 146 . | BV n = 150 . | |
| Baseline score, mean (SD) | 68.2 (18.1) | 67.0 (20.2) | 71.3 (17.9) | 71.2 (17.9) | 0.79 (0.19) | 0.76 (0.20) |
| Week 24, mean score (SD) | 76.5 (16.9) | 69.1 (17.1) | 80.5 (15.1) | 76.9 (15.5) | 0.83 (0.17) | 0.76 (0.18) |
| Change from baseline | ||||||
| LSM change from baseline (95% CI) | 7.29 (3.94-10.64) | −1.31 (−5.17 to 2.55) | 8.53 (5.42-11.64) | 2.41 (−1.05 to 5.87) | 0.04 (0.00-0.08) | −0.05 (−0.09 to −0.01) |
| LSM difference (95% CI) | 8.60 (3.89-13.31); P = .0004 | 6.12 (1.91-10.34); P = .0046 | 0.09 (0.04-0.14); P = .0004 | |||
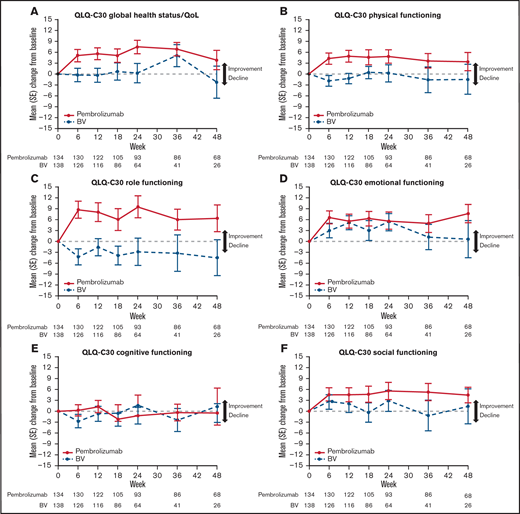
Although BV-treated patients reported little change over time across domains, pembrolizumab-treated patients reported improved empirical mean changes in QLQ-C30 domains (global and 5 functional domains) beginning at week 6 and lasting through week 24 (except again in the cognitive functioning domain, which was unchanged) (Figure 2). The 2 treatment groups diverged most notably in the role functioning domain, with pembrolizumab-treated patients attaining improved and sustained scores beginning at week 6 and scores worsening in BV-treated patients (Figure 2D).
(A–F) QLQ-C30 empirical mean change from baseline over 48 weeks. SE, standard error.
(A–F) QLQ-C30 empirical mean change from baseline over 48 weeks. SE, standard error.
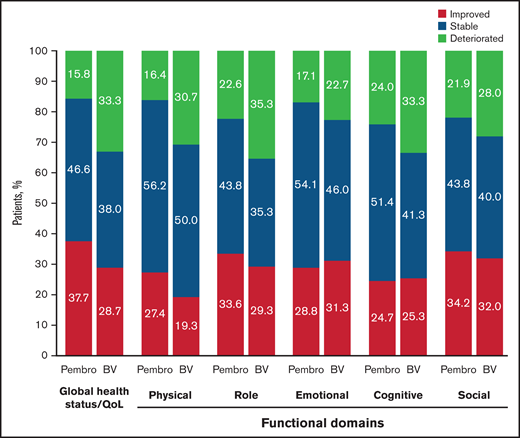
Clinically meaningful differences between pembrolizumab and BV were observed for patient changes in QLQ-C30 domains (Figure 3). A higher proportion of pembrolizumab-treated patients than BV-treated patients demonstrated clinically meaningful improvement at week 24 in GHS/QoL, physical functioning, role functioning, and social functioning. This difference was significant for GHS/QoL (difference between proportions with improvement [95% CI], 9.5% [0.1-18.9]; 2-sided nominal P = .024) and physical functioning (11.3% [2.3-20.3]; 2-sided nominal P = .007). When the analysis population was expanded to include patients with either clinically meaningful improvement or stability of PROs, pembrolizumab-treated patients still showed significant advantage in GHS/QoL (25.3% [14.4-35.6]; 2-sided nominal P < .001) and physical functioning (18.6 [8.0-29.0]; 2-sided nominal P < .001) compared with BV-treated patients.
Improved/stable/worsening of QLQ-C30 scores at week 24. Pembro, pembrolizumab.
TTD of PROs
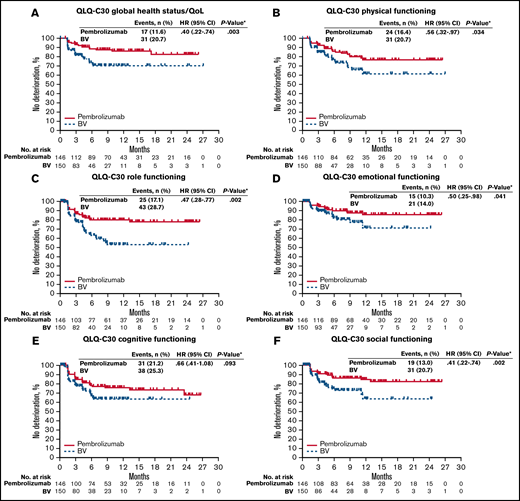
Consistent with the significant LSM changes in PROs from baseline compared with BV, pembrolizumab significantly prolonged TTD in the QLQ-C30 GHS/QoL score (HR, 0.40; 95% CI, 0.22-0.74; 2-sided nominal P = .003). TTD was significantly prolonged in each domain, except cognitive functioning, for patients treated with pembrolizumab compared with BV (Figure 4).
Kaplan-Meier estimates of time to true deterioration. (A) QLQ-C30 global health status/QoL. (B) QLQ-C30 physical functioning. (C) QLQ-C30 role functioning. (D) QLQ-C30 emotional functioning. (E) QLQ-C30 cognitive functioning. (F) QLQ-C30 social functioning. Time to true deterioration is defined as the time to first onset of 10 or more decrease from baseline with confirmation under right-censoring rule (the last observation). *Two-sided P value based on log-rank test.
Kaplan-Meier estimates of time to true deterioration. (A) QLQ-C30 global health status/QoL. (B) QLQ-C30 physical functioning. (C) QLQ-C30 role functioning. (D) QLQ-C30 emotional functioning. (E) QLQ-C30 cognitive functioning. (F) QLQ-C30 social functioning. Time to true deterioration is defined as the time to first onset of 10 or more decrease from baseline with confirmation under right-censoring rule (the last observation). *Two-sided P value based on log-rank test.
Discussion
First-line treatment of cHL is curative in ∼85% of cases,21 but because of the poor prognosis associated with subsequent salvage treatments,22 along with accumulating toxicity that occurs with subsequent rounds of chemotherapy,4 the management of patients with R/R cHL is noncurative except in the subset of patients with stem cell transplantation. HRQoL is an important consideration for the management of patients with R/R cHL because clinicians must select the treatment regimen with the appropriate risk/benefit profile for each setting and each patient. The KEYNOTE-204 study of pembrolizumab monotherapy in patients with R/R cHL was positive from an efficacy standpoint, with a significant PFS benefit over BV monotherapy (13.2 months vs 8.3 months; median follow-up, 25.7 months).17 However, given that exposure to treatment was significantly longer with pembrolizumab than with BV in this study,17 it becomes critical to highlight the impact of pembrolizumab on patients through the use of HRQoL assessments while patients are receiving treatment.
In these exploratory analyses of PROs from KEYNOTE-204, pembrolizumab improved HRQoL among patients and prolonged TTD compared with BV. Patients treated with pembrolizumab reported improvements in QLQ-C30 GHS/QoL and physical functioning scores and EQ-5D VAS and utility scores from baseline to week 24 compared with BV. Furthermore, a positive effect was seen in QLQ-C30 GHS/QoL scores regardless of disease status in this study, consistent with results from KEYNOTE-087, in which QLQ-C30 GHS/QoL and EQ-5D VAS scores were improved or stable in ≥75% of patients receiving pembrolizumab from baseline to week 24 regardless of response to treatment.18 Notably, at the prespecified analysis time point of 24 weeks in the present study, 84.3% of patients receiving pembrolizumab had improved or stable QLQ-C30 GHS/QoL scores from baseline (vs 132/151 or 87% from KEYNOTE-087),18 and 83.6% had improved or stable QLQ-C30 physical functioning scores (Figure 3).
The favorable PRO data for pembrolizumab in cHL are consistent with data for other studies of anti–PD-1 therapy. Pembrolizumab has demonstrated improvements in PROs compared with chemotherapy in non–small cell lung cancer, urothelial cancer, and melanoma.23-25 Furthermore, improvement in PROs in the present study is supported by results from the CheckMate-205 study of patients with cHL whose ASCT therapy failed; in CheckMate-205, patients receiving anti–PD-1 therapy with nivolumab demonstrated clinically meaningful improvements in mean EQ-5D VAS at week 9 and trends toward improvements across HRQoL metrics while on treatment.26 In a separate observational study of patients receiving third-line or later treatment of R/R cHL, nivolumab evoked meaningful increases in functioning scores and reductions in symptom burden during treatment based on RAND Short Form-36 and EQ-5D PROs.27 A recent real-world study of patients with advanced melanoma determined from changes in QLQ-C30 GHS/QoL and EQ-VAS scores that pembrolizumab improved 24-week HRQoL over ipilimumab and nivolumab combination therapy.28
As in the present study, the AETHERA cHL study also reported decreased HRQoL with BV treatment, reaching clinical significance beginning 15 months after treatment start.10 Of note, even though patients remained on pembrolizumab approximately twice as long as on BV,17 pembrolizumab treatment still improved HRQoL in this study.
Ongoing use of PROs to evaluate treatments may help clinicians determine the optimal use of available therapies with the goals of maintaining or improving HRQoL for patients. HRQoL is an important determinant for the management of disease, and a treatment with favorable HRQoL should be emphasized earlier in the disease course over treatments with less favorable HRQoL.
The present analyses were limited by the open-label trial design (which could have influenced responses from patients) and the lack of formal hypothesis testing for HRQoL end points. However, oncology clinical trials are increasingly single-arm or open-label comparative studies and include PRO measures, and evidence regarding the meaningfulness or degree of potential bias in them varies.29,30 Data from the present study suggest that bias attributed to the open-label design was not observed, at least not at the time of trial initiation, because the treatment arms had similar baseline compliance rates and mean scores for QLQ-C30 and EQ-5D. High rates of compliance also observed for QLQ-C30 and EQ-5D across all time points support the feasibility of reliably collecting these data in clinical trials. Furthermore, the consistency between PRO results and the positive clinical efficacy end point (PFS) for pembrolizumab-treated patients in KEYNOTE-204 indicate that these measures are not at odds with determining treatment benefit in cHL.17 PROs at later time points will be necessary to assess the effects of longer-term treatment on HRQoL.
The results presented here support the usefulness of PROs in determining clinically meaningful differences in patients with R/R cHL and suggest that pembrolizumab treatment is associated with better HRQoL than BV treatment. The PRO data from this study, along with the significant improvement in PFS and the clinically meaningful improvements in ORR and duration of response over BV, suggest that pembrolizumab should be considered the preferred treatment option for patients with R/R cHL who have experienced relapse following ASCT or who are ineligible for ASCT.
Acknowledgments
The authors thank the patients and their families, all investigators and site personnel integral to the study's completion, and Patricia Marinello (employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA) for contributions to the development of the study.
Medical writing and editorial assistance were provided by Eileen McIver and Matthew Grzywacz of ApotheCom (Yardley, PA, USA). This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. This work was supported by research funding from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Authorship
Contribution: P.L.Z. analysis of the data, interpretation of the results, and critically reviewing or revising the manuscript for important intellectual content; R.R. and J.S.R.d.O. acquisition of the data, interpretation of the results, and critically reviewing or revising the manuscript for important intellectual content; A.S. conception, design, or planning of the study, analysis of the data, interpretation of the results, and critically reviewing or revising the manuscript for important intellectual content; E.P.-K. interpretation of the results and critically reviewing or revising the manuscript for important intellectual content; R.G. acquisition and analysis of the data, interpretation of the results, and critically reviewing or revising the manuscript for important intellectual content; N.A.J., V.B. G.F.P., A.M., and M.O. acquisition of the data and critically reviewing or revising the manuscript for important intellectual content; M.D. interpretation of the results, drafting of the manuscript, and critically reviewing or revising the manuscript for important intellectual content; N.S. interpretation of the results and critically reviewing or revising the manuscript for important intellectual content; Y.Z. conception, design or planning of the study, analysis of the data, and critically reviewing or revising the manuscript for important intellectual content; M.R. conception, design or planning of the study, interpretation of the results, and critically reviewing or revising the manuscript for important intellectual content; T.L.S., A.N., and J.K. interpretation of the results and critically reviewing or revising the manuscript for important intellectual content; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: P.L.Z. reports consultancy for MSD, Eusapharma, and Novartis; has attended advisory boards for Celltrion, Gilead, Janssen-Cliag, Bristol-Myers Squibb, Servier, MSD, TG Therapeutics, Takeda, Roche, Eusapharma, Kyowa Kirin, Novartis, Incyte and Belgene; and is a member of speaker bureaus for Secura Bio, Celltrion, Gilead, Janssen-Cliag, Bristol-Myers Squibb, Servier, Sandoz, MSD, TG Therapeutics, Takeda, Roche, Eusapharma, Kyowa Kirin, Novartis, ADC Therapuetics, Incyte and Belgene. R.R. reports consultant/advisory role for Seattle Genetics, Sandoz-Novartis, Pharmacyclics/Janssen, and Bristol Myers Squibb and research funding from Merck, Seattle Genetics, Janssen and Genentech. A.S. reports consultant/advisory role for Bristol Myers Squibb, Servier, Gilead Sciences, Pfizer, Eisai, Bayer, MSD, Sanofi, and ArQule and is a member of speaker bureaus for Takeda, Roche, AbbVie, Amgen, Celgene, AstraZeneca, ArQule, Lilly, Sandoz, Novartis, Bristol Myers Squibb, Servier, Gilead Sciences, Pfizer, Eisai, Bayer, and MSD. E.P.-K. received honoraria from Roche and Takeda and has received travel accommodations/expenses from Roche, Takeda, and Celgene. R.G. received honoraria from MSD, AbbVie, Novartis, Astellas, and Takeda. N.A.J. received honoraria from Merck, Bristol Meyers Squibb, Roche, and Lundbeck and has a consultant advisory role for Merck, Bristol Meyers Squibb, Roche, Lundbeck, AbbVie, Johnson & Johnson, and Seattle Genetics. V.B. reports consultant/advisory role and is a member of speaker bureaus for AstraZeneca. G.F.P. reports consultant/advisory role and is a member of speaker bureaus for Janssen, Takeda, AstraZeneca, and AbbVie. M.D. reports consultant/advisory role (self and institution), received honoraria, and is a member of speaker bureaus for MSD and Takeda. M.O. received honoraria from Takeda and Amgen; received research funding from Janssen, Celgene, Takeda, Archigen, Roche, Bayer, MSD, AbbVie, and Novartis; and received travel accommodations/expenses from Bristol Myers Squibb, Jazz, Roche, Sanofi, Abdi İbrahim, Janssen, Takeda, AbbVie, and Amgen. N.S. received research funding (self and institution) from Ono, A2 Healthcare, Astellas, Janssen, Merck Sharp & Dohme, Otsuka, Pfizer, PPD-SNBL, Sumitomo Dainippon, Daiichi Sankyo, and Bristol Myers Squibb. Y.Z. is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. M.R. is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. T.L.S. is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. A.N. is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. J.K. reports consultancy/advisory role for AbbVie, Bristol Myers Squibb, Gilead Sciences, Karyopharm Therapeutics, Merck, Roche/Genentech, and Seattle Genetics; received research funding from Janssen and Roche; and received honoraria from AbbVie, Bristol Myers Squibb, Amgen, AstraZeneca, Celgene, Gilead Sciences, Janssen, Karyopharm Therapeutics, Merck, Novartis, Roche, and Seattle Genetics. The remaining authors report no conflict of interest.
Correspondence: Pier Luigi Zinzani, Professor of Hematology, IRCCS Azienda Ospedaliero-Universitaria di Bologna Istituto di Ematologia “Seràgnoli,” and Dipartimento di Medicina Specialistica, Diagnostica e Sperimentale Università di Bologna, via Massarenti 9, Bologna, Italy, 40126; e-mail: pierluigi.zinzani@unibo.it or lisa.argnani@unibo.it.
References
Author notes
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts. After approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
The full-text version of this article contains a data supplement.