TO THE EDITOR:
Subsequent malignant neoplasms (SMNs) are well described after chemotherapy, radiation therapy, and hematopoietic stem cell transplantation (HSCT).1-3 Cancer survivors, particularly those with cancer predisposition syndromes, are also at risk of second primary malignancies unrelated to their treatment.4,5 The cumulative incidence of SMNs continues to increase as more childhood cancer survivors reach adulthood.2 Among survivors of childhood acute lymphoblastic leukemia (ALL), those with relapsed disease and/or those requiring HSCT have the highest 20-year cumulative incidence of SMNs at ∼4.2%.6 There is a paucity of literature regarding how cellular immune-based therapies influence the risk of SMNs.
Chimeric antigen receptor (CAR) T-cell therapy is associated with well-described unique inflammatory toxicities in the early postinfusion period, but less is known about potential long-term toxicities. To monitor for delayed adverse events related to lentiviral/retroviral-based technologies used in CAR T-cell manufacturing, the US Food and Drug Administration requires 15 years of long-term follow-up after administration of any investigational human gene therapy product.7 This surveillance includes monitoring for risks associated with vector integration, which may disrupt tumor suppressor genes or activate protooncogenes near integration sites, thereby theoretically increasing the risk of SMNs. Although malignant transformation directly related to traditional CAR T-cell vector transduction has not been reported,8,9 long-term follow-up remains limited. The issue of delayed adverse events is particularly relevant in children and young adults treated with CAR T cells, given their longer potential lifespans and thus higher cumulative risk of SMNs. After a decade since the first child with B-cell ALL (B-ALL) was treated with CD19 CAR T cells, and with longitudinal data increasingly available, we sought to identify the incidence of SMNs in a large cohort of children and young adults after treatment with CD19 CAR T cells.
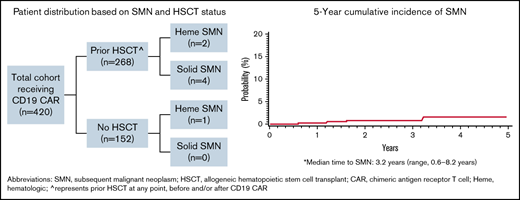
Based on a multicenter retrospective analysis of children and young adults with B-ALL who received CD19 CAR T cells between 2012 and 2019,10 we identified an SMN in 7 (1.7%) of 420 patients. SMN diagnoses included cholangiocarcinoma,11 synovial sarcoma, malignant melanoma, papillary thyroid carcinoma, and 3 myeloid neoplasms (Table 1). Median times to SMN diagnosis after initial B-ALL diagnosis and after CD19 CAR T-cell infusion were 8.2 years (range, 1.1-12.5 years) and 3.2 years (range, 0.6-8.2 years), respectively, leading to a 5-year cumulative incidence of SMNs of 1.5% (95% confidence interval, 0.4% to 4%). Six had undergone allogeneic HSCT before diagnosis of the SMN, and 6 had received prior radiation therapy (total-body irradiation [TBI], n = 5; central nervous system irradiation, n = 1). Additionally, 1 patient received ongoing ALL-directed therapy for relapse after CD19 CAR T-cell therapy, before SMN diagnosis (synovial sarcoma), and 1 patient with a myeloid SMN had a germ line TP53 mutation, a well-described cancer predisposition syndrome. Among those with SMNs, 2 patients received a CD19/28ζ-based CD19 CAR T-cell therapy; 4 received either commercial tisagenlecleucel or the investigational CD19/41BB predecessor, and 1 received an alternative CD19/BB CAR T-cell therapy. No patient with testing had evidence of replication-competent retrovirus/lentivirus, but assessment of vector integration into SMN sites was limited. With a median follow-up of 1177 days (range, 220-2981 days) after CD19 CAR T-cell therapy, 5 (71%) of 7 patients remain alive and in remission from their SMNs, including 2 of 3 patients with myeloid SMNs. Additional details on the presentation, diagnosis, and management are listed in Tables 2 and 3. Institutional review board approval was obtained at all participating institutions before retrospective analysis. The study was conducted according to the Declaration of Helsinki.
Overview of subsequent malignant neoplasms observed post–CD19 CAR T-cell therapy
| . | Hematologic malignancy (n = 3) . | Solid tumor (n = 4) . |
|---|---|---|
| Diagnosis | AML (n = 2) MDS (n = 1) | Cholangiocarcinoma Synovial sarcoma Melanoma Thyroid carcinoma |
| Median (range) time from CAR T-cell infusion to diagnosis of SMN, y | 1.2 (0.6-1.6) | 5.9 (3.2-8.2) |
| Median (range) time from initial diagnosis to diagnosis of SMN, y | 3.7 (1.1-4.2) | 9.6 (8.2-12.5) |
| History of alloHSCT before SMN | Yes (n = 2) | Yes (n = 4) |
| Current status | 2 patients remain alive in remission after alloHSCT; 1 died of progressive disease | 3 patients remain alive in remission; 1 died of surgical complications |
| . | Hematologic malignancy (n = 3) . | Solid tumor (n = 4) . |
|---|---|---|
| Diagnosis | AML (n = 2) MDS (n = 1) | Cholangiocarcinoma Synovial sarcoma Melanoma Thyroid carcinoma |
| Median (range) time from CAR T-cell infusion to diagnosis of SMN, y | 1.2 (0.6-1.6) | 5.9 (3.2-8.2) |
| Median (range) time from initial diagnosis to diagnosis of SMN, y | 3.7 (1.1-4.2) | 9.6 (8.2-12.5) |
| History of alloHSCT before SMN | Yes (n = 2) | Yes (n = 4) |
| Current status | 2 patients remain alive in remission after alloHSCT; 1 died of progressive disease | 3 patients remain alive in remission; 1 died of surgical complications |
AML, acute myeloid leukemia; alloHSCT, allogeneic HSCT; MDS, myelodysplastic syndrome.
Presentation, diagnosis, and management of solid tumor SMNs
| . | Cholangiocarcinoma11 . | Synovial sarcoma . | Melanoma . | Thyroid carcinoma . |
|---|---|---|---|---|
| Oncologic history | History of multiply relapsed B-ALL, initially diagnosed at age 18 y; received CD19 CAR T cells with subsequent consolidative HSCT from 10/10 matched sibling donor, with TBI -based myeloablative conditioning regimen (TBI and CTX) | History of multiply relapsed B-ALL, initially diagnosed at age 20 y; underwent haploidentical HSCT from parent, with TBI-based myeloablative conditioning regimen (TBI and CTX) in CR1 and CD19 CAR T-cell therapy in CR2 | History of multiply relapsed B-ALL, initially diagnosed at age 21 y; underwent double cord blood HSCT, with TBI-based myeloablative conditioning regimen (fludarabine, CTX, and TBI) in first relapse. Received CD19 CAR T cells in second relapse | History of multiply relapsed/refractory B-ALL, initially diagnosed at age 8 y; received CD19 CAR T cells with subsequent consolidative HSCT from 9/10 matched unrelated donor, with TBI-based myeloablative conditioning regimen (TBI, CTX, and thiotepa) |
| Total radiation exposure (TBI), cGy | 3600 | 1200 (plus 400 testicular boost) | 1320 | 1200 |
| Brief CAR T-cell therapy course | Received lymphodepleting chemotherapy with CTX and fludarabine before CD19 CAR T cells; achieved MRD− CR and proceeded directly to consolidative HSCT | Received lymphodepleting chemotherapy with CTX and fludarabine before CD19 CAR T cells; achieved MRD− CR; developed CD19− relapse 6 mo post–CD19 CAR T-cell therapy, and received ALL-directed therapy before SMN diagnosis | Received lymphodepleting chemotherapy with CTX and fludarabine before CD19 CAR T cells; achieved MRD− CR; continued B-cell aplasia (>7 y) | Received lymphodepleting chemotherapy with CTX and fludarabine before CD19 CAR T cells; achieved MRD− CR and proceeded directly to consolidative HSCT |
| CAR T-cell construct | CD19/28z | CD19/BB | CD19/BB (tisagenlecleucel/CTL019) | CD19/28z |
| HSCT (before/after CD19 CAR T-cell therapy) | Yes, after | Yes, before | Yes, before | Yes, after |
| SMN presenting symptoms | Presented at routine follow-up 3 y post–CD19 CAR T-cell therapy and post-HSCT at age 26 y with asymptomatic mild elevation of transaminases | Dental pain and swelling noted after wisdom teeth removal 5 y post–CD19 CAR T-cell therapy at age 29 y | Growing mole on right buttock 6 y post–CD19 CAR T-cell therapy | Presented for follow-up 8 y post–CD19 CAR T-cell therapy and post-HSCT with suspicious thyroid nodule |
| Diagnosis | MRI with MRCP: hilar biliary obstruction extending into left hepatic duct with no obstructing mass/stone identified ERCP: focal biliary stricturing in common and left hepatic ducts Diagnostic laparoscopy with exploration and resection: frozen section of left hepatic duct positive for carcinoma, with final surgical pathology results showing moderately differentiated cholangiocarcinoma | Biopsy: synovial sarcoma, biphasic type (FNCLCC grade 2) | Biopsy: malignant melanoma, superficial spreading type, in radial growth phase | Thyroidectomy: 1.5-cm nodule that contained papillary carcinoma of thyroid with extrathyroidal extension and microscopic strap muscle invasion |
| Management | Underwent left hepatectomy with extrahepatic bile duct resection and portal lymphadenectomy | Underwent partial left maxillary teeth extraction, left mandibular reconstruction, soft tissue flap, bilateral level 1 and left level 2-4 lymph node dissection as well as 660-cGy proton radiation therapy | Local resection with negative margins | Thyroidectomy and 131I radioactive iodine ablation |
| Outcome | Died on postoperative day 3 from fulminant hepatic failure | Continued remission from synovial sarcoma with active B-ALL | Continued remission from both B-ALL and malignant melanoma | Continued remission from both B-ALL and thyroid carcinoma |
| . | Cholangiocarcinoma11 . | Synovial sarcoma . | Melanoma . | Thyroid carcinoma . |
|---|---|---|---|---|
| Oncologic history | History of multiply relapsed B-ALL, initially diagnosed at age 18 y; received CD19 CAR T cells with subsequent consolidative HSCT from 10/10 matched sibling donor, with TBI -based myeloablative conditioning regimen (TBI and CTX) | History of multiply relapsed B-ALL, initially diagnosed at age 20 y; underwent haploidentical HSCT from parent, with TBI-based myeloablative conditioning regimen (TBI and CTX) in CR1 and CD19 CAR T-cell therapy in CR2 | History of multiply relapsed B-ALL, initially diagnosed at age 21 y; underwent double cord blood HSCT, with TBI-based myeloablative conditioning regimen (fludarabine, CTX, and TBI) in first relapse. Received CD19 CAR T cells in second relapse | History of multiply relapsed/refractory B-ALL, initially diagnosed at age 8 y; received CD19 CAR T cells with subsequent consolidative HSCT from 9/10 matched unrelated donor, with TBI-based myeloablative conditioning regimen (TBI, CTX, and thiotepa) |
| Total radiation exposure (TBI), cGy | 3600 | 1200 (plus 400 testicular boost) | 1320 | 1200 |
| Brief CAR T-cell therapy course | Received lymphodepleting chemotherapy with CTX and fludarabine before CD19 CAR T cells; achieved MRD− CR and proceeded directly to consolidative HSCT | Received lymphodepleting chemotherapy with CTX and fludarabine before CD19 CAR T cells; achieved MRD− CR; developed CD19− relapse 6 mo post–CD19 CAR T-cell therapy, and received ALL-directed therapy before SMN diagnosis | Received lymphodepleting chemotherapy with CTX and fludarabine before CD19 CAR T cells; achieved MRD− CR; continued B-cell aplasia (>7 y) | Received lymphodepleting chemotherapy with CTX and fludarabine before CD19 CAR T cells; achieved MRD− CR and proceeded directly to consolidative HSCT |
| CAR T-cell construct | CD19/28z | CD19/BB | CD19/BB (tisagenlecleucel/CTL019) | CD19/28z |
| HSCT (before/after CD19 CAR T-cell therapy) | Yes, after | Yes, before | Yes, before | Yes, after |
| SMN presenting symptoms | Presented at routine follow-up 3 y post–CD19 CAR T-cell therapy and post-HSCT at age 26 y with asymptomatic mild elevation of transaminases | Dental pain and swelling noted after wisdom teeth removal 5 y post–CD19 CAR T-cell therapy at age 29 y | Growing mole on right buttock 6 y post–CD19 CAR T-cell therapy | Presented for follow-up 8 y post–CD19 CAR T-cell therapy and post-HSCT with suspicious thyroid nodule |
| Diagnosis | MRI with MRCP: hilar biliary obstruction extending into left hepatic duct with no obstructing mass/stone identified ERCP: focal biliary stricturing in common and left hepatic ducts Diagnostic laparoscopy with exploration and resection: frozen section of left hepatic duct positive for carcinoma, with final surgical pathology results showing moderately differentiated cholangiocarcinoma | Biopsy: synovial sarcoma, biphasic type (FNCLCC grade 2) | Biopsy: malignant melanoma, superficial spreading type, in radial growth phase | Thyroidectomy: 1.5-cm nodule that contained papillary carcinoma of thyroid with extrathyroidal extension and microscopic strap muscle invasion |
| Management | Underwent left hepatectomy with extrahepatic bile duct resection and portal lymphadenectomy | Underwent partial left maxillary teeth extraction, left mandibular reconstruction, soft tissue flap, bilateral level 1 and left level 2-4 lymph node dissection as well as 660-cGy proton radiation therapy | Local resection with negative margins | Thyroidectomy and 131I radioactive iodine ablation |
| Outcome | Died on postoperative day 3 from fulminant hepatic failure | Continued remission from synovial sarcoma with active B-ALL | Continued remission from both B-ALL and malignant melanoma | Continued remission from both B-ALL and thyroid carcinoma |
CR1, first complete remission; CR2, second complete remission; CTX, cyclophosphamide; ERCP, endoscopic retrograde cholangiopancreatography; FNCLCC, Fédération Nationale des Centres de Lutte Contre Le Cancer; MRCP, magnetic resonance cholangiopancreatography; MRD, minimal residual disease; MRI, magnetic resonance imaging.
Presentation, diagnosis, and management of hematologic SMNs
| . | AML/MDS . | AML . | MDS . |
|---|---|---|---|
| Oncologic history | History of Li Fraumeni syndrome with very high-risk B-ALL diagnosed at age 18 y; attempt at curative frontline therapy with CD19 CAR T cells in hope of lesser toxicity compared with myeloablative regimen because of Li Fraumeni–associated risks; because of early loss of B-cell aplasia after CD19 CAR T-cell therapy at 4 mo, received CD19 CAR T-cell reinfusion, with subsequent development of MDS (chromosome 5 changes) 2 mo later; underwent unrelated cord blood HSCT for MDS using busulfan, fludarabine, and thiotepa–based conditioning regimen | History of disseminated B-lymphoblastic lymphoma (TCF-PBX1 translocation) diagnosed at age 10 y, including involvement of multiple cranial nerves; required emergency cranial XRT (2350 cGy) and boost (1500 cGy); isolated CNS relapse of B-lymphoblasts 2.5 y after initial diagnosis; received triple IT therapy and VCR/MTX as bridging therapy; received CD19 CAR T cells in first relapse | History of with B-ALL (unknown cytogenetics) diagnosed at age 10 y; first relapse was very early; received salvage chemotherapy followed by unrelated cord blood HSCT with TBI-based myeloablative conditioning regimen (TBI, etoposide, and CTX); second relapse was 2.5 y post-HSCT; received bridging chemotherapy while awaiting CD19 CAR T-cell infusion |
| Total radiation exposure (TBI), cGy | None | 2850 (CNS only) | 1200 |
| Brief CAR T-cell therapy course | Received lymphodepleting chemotherapy with CTX and fludarabine before CD19 CAR T cells, without significant complications; good count recovery but developed early loss of B-cell aplasia and received CD19 CAR T-cell reinfusion | Received lymphodepleting chemotherapy with CTX and fludarabine before CD19 CAR T cells; achieved MRD− CR; continued B-cell aplasia | Received lymphodepleting chemotherapy with CTX and fludarabine before CD19 CAR T cells; achieved MRD− CR; continued B-cell aplasia until time of SMN diagnosis |
| CAR T-cell construct | CD19/BB | CD19/BB | CD19/BB |
| HSCT (before/after CD19 CAR T-cell therapy) | Yes, after | NA | Yes, before |
| Presenting symptoms | Progressive cytopenias (trilineage) after full count recovery after first CD19 CAR T-cell treatment and early post-HSCT; fatigue | Developed worsening pancytopenia 18 mo post–CD19 CAR T-cell therapy | Progressive thrombocytopenia and new fevers 13 mo post–CD19 CAR T-cell therapy |
| Diagnosis | Bone marrow evaluation: MDS/AML with loss of original clone and new cytogenetics consisting of translocation(5;17)/AML | Bone marrow evaluation: AML NPM1+ and CEBPA+; negative for TCF3-PBX1 translocation | Bone marrow evaluation: MDS (RAEB-2), trisomy 8 and 9; MDS was XY and thought to be derived from cord blood |
| Management | Therapy with venetoclax/azacytidine to control disease; not eligible for second HSCT because of pulmonary GVHD/IPS complications from first HSCT | Therapy with CPX-351 (liposomal daunorubicin and cytarabine) and FLAG, then proceeded to 10/10 HLA-matched unrelated donor HSCT; myeloablative conditioning regimen with busulfan, fludarabine, and thiotepa | Therapy with azacitidine, then mismatched sibling HSCT; myeloablative conditioning regimen with busulfan and melphalan |
| Outcome | Patient died of refractory MDS/AML with minimal response to therapy and progressive IPS post-HSCT at ∼3 mo post-HSCT | Alive without disease 14 mo post-HSCT | Alive without disease 18 mo post–second HSCT |
| . | AML/MDS . | AML . | MDS . |
|---|---|---|---|
| Oncologic history | History of Li Fraumeni syndrome with very high-risk B-ALL diagnosed at age 18 y; attempt at curative frontline therapy with CD19 CAR T cells in hope of lesser toxicity compared with myeloablative regimen because of Li Fraumeni–associated risks; because of early loss of B-cell aplasia after CD19 CAR T-cell therapy at 4 mo, received CD19 CAR T-cell reinfusion, with subsequent development of MDS (chromosome 5 changes) 2 mo later; underwent unrelated cord blood HSCT for MDS using busulfan, fludarabine, and thiotepa–based conditioning regimen | History of disseminated B-lymphoblastic lymphoma (TCF-PBX1 translocation) diagnosed at age 10 y, including involvement of multiple cranial nerves; required emergency cranial XRT (2350 cGy) and boost (1500 cGy); isolated CNS relapse of B-lymphoblasts 2.5 y after initial diagnosis; received triple IT therapy and VCR/MTX as bridging therapy; received CD19 CAR T cells in first relapse | History of with B-ALL (unknown cytogenetics) diagnosed at age 10 y; first relapse was very early; received salvage chemotherapy followed by unrelated cord blood HSCT with TBI-based myeloablative conditioning regimen (TBI, etoposide, and CTX); second relapse was 2.5 y post-HSCT; received bridging chemotherapy while awaiting CD19 CAR T-cell infusion |
| Total radiation exposure (TBI), cGy | None | 2850 (CNS only) | 1200 |
| Brief CAR T-cell therapy course | Received lymphodepleting chemotherapy with CTX and fludarabine before CD19 CAR T cells, without significant complications; good count recovery but developed early loss of B-cell aplasia and received CD19 CAR T-cell reinfusion | Received lymphodepleting chemotherapy with CTX and fludarabine before CD19 CAR T cells; achieved MRD− CR; continued B-cell aplasia | Received lymphodepleting chemotherapy with CTX and fludarabine before CD19 CAR T cells; achieved MRD− CR; continued B-cell aplasia until time of SMN diagnosis |
| CAR T-cell construct | CD19/BB | CD19/BB | CD19/BB |
| HSCT (before/after CD19 CAR T-cell therapy) | Yes, after | NA | Yes, before |
| Presenting symptoms | Progressive cytopenias (trilineage) after full count recovery after first CD19 CAR T-cell treatment and early post-HSCT; fatigue | Developed worsening pancytopenia 18 mo post–CD19 CAR T-cell therapy | Progressive thrombocytopenia and new fevers 13 mo post–CD19 CAR T-cell therapy |
| Diagnosis | Bone marrow evaluation: MDS/AML with loss of original clone and new cytogenetics consisting of translocation(5;17)/AML | Bone marrow evaluation: AML NPM1+ and CEBPA+; negative for TCF3-PBX1 translocation | Bone marrow evaluation: MDS (RAEB-2), trisomy 8 and 9; MDS was XY and thought to be derived from cord blood |
| Management | Therapy with venetoclax/azacytidine to control disease; not eligible for second HSCT because of pulmonary GVHD/IPS complications from first HSCT | Therapy with CPX-351 (liposomal daunorubicin and cytarabine) and FLAG, then proceeded to 10/10 HLA-matched unrelated donor HSCT; myeloablative conditioning regimen with busulfan, fludarabine, and thiotepa | Therapy with azacitidine, then mismatched sibling HSCT; myeloablative conditioning regimen with busulfan and melphalan |
| Outcome | Patient died of refractory MDS/AML with minimal response to therapy and progressive IPS post-HSCT at ∼3 mo post-HSCT | Alive without disease 14 mo post-HSCT | Alive without disease 18 mo post–second HSCT |
AML, acute myeloid leukemia; CNS, central nervous system; CR, complete remission; FLAG, fludarabine, cytarabine, and filgrastim; GVHD, graft-versus-host disease; IPS, idiopathic pneumonia syndrome; IT, intrathecal; MDS, myelodysplastic syndrome; MRD, minimal residual disease; MTX, methotrexate; NA, not applicable; RAEB-2, refractory anemia with excess blasts type 2; VCR, vincristine; XRT, radiation therapy.
In the limited literature available, the incidence of SMNs in patients who received CD19 CAR T cells ranges from 2.2% to 16.3% and varies between pediatric and adult reports.12-14 To that end, the incidence of SMNs in our cohort was similar to that in the pooled safety analysis of tisagenlecleucel in children and young adults with B-ALL.12 Three (2.2%) of 137 patients included in the tisagenlecleucel safety analysis developed an SMN, with a median follow-up of 24 months, including 2 patients who developed myelodysplastic syndrome and 1 patient with a germ line TP53 mutation who developed glioblastoma. In our cohort, a trend toward a longer latency period for developing a solid tumor was apparent when compared with developing a hematologic malignancy. This is consistent with historical reports of SMNs after HSCT, where the risk of hematologic malignancies is highest in the first 10 years, but the risk continues to increase, including long latency periods (≥10 years) before development of solid tumors.3,15 Notably, 6 (85.7%) of 7 patients in our cohort had received prior radiation therapy, and 6 had undergone prior allogeneic HSCT, including all 4 patients who developed a solid tumor, with a majority receiving a TBI-based myeloablative regimen, a standard approach in B-ALL conditioning.16,17
A rare but important phenomenon after CD19 CAR T-cell therapy for B-ALL is a lineage switch (LS), in which there is an immunophenotypic transition from lymphoid- to myeloid-defining cell surface markers. Based on a recent effort from our group, LSs comprised 7.2% of all relapse phenotypes after CD19 CAR T-cell therapy.18 It is important to distinguish an LS from a therapy-related myeloid neoplasm, because the prognosis of patients who develop an LS after CAR T-cell therapy is dismal, as recently reported.18 In contrast, 2 of the 3 patients who developed myeloid SMNs in this report received successful salvage therapy. Immunophenotype alone is insufficient to distinguish between the 2 entities, and cytogenetic changes observed at the time of the event are critical, because the original cytogenetic drivers are likely to be preserved in an LS. All 3 myeloid SMNs in our report were differentiated from LSs based on the absence of the cytogenetic abnormalities noted in the B-ALL clone, including 1 SMN emerging from the prior sex-disparate stem cell source. Given the clinical relevance of separating LSs from SMNs, use of next-generation sequencing of B-cell receptor rearrangements for minimal residual disease tracking in B-ALL19,20 and genomic analyses with either DNA- or RNA-based deep sequencing21 should be considered when distinguishing between LSs and myeloid SMNs.
With the low incidence of SMNs in our series and in the context of extensive prior ALL-directed therapy, including chemotherapy, radiation therapy, and/or HSCT, direct attribution of SMNs to CAR T-cell therapy is challenging, particularly in those undergoing HSCT after CD19 CAR T-cell therapy. However, given our findings and the absence of reported events related directly to CD19 CAR T-cell vector integration, current surveillance for SMNs, which incorporates both the US Food and Drug Administration long-term follow-up guidelines and routine postcancer long-term monitoring (including heightened monitoring in those with cancer predisposition syndromes),5 is likely sufficient at this time.
Although the rate of SMNs in this cohort is low, ongoing surveillance over decades will be required to understand the full risk of subsequent neoplasms. Additionally, the risk of SMNs beyond CD19 CAR T-cell targeting, within other diseases, and with next-generation constructs is unknown and will be informed by additional studies. Indeed, 2 recently published reports detail the development of T-cell lymphomas derived from CD19 CAR T cells that were generated using the piggyBac transposon system,22,23 a delivery system developed to decrease the cost, logistics, and theoretic risk of insertional mutagenesis associated with viral vectors.
Demonstrating a low incidence of SMNs, this report adds to the limited literature surrounding delayed adverse events after CD19 CAR T-cell therapy and helps inform patient counseling and surveillance approaches. Monitoring for delayed toxicities is particularly relevant in children as CAR T-cell therapy continues to advance and moves earlier into frontline treatment for high-risk ALL and potentially spares the need for more intensive therapies that increase SMN risk. Although the extensive prior therapy received in our cohort, including TBI, is likely causative for SMNs, additional long-term follow-up studies after CAR T-cell therapy are needed to evaluate the cumulative incidence and type of SMNs after CAR T-cell therapy as childhood cancer survivors age into adulthood.
Acknowledgments: The authors thank the treating and referring centers, care providers, supporting staff, and referring physicians who cared for the patients included in this study.
This work was supported in part by the Center for Cancer Research and the Warren Grant Magnuson Clinical Center, Intramural Research Program, National Cancer Institute, National Institutes of Health (grant ZIA BC 011823; N.N.S.). L.G. is supported by grant CA046934.
Contribution: E.M.H., N.N.S., and A.J.L. wrote the first draft of the manuscript; and all authors contributed to data collection and provided relevant details, substantially contributed to the final version of the manuscript, and approved the submission.
The content of this publication does not necessarily reflect the views or policies of the US Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Conflict-of-interest disclosure: D.W.L. has consulted for Harpoon Therapeutics, has advised for Amgen and Bristol Myers Squibb, and has received research funding from Kite Pharma and Gilead. S.A.G. has received research funding from Novartis, Kite Pharma, Vertex, and Servier; has consulted for Novartis, Roche, GlaxoSmithKline, Humanigen, Cellular Biomedicine Group, Eureka, Janssen/Johnson & Johnson, and Jazz Pharmaceuticals; and has advised for Novartis, Adaptimmune, TCR2, Cellectis, Juno, Vertex, Allogene, Jazz Pharmaceuticals, and Cabaletta. M.R.V. has advised for Novartis, Equillium, Mederus, and Takeda. L.G. has consulted for Amgen, Novartis, and Roche; has advised for Amgen, Novartis, and Celgene; and holds equity in Amgen, Anchiano, Blueprint Medicines, Celgene, Clovis, Mirati, and Sanofi Paris. P.A.B. has advised for Novartis, Takeda, Amgen, Kura, and Kite. S.R.R. has received research funding from Pfizer. M.A.P. has advised for Mesoblast, Novartis, Equillium, Medexus, and Vertex; has received research funding from Adaptive and Miltenyi; and has received honoraria from Novartis, Miltenyi, and Bellicum. R.A.G. has consulted for Novartis and received patents and royalties from Bristol Myers Squibb. T.W.L. has advised for Bayer, Cellectis, Novartis, Deciphera, Juno, and Y-mAbs Therapeutics; has received honoraria from Bayer, Cellectis, Novartis, Deciphera, Juno, and Y-mAbs Therapeutics; and has received research support from Pfizer and Bayer. The remaining authors declare no competing financial interests.
Correspondence: Adam J. Lamble, Division of Hematology/Oncology, University of Washington, Seattle Children’s Hospital, Seattle, WA; e-mail: adam.lamble@seattlechildrens.org.
References
Author notes
Contact the corresponding author for data sharing: adam.lamble@seattlechildrens.org.
The current affiliation for A.T. is Janssen Research & Development, LLC, Raritan, NJ.
The current affiliation for P.A.B. is Bristol Myers Squibb, New York, NY.