Key Points
There is no clinically relevant risk of diabetes mellitus for non-Hodgkin lymphoma patients treated with steroid-containing chemotherapy.
For diabetic patients there is a temporary increased risk of insulin use and CVD in the first year following steroid-containing chemotherapy.
Abstract
First-line treatments for lymphomas often include high doses of prednisolone, but the risks of new-onset diabetes mellitus (DM) or worsening of preexisting DM following treatment with cyclic high dose corticosteroids is unknown. This cohort study matched non-Hodgkin lymphoma (NHL) patients treated with steroid-containing immunochemotherapy (ie, R-CHOP[-like] and R-CVP) between 2002 and 2015 to individuals from the Danish population to investigate the risks of new-onset DM. For patients with preexisting DM, the risks of insulin dependency and anthracycline-associated cardiovascular diseases (CVDs) were assessed. In total, 5672 NHL patients and 28 360 matched comparators were included. Time-varying incidence rate ratios (IRRs) showed increased risk of DM in the first year after treatment compared with matched comparators, with the highest IRR being 2.7. The absolute risks were higher among patients in the first 2 years, but the difference was clinically insignificant. NHL patients with preexisting DM had increased risks of insulin prescriptions with 0.5-, 5-, and 10-year cumulative risk differences of insulin treatment of 15.3, 11.8, and 6.0 percentage units as compared with the DM comparators. In a landmark analysis at 1 year, DM patients with lymphoma had decreased risks of insulin dependency compared with comparators. Time-varying IRRs showed a higher CVD risk for NHL patients with DM as compared with comparators in the first year after treatment. NHL patients treated with steroid-containing immunochemotherapy regimens have a clinically insignificant increased risk of DM in the first year following treatment, and patients with preexisting DM have a temporary increased risk of insulin prescriptions and CVD.
Introduction
Intermittent treatment with high doses of prednisolone has been used for decades to treat lymphomas due to its toxic effects on lymphocytes and beneficial effects on chemo-induced nausea and fatigue.1-5 However, prednisolone has important side effects, one of which is steroid-induced diabetes mellitus (DM).6 Commonly used first-line steroid-containing immunochemotherapy regimens are rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) and rituximab, cyclophosphamide, vincristine, and prednisolone (R-CVP).7-9 Lymphoma patients treated with R-CHOP and R-CVP typically receive 100 mg prednisolone daily for 5 consecutive days in up to 8 cycles.9 The high cure rates of 60% to 70% for diffuse large B-cell lymphoma (DLBCL) patients imply that many DLBCL patients with durable remissions will have life expectancies in the range of age- and sex- matched individuals without lymphoma.10,11 Most patients with follicular lymphoma (FL) have incurable disease with conventional therapies, but with 10-year overall survival (OS) rates of 80% and long disease-free periods without treatment, survivorship care is equally important for these patients.12 Life expectancy with FL is favorable, with the important exception of patients with early relapse (within 2 years of first-line treatment).13,14 Collectively, these data underscore the importance of research focusing on lymphoma survivorship.10,15 -18
Due to the exposure to a high cumulative dosage of steroids during first-line treatment, patients with non-Hodgkin lymphoma (NHL) could face increased risk of new-onset steroid-induced DM or dysregulation of a preexisting DM.6,19 -22 Preexisting DM with late complications such as atherosclerosis may also increase the risk of anthracycline-associated cardiac complications.23 Finally, the presence of DM prior to lymphoma diagnosis may also impact lymphoma treatment, with diabetic patients at risk of receiving dose reductions and suboptimal treatment resulting in inferior disease control.24-27 Few studies have evaluated the association between steroid treatment and DM in patients with lymphoma, but they mainly used temporary hyperglycemia in the definition of DM.20,25
This nationwide observational cohort study evaluated the risk of new-onset DM, dysregulated preexisting diabetes, and anthracycline-associated cardiovascular diseases (CVDs) in lymphoma patients according to the use of steroid-containing regimens.
Methods
This study was an observational matched cohort study based on nationwide Danish health registers. NHL patients were identified using the Danish Lymphoma Register (LYFO), which contains data on adult lymphoma patients since the year 2000, including information about lymphoma type, clinicopathological features, treatment, relapse, progression, treatment response, and death. Information is prospectively entered into LYFO at local sites. In a previous validation of LYFO, data completeness was between 92% and 100% and data accuracy between 87% and 100%.28 To ensure data completeness of information on relapse, LYFO was linked to the Danish Pathology Register using the unique Civil Personal Register numbers for Danish citizens.29 Biopsies in the Danish Pathology Register showing lymphoma obtained at least 3 months after start of first-line immunochemotherapy treatment were considered as treatment failure or relapse in addition to the relapses/progressions reported in LYFO.
In this study, newly diagnosed NHL patients ≥18 years were included if they had been treated between 2002 and 2015 with first-line steroid-containing immunochemotherapy, such as R-CHOP(-like) and R-CVP, for at least 3 cycles. Inclusion date was at initiation of first-line treatment (index date).
Patients and matched comparators with a diagnosis of any type of DM at least 6 months prior to the index date were included in analyses investigating the risk of insulin prescriptions (as a marker of dysregulated DM) and anthracycline-related CVDs. In the analysis of CVDs, only patients treated with anthracycline-containing immunochemotherapy were included (R-CHOP/CHOEP [CHOP+etoposide]).
Patients and comparators with DM prior to the start of the study were excluded in analyses of incident DM. In analyses of insulin prescriptions, patients and comparators with DM were excluded if they had received any insulin prescriptions prior to start of the study. For analyses of CVDs, patients and comparators were excluded if they had any CVDs prior to the start of the study.
NHL patients observed without treatment (watch and wait) were included in a subanalysis to explore the risk of DM and to compare the surveillance effects of frequent hospital visits to the risk of being diagnosed with DM.
Matching
A matched cohort of Danish citizens without lymphoma was constructed by matching 5 individuals from the Danish general population to each patient on birth year, sex, and Charlson Comorbidity Index score (CCIS). For the analysis including individuals with preexisting DM, patients were matched on birth year, sex, CCIS, and duration of DM (numbers of years up until 9 years, then ≥10 years). More information about the matching can be found in Appendix 1. Follow-up for matched comparators started on the day of the inclusion date for the index NHL patient.
Patients characteristics
Comorbidity assessment was based on the Danish National Patient Register and the International Classification of Diseases 10th revision (ICD-10) codes, but only comorbidities existing at least 6 months before index date were considered to limit surveillance bias in lymphoma patients and to avoid including the diagnosis of lymphoma in the score (see Appendix 2 for comorbidities).30
Educational level was identified for all individuals at the year of the index date and stratified into low (basic school or high school education) or high (vocational education, short/medium length higher education, or long higher education).
Outcome
The primary outcome of the study of incident DM was the rate of new-onset DM in NHL patients relative to the background population. DM outcomes were retrieved from the Danish National Patient Register, which registers data on all hospital admissions since 1977 and all hospital-based outpatient activities since 1995.31 For every hospital admission or outpatient activity, diagnoses are registered based on ICD-10 codes. To capture DM outcomes managed by general practitioners without hospital referrals, data related to redeemed prescriptions of DM drugs were retrieved from the National Prescription Register, where information on drug prescriptions has been held since 1994.32 We defined DM as any DM registered in the Danish National Patient Register (type 1, type 2, or other; ICD-10 codes E10-E14) or any antidiabetic treatment registered in the National Prescription Register (insulin or oral antidiabetic medicine; Anatomical Therapeutic Chemical Classification System codes A10A and A10B, respectively).
Secondary outcomes of this study included incident insulin prescriptions in patients with preexisting non–insulin-dependent DM. Insulin prescriptions were defined as any redeemed prescriptions of insulin in the National Prescription Registry (Anatomical Therapeutic Chemical Classification System code A10A).
Anthracycline-associated cardiovascular complications, including a separate analysis of congestive heart failure, were also included to investigate the relationship between treatment with anthracycline- and steroid-containing chemotherapy and the risk of CVDs in patients with preexisting DM (see Appendix 3 for a list of ICD-10 codes for CVDs).
Statistics
Baseline characteristics were described and summarized for all patients and matched comparators, and continuous variables were presented using medians with interquartile ranges.
In the time-to-event analyses, patients and comparators were followed from the index date until the event of interest (DM, insulin prescription, or anthracycline-related CVD, respectively), death, relapse, NHL diagnosis (for matched comparators), immunochemotherapy treatment (for the watch and wait patients), or censoring (emigration, missing, or end of study on 31 December 2018), whichever came first. OS was defined as the time from index date until death from any cause.
The Aalen-Johansen estimator was used to compute the cumulative risk of an event treating death, relapse, and NHL diagnosis (in matched comparators) as competing events. Risk differences at specific time points were calculated using pseudo-observations.33 P values testing for a difference in cumulative risk for the whole period were obtained using Gray’s test. A landmark analysis was performed for all patients alive at 1 year after treatment to investigate the cumulative incidence of new insulin prescriptions after 1 year. In this analysis, patients were allowed insulin prescriptions between the original index date and the landmark analysis at 1 year and were rematched to new controls at 1 year.
To identify and account for risk factors, crude and adjusted cause-specific hazard ratios were obtained using Cox regression. Although similar in patients and comparators, sex and age were adjusted for in the adjusted Cox regression to avoid noncollapsibility issues.34 The proportional hazards assumption was assessed visually using scaled Schoenfeld residuals. For analyses in which the proportional hazards assumption was violated, we estimated time-varying incidence rates (IRs) per 1000 person years and incidence rate ratios (IRRs) with 95% confidence intervals using the spline-based Poisson regression approach described by Carstensen.35 We used time splits of 2-month intervals for follow-up time and smoothed the time-varying rates by using natural cubic splines with 5 knots. Both the Cox regression and time-varying Poisson models aim to estimate the marginal rates of the outcomes (ie, the rates [and rate ratios] we would observe in the absence of competing events). This contrasts with the Aalen-Johansen estimates, where the competing events are incorporated into the estimation and where the results with respect to the exposure-outcome association could in part or whole be explained by differences in the distribution of the competing events
The study population was defined using SAS Software 9.4, and all statistical analyses were made using R version 3.4.1.36,37 The study was approved by the Danish Data Protection Agency (3-3013-2536/1).
Results
A total of 5672 NHL patients and 28 360 comparators were included. Median age at baseline was 66, and the male-to-female ratio was 1.3. DM was prevalent at the time of NHL diagnosis in 523 patients. Median follow-up was 8.5 years (reverse Kaplan-Meier method), and the 5-year OS was 71.8% (95% confidence interval [CI], 70.6-73.1) for lymphoma patients without DM and 88.1% (95% CI, 87.7-88.5) for matched comparators without DM. A table of NHL subtypes can be found in supplemental Table 1. A consort diagram for patient selection for each main analysis is shown in supplemental Figure 1.
Risk of incident diabetes
A total of 5175 NHL patients did not have preexisting DM and were matched to 25 875 comparators without DM. NHL patients had a lower baseline prevalence of CVD, circulatory diseases, cerebrovascular diseases, and chronic obstructive pulmonary disease compared with the matched comparators (Table 1).
Baseline characteristics of NHL patients and matched comparators for the risk of diabetes mellitus analysis
| . | NHL patients (n = 5175) . | Comparators (n = 25 875) . |
|---|---|---|
| Male | 2875 (55.56%) | 14 375 (55.56%) |
| Median age (IQR) | 65 (17) | 65 (17) |
| CVD | 721 (13.93%) | 4055 (15.67%) |
| Cerebrovacular disease | 218 (4.21%) | 1552 (6.00%) |
| Circulatory disease | 1418 (27.40%) | 7661 (29.61%) |
| Chronic obstructive pulmonary disease | 192 (3.71%) | 1289 (4.98%) |
| Liver disease | 62 (1.20%) | 352 (1.36%) |
| Renal disease | 115 (2.22%) | 599 (2.31%) |
| Higher education | 3048 (60.86%) | 14 999 (60.75%) |
| Missing | 167 | 1184 |
| Performance score | ||
| 0 | 2925 (56.52%) | |
| 1 | 1602 (30.96%) | |
| 2 | 379 (7.32%) | |
| 3 | 195 (3.77%) | |
| 4 | 74 (1.43%) | |
| Ann Arbor stage | ||
| 1 | 854 (16.69%) | |
| 2 | 767 (14.99%) | |
| 3 | 1137 (22.22%) | |
| 4 | 2359 (46.10%) | |
| Missing | 58 | |
| Treatment | ||
| R-CVP | 917 (17.72%) | |
| R-CHOP(-like) | 4258 (82.28%) | |
| . | NHL patients (n = 5175) . | Comparators (n = 25 875) . |
|---|---|---|
| Male | 2875 (55.56%) | 14 375 (55.56%) |
| Median age (IQR) | 65 (17) | 65 (17) |
| CVD | 721 (13.93%) | 4055 (15.67%) |
| Cerebrovacular disease | 218 (4.21%) | 1552 (6.00%) |
| Circulatory disease | 1418 (27.40%) | 7661 (29.61%) |
| Chronic obstructive pulmonary disease | 192 (3.71%) | 1289 (4.98%) |
| Liver disease | 62 (1.20%) | 352 (1.36%) |
| Renal disease | 115 (2.22%) | 599 (2.31%) |
| Higher education | 3048 (60.86%) | 14 999 (60.75%) |
| Missing | 167 | 1184 |
| Performance score | ||
| 0 | 2925 (56.52%) | |
| 1 | 1602 (30.96%) | |
| 2 | 379 (7.32%) | |
| 3 | 195 (3.77%) | |
| 4 | 74 (1.43%) | |
| Ann Arbor stage | ||
| 1 | 854 (16.69%) | |
| 2 | 767 (14.99%) | |
| 3 | 1137 (22.22%) | |
| 4 | 2359 (46.10%) | |
| Missing | 58 | |
| Treatment | ||
| R-CVP | 917 (17.72%) | |
| R-CHOP(-like) | 4258 (82.28%) | |
IQR, interquartile range.
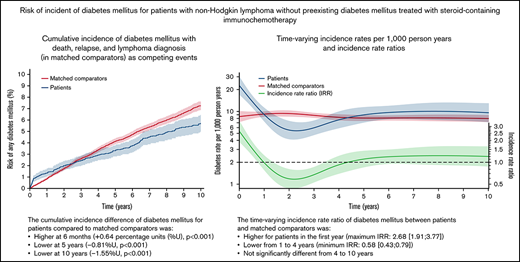
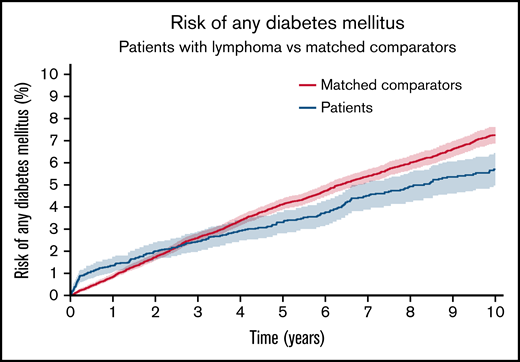
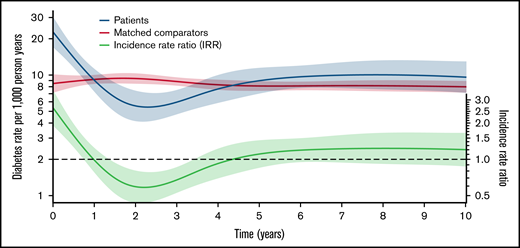
NHL patients treated with steroid-containing immunochemotherapy had 0.5-, 5-, and 10-year cumulative risks of developing DM of 1.1% (95% CI, 0.8-1.4), 3.3% (95% CI, 2.8-3.8), and 5.7% (95% CI, 5.0-6.4). The cumulative incidence difference of DM between NHL patients and comparators was higher at 6 months (+0.64 percentage units [%U], P < .001) but lower at 5 years (−0.81%U, P < .001) and 10 years (−1.55%U, P < .001) compared with the matched comparators (Figure 1). The IR of DM among matched comparators was almost constant over time (rate range of 8.0 to 9.4 events per 1000 person years). The IRR of DM for patients vs comparators was higher for patients in the first year following treatment (maximum IRR between 0 and 1 years: 2.68 [95% CI, 1.91-3.77]) and lower from 1 to 4 years (minimum IRR, 0.58 [95% CI, 0.43-0.79]). There was no significant difference from 4 to 10 years (Figure 2).
The cumulative incidence of any DM for NHL patients without preexisting DM treated with steroid-containing immunochemotherapy compared with matched comparators without preexisting DM. Aalen-Johansen estimator treating death, relapse, and NHL diagnosis (in comparators) as competing events. P value from Gray’s test. The y-axis is limited to 10%.
The cumulative incidence of any DM for NHL patients without preexisting DM treated with steroid-containing immunochemotherapy compared with matched comparators without preexisting DM. Aalen-Johansen estimator treating death, relapse, and NHL diagnosis (in comparators) as competing events. P value from Gray’s test. The y-axis is limited to 10%.
The incidences rates (red for matched comparators; blue for NHL patients) of new-onset diabetes mellitus per 1000 person years and the incidence rate ratio (IRR; green) between patients and matched comparators >10 years from start of lymphoma treatment (index date). The dotted black line shows an IRR of 1.0 for reference.
The incidences rates (red for matched comparators; blue for NHL patients) of new-onset diabetes mellitus per 1000 person years and the incidence rate ratio (IRR; green) between patients and matched comparators >10 years from start of lymphoma treatment (index date). The dotted black line shows an IRR of 1.0 for reference.
Comparing NHL patients managed by watch and wait to NHL patients treated with steroid-containing immunochemotherapy, the crude hazard ratio (HR) was 1.13 (95% CI, 0.86-1.49) for risk of incident DM for treated patients. After adjusting for sex, age, lymphoma type, performance status, educational level, and comorbidities, the HR was 1.14 (95% CI, 0.86-1.51).
Insulin prescriptions
A total of 382 NHL patients with preexisting non–insulin-dependent DM were identified and matched to 1910 comparators with preexisting non–insulin-dependent DM. Median age was 70 years. NHL patients had a lower baseline occurrence of cerebrovascular diseases, renal diseases, liver diseases, and chronic obstructive pulmonary diseases compared with matched comparators (supplemental Table 2).
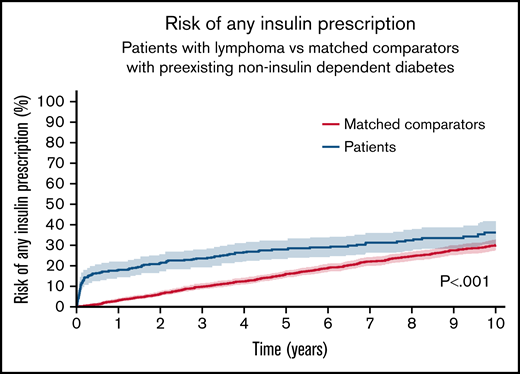
NHL patients treated with steroid-containing immunochemotherapy had 0.5-, 5-, and 10-year cumulative risks of any insulin prescription of 16.5% (95% CI, 12.8-20.2), 27.8% (95% CI, 23.3-32.3), and 36.1% (95% CI, 30.4-41.9). The cumulative incidence difference of any insulin prescription for NHL patients vs comparators was higher at 6 months (+15.3%U, P < .001), 5 years (+11.8%U, P < .001), and 10 years (+6.0%U, P = .06) (Figure 3). In the landmark analysis for patients surviving at least 1 year, patients had a decreased risk of new insulin prescriptions compared with comparators (supplemental Figure 2, P < .001).
The cumulative risk of any insulin prescription for NHL patients with preexisting non–insulin-dependent DM treated with steroid-containing immunochemotherapy compared with matched comparators with preexisting non–insulin-dependent DM. Aalen-Johansen estimator treating death, relapse, and NHL diagnosis (in comparators) as competing events. P value from Gray’s test.
The cumulative risk of any insulin prescription for NHL patients with preexisting non–insulin-dependent DM treated with steroid-containing immunochemotherapy compared with matched comparators with preexisting non–insulin-dependent DM. Aalen-Johansen estimator treating death, relapse, and NHL diagnosis (in comparators) as competing events. P value from Gray’s test.
Anthracycline-related cardiovascular complications
A total of 290 NHL patients treated with anthracycline- and steroid-containing immunochemotherapy and 1450 comparators with preexisting DM and no previous CVDs were included. Median age was 68 years.
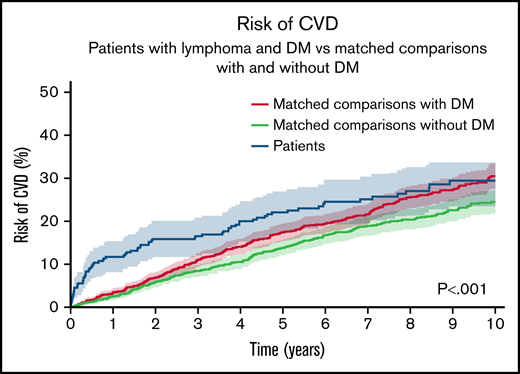
NHL patients had 0.5-, 5-, and 10-year cumulative risks of CVD of 9.7% (95% CI, 6.3-13.1), 22.0% (95% CI, 17.2-26.9), and 29.4% (95% CI, 23.4-35.4). The cumulative incidence difference of CVD for NHL patients vs comparators was higher at 6 months (+8.00%U, P < .001) and 5 years (+4.68%U, P = .08). No difference could be observed at 10 years. Both NHL patients and matched comparators with DM had higher risks than comparators without DM (Figure 4). For cumulative risks of congestive heart failure only, please see supplemental Figure 3.
The cumulative risk of new-onset CVD for NHL patients with preexisting DM treated with steroid- and anthracycline-containing immunochemotherapy compared with matched comparators with preexisting DM and matched comparators without DM. Aalen-Johansen estimator treating death, relapse, and NHL diagnosis (in comparators) as competing events. P value from Gray’s test.
The cumulative risk of new-onset CVD for NHL patients with preexisting DM treated with steroid- and anthracycline-containing immunochemotherapy compared with matched comparators with preexisting DM and matched comparators without DM. Aalen-Johansen estimator treating death, relapse, and NHL diagnosis (in comparators) as competing events. P value from Gray’s test.
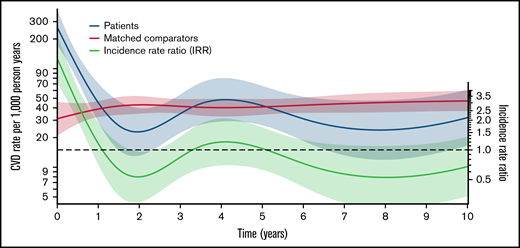
The IR of CVD among matched comparators only varied slightly during follow-up (rate range of 31 to 47 events per 1000 person years). The IRR of CVD for patients vs comparators was higher for patients in the first year following treatment (maximum IRR between 0-1 years, 8.44 [95% CI, 4.77-14.93]) and lower from 1 to 3 years (minimum IRR, 0.53 [95% CI, 0.29-0.97]). There was no significant difference from 3 to 10 years (Figure 5).
The incidences rates (red for matched comparators; blue for NHL patients with preexisting DM treated with steroid- and anthracycline-containing immunochemotherapy) of new-onset CVD per 1000 person years and the incidence rate ratio (IRR; green) between patients and matched comparators >10 years from start of lymphoma treatment (index date). The dotted black line shows an IRR of 1.0 for reference.
The incidences rates (red for matched comparators; blue for NHL patients with preexisting DM treated with steroid- and anthracycline-containing immunochemotherapy) of new-onset CVD per 1000 person years and the incidence rate ratio (IRR; green) between patients and matched comparators >10 years from start of lymphoma treatment (index date). The dotted black line shows an IRR of 1.0 for reference.
Discussion
In the present study, NHL patients treated with steroid-containing immunochemotherapy did not face a clinically relevant increased risk of DM compared with a matched population or lymphoma patients managed by watch and wait. The IRR of DM for patients compared with matched comparators varied over time and was increased during the first year following lymphoma treatment, which may be due to triggering of a prediabetic state to overt diabetes by steroids: patients who would potentially have developed DM at some point in the future.6 This could also explain the lower IRR in the following years. When studying the absolute risk of DM, in terms of cumulative risk, the probability of being diagnosed with DM was lower for patients than for comparators after 2 years. This result may reflect higher rates of the competing events (death and relapse) among patients with lymphoma.
The risk of type 2 DM is highly influenced by lifestyle behaviors, such as diet and exercise, with unhealthy dietary habits and lack of exercise being risk factors.38 Being diagnosed with a life-threating diagnosis, such as lymphoma, could potentially improve the lymphoma patients’ awareness of health in general and thereby lead to a healthier lifestyle and, subsequently, a lower risk of diabetes. However, studies regarding the impact of cancer on lifestyle behaviors are inconsistent in regard to postcancer dietary habits, whereas for exercise, some studies find that daily exercise is reduced following a cancer diagnoses.39-44 Although this study does not include information on lifestyle factors, information on educational level is included, and this has been shown to correlate well with lifestyle.45 There was no difference in educational level between patients without DM and matched comparators without DM, suggesting similar life styles.
Treatment with high-dose steroids can lead to a risk of steroid-induced DM or dysregulation of preexisting DM due to an increased resistance to insulin in muscle, liver, and fat tissue and an increased glucose production in the liver.18 To our knowledge, no studies have shown β-cell toxicity following R-CHOP treatment. In our study, NHL patients did not have a statistically significant higher cumulative incidence of type 1 diabetes following immunochemotherapy treatment compared with comparators, suggesting no increased risk of β-cell toxicity after treatment (P = .69, supplemental Figure 4).
A previous retrospective study of 80 lymphoma patients investigated the risk of DM following R-CHOP treatment and found that 32.5% developed DM during chemotherapy treatment.20 The study defined DM by the American Diabetes Association’s criteria based on blood glucose values (fasting plasma glucose level ≥126 mg/dL, random plasma glucose level ≥200 mg/dL along with symptoms of hyperglycemia, or HbA1c level of ≥6.5%). Even though temporary hyperglycemia is a good measurement for steroid-induced DM, it may not predict whether DM persists in the long term. Only 3 patients were treated with insulin for hyperglycemia in that study, and the treatment was only short term during the first or second cycle of chemotherapy.20
Other studies have shown an increased risk of hyperglycemia for NHL patients treated with R-CHOP.25,46 The retrospective study by Lamar et al found that 47% of the 160 included patients had at least 1 hyperglycemic episode based on random plasma glucose values during treatment with R-CHOP–like regimens. The study population consisted of 27% patients with preexisting DM, which was a risk factor for hyperglycemia, with a HR of 12.7.25 Our study did not include hyperglycemia as an event as our study relied on register data, and blood glucose levels at fixed time points were not available.
Our study assessed the cumulative risk of any insulin prescriptions among NHL patients with DM and no prior use of insulin and found a difference in the cumulative incidence of any insulin prescriptions after 0.5 and 5 years of follow-up of 15.3 and 11.8 percentage units, respectively, compared with matched comparators (Figure 3). Our study may overestimate the incidence of insulin use among NHL patients because insulin could have been temporarily used to control hyperglycemia in relation to an ongoing infection or other complications. However, it remains likely that the insulin prescriptions were prescribed to control steroid-induced hyperglycemia rather than for other complications that lead to increased stress and hyperglycemia because the study analyzed prescriptions for home use and not in-hospital use of insulin. The study also investigated the risk of new insulin prescriptions 1 year after treatment and found that the increased risk for patients, which was predominantly found right after treatment, was reversed. This suggests that the increased risk of new insulin prescriptions for NHL patients with DM treated with R-CHOP(-like) treatment is temporary and that patients needing insulin treatment after treatment for NHL may only need it temporarily.
Our study investigated the risk of anthracycline-related CVDs among NHL patients with preexisting diabetes as NHL patients treated with anthracycline-containing immunochemotherapy are at an increased risk of CVDs both due to the treatment and the preexisting diabetes.23 There was an increased risk of new-onset CVDs among NHL patients with DM in the first year after treatment. After the first year, the rate was similar for patients and comparators with diabetes. The risk of CVDs might be overestimated among patients due to surveillance bias of diabetic lymphoma patients, but the diabetic comparators are also frequently screened for CVDs during follow-up for diabetes. The 1-year cumulative risk of CVD among NHL patients with preexisting DM treated with any number of cycles was 11.7% in the present study. This was significantly higher compared with the 1-year cumulative risks found in our previous study for patients with DLBCL and FL treated with R-CHOP/R-CHOEP (CHOP+etoposide) with unknown DM status. In that study, we found that patients treated with 3 to 5 cycles of steroid-containing immunochemotherapy had cumulative risks of CVD of 2.5%, 6 cycles had 2.1%, and >6 cycles had 5.9%.23
Although the NHL patients and comparators were matched on age, sex, and CCIS, there is still a risk of unmeasured confounding by indication as especially diabetic NHL patients selected for at least 3 cycles of R-CHOP are likely more healthy on average as compared with the matched comparators. This may reduce the risk difference between diabetic patients treated with R-CHOP and comparators in regard to cardiovascular events. NHL patients in our study had a lower incidence of baseline comorbidities even after matching on CCIS as the matching was relaxed for patients with no exact matches ( Appendix 1).
Prednisolone has been part of the CHOP regimen since its introduction.47,48 The randomized controlled trials, which established the CHOP regimen as the gold standard, did not report on the incidences of hyperglycemia or steroid-induced diabetes.15,17,47 Furthermore, even though the dosage of 100 mg prednisolone is now the standard dosage used in the CHOP regimen, there were discrepancies in the early literature, with standard dosages being described as 100 mg per day or in the range of 100 mg/m2 per day to 50 mg/m2 per day.49 A dose-reduced regimen, R-mini-CHOP was introduced, which contains a 50% dose reduction for the included drugs, except for prednisolone, which is only slightly lower than standard R-CHOP (40 mg/m2).50 Although different trials have used different dosages, no dose comparison of prednisolone in CHOP has been evaluated; however, in our clinical experience, it is common to reduce steroid doses in the elderly due to diabetes or due to common side effects such as insomnia, confusion, depression, or other mental adverse events. A dose reduction of prednisolone for elderly patients in our study would underestimate the relationship between steroid-containing treatment and DM in our study.
This study is, to our knowledge, the largest study assessing the relationship between steroid-containing immunochemotherapy treatment for NHL patients and DM. Based on this study, we conclude that patients treated with steroid-containing immunochemotherapy for NHL do not experience a clinically relevant higher risk of DM compared with a matched population. For patients with preexisting non–insulin-dependent DM, the risk of any insulin prescriptions was higher for patients, suggesting that steroids lead to further dysregulation of DM and important treatment changes when managing DM. However, the increased risk of insulin prescriptions was only temporary in time and even decreased after 1 year compared with the risk in comparators. Patients with lymphoma and preexisting DM had a temporary higher risk of CVDs in the first year after treatment with anthracycline- and steroid-containing immunochemotherapy compared with comparators with DM. This was significantly higher than what was observed in a previous study of unselected DLBCL and FL patients treated with similar regimens, suggesting that awareness of new cardiovascular symptoms is particularly relevant for diabetic NHL patients.
Acknowledgments
This study was supported by research funding from ømrermester Jørgen Holm og hustru Elisa F. Hansens Mindelegat to J.B., Danish Cancer Society to T.C.E.-G., and Trigon Foundation to T.C.E.-G.
Authorship
Contribution: J.B., M.T.S., L.H.J., T.C.E.-G., A.K.Ø., H.F., P.V., S.E., and K.E.S. contributed to conception and design of the study; J.B. performed the management and coordination responsibilities; J.B., M.T.S., A.K.Ø., H.F., J.J., M.R.C., C.B.P., P.B., A.O.G., R.S.P., L.H.J., and T.C.E.-G. contributed to acquisition of data; J.B., L.H.J., and S.E. performed analysis of data; J.B. drafted the manuscript; and all authors contributed to interpretation of the results, revised the manuscript, and read and approved the final manuscript.
Conflict-of-interest disclosure: H.F. received support outside this work from Alexion, Gilead, Abbvie, Janssen Pharmaceuticals, and Novartis. T.C.E.-G. reports previous employment at Roche Ltd (Basel) and speakers fees from Abbvie. The remaining authors declare no competing interests.
Correspondence: Joachim Baech, Department of Hematology, Clinical Cancer Research Center, Aalborg University Hospital, Mølleparkvej 4, DK-9000 Aalborg, Denmark; e-mail: j.baech@rn.dk.
Appendix 1: matching
CCIS was calculated at 6 months prior to inclusion and was based on an ICD-10 updated CCIS and further updated by including prescriptions for insulin and oral antidiabetic medicine to identify DM observed in the primary sector.51 Matching on CCIS was done on the specific score whenever possible. When matching on specific score was not available due to missing possible matches, patients with CCIS < 6 were allowed to differ 1 in CCIS (+1/−1), patients with CCIS ≥ 6 and <10 were allowed to differ 2 (+2/−2), and patients with CCIS ≥ 10 were matched on +20/−2 CCIS.
In the analysis of CVD, patients were matched to both a population with DM and one without DM to show the increased risk of CVD in comparison with a DM-free population.
Appendix 2: comorbidities
Cardiovascular diseases: I20 to I25, I46, I47, I48, I49.0, I50.
Cerebrovascular diseases: I60 to I69.
Circulatory: I10 to I82.
Chronic obstructive pulmonary disease: J43 to J44, J84.
Liver disease: K70 to K77.
Renal disease: N00 to N19, N28.9, N08.3, I12.
Appendix 3: cardiovascular diseases
Ischaemic heart diseases: I20 to I25.
Cardiac arrest: I46.
Heart failure: I50.
Atherosclerosis: I70.
Aortic aneurysm and dissection: I71.
References
Author notes
For register studies conducted on Statistics Denmark, data are not allowed to be shared with anyone according to rules set forth by the Danish authorities.
The full-text version of this article contains a data supplement.