TO THE EDITOR:
Ruxolitinib, approved for the treatment of primary and secondary myelofibrosis (MF), can reduce spleen volume and disease-related symptoms, with responses usually observed within the first 3 to 6 months of treatment. Nevertheless, a proportion of patients does not respond adequately to treatment and develops a progressive disease. To date, there is no consensus definition of ruxolitinib failure.1,2 Unfortunately, ∼25% of patients were able to continue with the drug after 5 years,3 and the outcome of patients who discontinue the treatment has been reported to be very poor with a median overall survival (OS) of 14 months.4,5 Considering the availability of new drugs in the setting of resistant patients, the identification of patients who will fail completely or become suboptimal to treatment remained an unmet need. Recently, a clinical prognostic score was investigated to determine survival after 6 months of treatment with ruxolitinib in patients with MF.6 Several risk factors were identified, such as ruxolitinib dose <20 mg twice daily at baseline, 3 and 6 months, palpable spleen length reduction from baseline ≤30% at 3 and 6 months, transfusion need at 3 and/or 6 months and at all time points analyzed. A model was created, namely RR6 (response to ruxolitinib after 6 months), able to stratify patients in 3 different categories: low, intermediate, and high risk. The model was validated in a small cohort of 40 patients.
We applied the RR6 prognostic model in a retrospective, single-center experience to ruxolitinib-treated patients with MF, to confirm its predictive ability. An overall cohort of 140 patients with MF was analyzed, with a median age at diagnosis of 63 years. A slight female predominance was observed (52.9% vs 47.1%) (Table 1). Before ruxolitinib start, 10.7% of patients were transfusion dependent. Median follow-up from diagnosis was 30.5 months (range, 6-120), whereas the median time on ruxolitinib was 26 months (range, 6-120). Ruxolitinib starting dose was 20 mg twice daily in 41% of patients, 15 mg twice daily in 27.5% of patients, 10 mg twice daily in 19.6% of patients, and 5 mg twice daily in 10.9% of patients. After 3 months, 33 patients (26.2%) were continuing full dose (20 mg twice daily), whereas after 6 months only 24 patients (20%) were still taking 20 mg twice daily. Seventy-seven patients were evaluable at 24 months; 12 patients (15.6%) were assuming 20 mg twice daily; 60 patients (78%) were transfusion independent; and 37 patients (48%) still maintained the spleen response >30%. We obtained local ethical approval for the study, which was conducted according to the Declaration of Helsinki.
Characteristics of analyzed patients at ruxolitinib start
| Features . | Low risk (N = 10) . | Intermediate risk (N = 92) . | High risk (N = 38) . | P . |
|---|---|---|---|---|
| Sex (%) | .98 | |||
| Male, n | 5 (3.6) | 49 (35) | 20 (14.3) | |
| Female, n | 5 (3.6) | 43 (30.7) | 18 (12.9) | |
| Age at diagnosis, median, y (range) | 54 (32-84) | 64 (16-82) | 62.5 (38-81) | .15 |
| Primary MF, n (%) | 3 (2.1) | 42 (30) | 20 (14.3) | .29 |
| Post–polycythemia vera, n (%) | 4 (2.9) | 26 (18.6) | 5 (3.6) | |
| Post–essential thrombocythemia, n (%) | 3 (2.1) | 24 (17.1) | 13 (9.3) | |
| Early MF, n (%) | 2 (1.4) | 14 (10.1) | 3 (2.2) | |
| Overt MF (%) | 8 (5.8) | 78 (56.5) | 35 (23.9) | |
| Grade of fibrosis (%) | .50 | |||
| 0 to 1, n | 2 (1.4) | 13 (9.3) | 6 (4.3) | |
| 2, n | 4 (2.9) | 36 (25.9) | 12 (8.6) | |
| 3, n | 4 (2.9) | 43 (30.9) | 20 (13.7) | |
| IPSS | ||||
| Low | 2 | 5 | 1 | |
| Int-1 | 1 | 12 | 4 | .24 |
| Int-2 | —* | 20 | 9 | |
| High | — | 5 | 6 | |
| MYSEC-PM | ||||
| Low | 1 | 5 | — | |
| Int-1 | 5 | 15 | 2 | .35 |
| Int-2 | 1 | 22 | 13 | |
| High | — | 8 | 3 | |
| Symptoms at diagnosis, n (%) | 3 (2.1) | 42 (30) | 13 (9.3) | .36 |
| JAK2 mutated, n (%) | 6 (4.3) | 67 (47.9) | 25 (17.9) | .56 |
| CALR mutated, n (%) | 1 (0.7) | 8 (5.7) | 6 (4.3) | .46 |
| TTS, median (range) | 0 (0-55) | 10 (0-70) | 15 (0-50) | .35 |
| Transfusion dependent, n (%) | /* | 23 (16.5) | 21 (15.1) | .42 |
| Palpable spleen below LCM, median, cm (range) | 5.5 (0-15) | 6 (0-23) | 4.5 (0-16) | .015 |
| Dose at ruxolitinib start (%) | .013 | |||
| 5 mg bid, n | / | 10 (7.2) | 5 (3.6) | |
| 10 mg bid, n | / | 20 (14.5) | 7 (5.1) | |
| 15 mg bid, n | / | 28 (20.3) | 11 (7.2) | |
| 20 mg bid, n | 10 (7.2) | 34 (23.9) | 15 (10.9) | |
| Features . | Low risk (N = 10) . | Intermediate risk (N = 92) . | High risk (N = 38) . | P . |
|---|---|---|---|---|
| Sex (%) | .98 | |||
| Male, n | 5 (3.6) | 49 (35) | 20 (14.3) | |
| Female, n | 5 (3.6) | 43 (30.7) | 18 (12.9) | |
| Age at diagnosis, median, y (range) | 54 (32-84) | 64 (16-82) | 62.5 (38-81) | .15 |
| Primary MF, n (%) | 3 (2.1) | 42 (30) | 20 (14.3) | .29 |
| Post–polycythemia vera, n (%) | 4 (2.9) | 26 (18.6) | 5 (3.6) | |
| Post–essential thrombocythemia, n (%) | 3 (2.1) | 24 (17.1) | 13 (9.3) | |
| Early MF, n (%) | 2 (1.4) | 14 (10.1) | 3 (2.2) | |
| Overt MF (%) | 8 (5.8) | 78 (56.5) | 35 (23.9) | |
| Grade of fibrosis (%) | .50 | |||
| 0 to 1, n | 2 (1.4) | 13 (9.3) | 6 (4.3) | |
| 2, n | 4 (2.9) | 36 (25.9) | 12 (8.6) | |
| 3, n | 4 (2.9) | 43 (30.9) | 20 (13.7) | |
| IPSS | ||||
| Low | 2 | 5 | 1 | |
| Int-1 | 1 | 12 | 4 | .24 |
| Int-2 | —* | 20 | 9 | |
| High | — | 5 | 6 | |
| MYSEC-PM | ||||
| Low | 1 | 5 | — | |
| Int-1 | 5 | 15 | 2 | .35 |
| Int-2 | 1 | 22 | 13 | |
| High | — | 8 | 3 | |
| Symptoms at diagnosis, n (%) | 3 (2.1) | 42 (30) | 13 (9.3) | .36 |
| JAK2 mutated, n (%) | 6 (4.3) | 67 (47.9) | 25 (17.9) | .56 |
| CALR mutated, n (%) | 1 (0.7) | 8 (5.7) | 6 (4.3) | .46 |
| TTS, median (range) | 0 (0-55) | 10 (0-70) | 15 (0-50) | .35 |
| Transfusion dependent, n (%) | /* | 23 (16.5) | 21 (15.1) | .42 |
| Palpable spleen below LCM, median, cm (range) | 5.5 (0-15) | 6 (0-23) | 4.5 (0-16) | .015 |
| Dose at ruxolitinib start (%) | .013 | |||
| 5 mg bid, n | / | 10 (7.2) | 5 (3.6) | |
| 10 mg bid, n | / | 20 (14.5) | 7 (5.1) | |
| 15 mg bid, n | / | 28 (20.3) | 11 (7.2) | |
| 20 mg bid, n | 10 (7.2) | 34 (23.9) | 15 (10.9) | |
indicates zero.
bid, twice daily; CALR, calreticulin; JAK2, Januse Kinase 2; LCM, low costal margin; TTS, total symptom score.
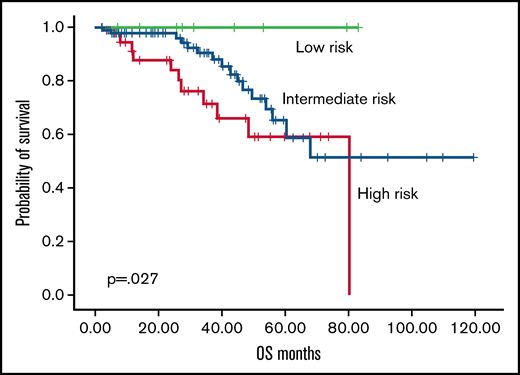
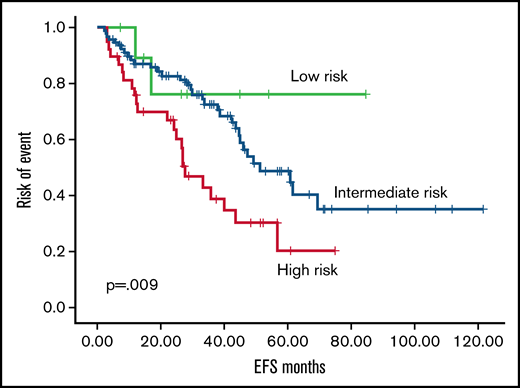
According to the RR6 model, 7.1%, 70.8%, and 27.1% of patients were classified as low, intermediate, and high risk. The OS rate from 6 months after ruxolitinib start was 100%, 82.6%, and 71%, respectively, with an estimated median OS not reached for low risk, 80 months for intermediate risk, and 50 months for high risk (P = .027; Figure 1). In the RR6 intermediate risk, female patients showed an increased estimated median OS (99 vs 63 months in male patients; P = .027). High-risk RR6 had a significantly increased risk of mortality in patients with post–polycythemia vera MF (P = .002) and in patients with overt MF at diagnosis (P = .046). Events considered for event-free survival (EFS) were death, allogeneic stem cell transplantation, progression of disease, loss of response, or toxicity, which required ruxolitinib discontinuation. High risk according to RR6 model showed an estimated median EFS of 27 months vs 51 months for low-/intermediate-risk patients (P = .009; Figure 2). Multivariate analysis performed in all parameters that showed a significance (RR6 model, symptoms at baseline, JAK2 mutation, overt MF) confirmed RR6 stratification role in predicting outcomes in ruxolitinib-treated patients with MF in terms of both OS (P = .002) and EFS (P = .003) for high-risk patients.
OS according to RR6 model. Overall survival according to RR6 model (low risk, green; intermediate risk, blue; high risk, red).
OS according to RR6 model. Overall survival according to RR6 model (low risk, green; intermediate risk, blue; high risk, red).
EFS according to RR6 model. Event free survival according to RR6 model (low risk, green; intermediate risk, blue; high risk, red).
EFS according to RR6 model. Event free survival according to RR6 model (low risk, green; intermediate risk, blue; high risk, red).
The early identification of failure or suboptimal response to ruxolitinib among patients with MF treated in first line or after conventional treatment remains an unmet need. Although the introduction of commercial new inhibitors or the possibility to include patients in ongoing trials with new drugs exploring different pathways, no clear definition and possible timing of intervention have been clarified by the International Working Group-Myeloproliferative Neoplasms Research and Treatment or European LeukemiaNet consortium.7 All the prognostic models for patients with MF did not provide a specifically indication regarding the outcome of patients during treatment with ruxolitinib. Indeed, a poor outcome has been described in patients with MF during treatment with ruxolitinib in the absence of a clinical response, owing to possible clonal evolution, persistent thrombocytopenia, and possible leukemic transformation.8,9
We validated the RR6 model in a large series of patients with MF treated with ruxolitinib: our results showed that the model identifies candidates for second-line treatment, among patients classified as high risk with a low median OS and EFS. In our experience, the RR6 model clearly indicated the need of a careful monitoring of postpolycythemia patients with MF among the high-risk categories.
In conclusion, the RR6 model could be applied for early identification of patients with MF with inferior survival and for candidates to alternative treatments and/or allogeneic procedures.
Contribution: E.S., C.I., C.L., P.M., M.L.B., I.C. followed patients, analyzed data; G.M.A. performed statistical analysis; M.M. approved the final version; and M.B. designed the research and wrote the paper.
Conflict-of-interest disclosure: M.B. received honoraria by Novartis, Incyte, Pfizer, BMS, Abbvie. The remaining authors declare no competing financial interests.
Correspondence: Massimo Breccia, Hematology, Department of Translational and Precision Medicine, Sapienza University, Via Benevento 6, 00161 Rome, Italy; e-mail: breccia@bce.uniroma1.it.
References
Author notes
Contact the corresponding author for data sharing: breccia@bce.uniroma1.it.