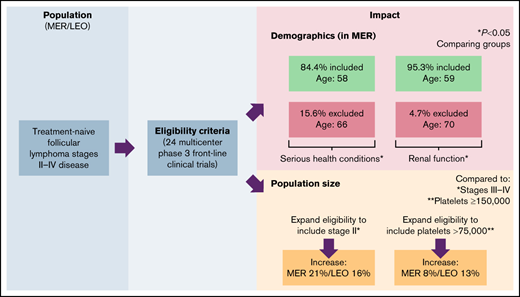
Key Points
Excluding patients with renal dysfunction, prior malignancies, and serious health conditions resulted in a younger trial population.
Liberalizing stage and platelet criteria could increase the pool of trial-eligible patients without impacting patient outcomes.
Abstract
Cancer clinical trial eligibility criteria may create patient populations studied in trials that do not reflect the patient populations treated in the real-world setting. Follicular lymphoma (FL) is an indolent lymphoma with heterogeneous presentations across a broad range of individuals, resulting in many acceptable management strategies. We evaluated how first-line clinical trial eligibility criteria impacted the demographic makeup and outcomes of patients with FL for whom systemic therapy might be considered. We compared the characteristics of 196 patients with FL from a single institution to eligibility criteria from 10 first-line FL trials on clinicaltrials.gov. Next, we tabulated eligibility criteria from 24 first-line FL protocols and evaluated their impact on 1198 patients with FL with stages II to IV disease from the prospective Molecular Epidemiology Resource (MER) and Lymphoma Epidemiology of Outcomes (LEO) cohort studies. We found that 39.8% and 52.7% of patients with FL might be excluded from clinical trials based on eligibility criteria derived from clinicaltrials.gov and protocol documents, respectively. Patients excluded because of renal function, prior malignancy, and self-reported serious health conditions tended to be older. Expanding stage requirement from III-IV to II-IV, and platelet requirement from ≥150 000 to ≥75 000 increased population size by 21% and 8%, respectively, in MER and by 16% and 13%, respectively, in LEO, without impacting patient demographics or outcomes. These data suggest that management of older individuals with FL may not be fully informed by recent clinical trials. Moreover, liberalizing stage and platelet criteria might expand the eligible population and allow for quicker trial accrual without impacting outcomes.
Introduction
Follicular lymphoma (FL) is a heterogeneous disease, affecting both men and women, with a broad age distribution. There are no clear risk factors other than family history and certain autoimmune conditions, and there are no strong biomarkers that inform prognosis or management.1 Additionally, although generally agreed on indications for treatment initiation exist including compromised organ function because of progressive or bulky disease, presence of systemic B symptoms, cytopenias, and an increase in disease tempo,2,3 there remains considerable heterogeneity in terms of which patients are treated and when they are treated. Furthermore, although anti-CD20–based therapy is preferred for initial treatment of FL, the exact therapy is highly variable and requires evaluation of patient preference and side effect profile, among other considerations. As such, the sample of patients with FL represented in clinical trials should be as broad as possible to accommodate the broad swath of patients that FL can potentially impact.
Cancer clinical trial eligibility criteria, which exist to define and protect potential study participants,4-7 may unintentionally create populations with different demographic and clinical characteristics than the broader population of patients seen in oncology offices and may contribute to this divergence in outcomes. Injudicious eligibility criteria can jeopardize generalizability of study results with little to no commensurate gain in internal validity or participant safety8-10 and might also have implications regarding equitable access to investigational therapies. Inconsistent eligibility criteria between trials further complicates intertrial comparisons. Overly restrictive eligibility criteria can slow the process of clinical research, which increases research costs and deprives society of new treatments. Given the rapid rate of drug development and approvals over the past decade, these issues are particularly relevant to FL.
Interestingly, despite the potential negative consequences of imprudent criteria, newer clinical trials often include more restrictive eligibility criteria than older trials. For instance, in a study of 74 thoracic oncology clinical trials activated between 1986 and 2016, researchers found that the median number of eligibility criteria in trials activated between 1986 and 1995 was 16 compared with 27 in trials activated between 2006 and 2016.11 Meanwhile, common eligibility criteria may not always effect their intended purpose. For example, exclusion of patients with a history of malignancy, which might be intended to avoid negatively biasing the trial results has not been shown to impact study outcomes.12,13
In this study, we hypothesized that eligibility criteria may be responsible for selection of research populations with distinct demographic and clinical characteristics and outcomes. We additionally evaluated whether certain criteria were duplicative and whether liberalizing criteria might enlarge the pool of eligible patients with FL for trial participation.
Methods
Clinical trials
We used the Advanced Search function to download data on 10 front-line phase 2 and 3 FL clinical trials that opened between 2002 and 2017 using clinicaltrials.gov, and we abstracted clinical trial eligibility criteria. A list of studies included is provided in supplemental Table 1. Eligibility criteria were aggregated and summarized into broad categories.
Subsequently, we abstracted clinical trial eligibility criteria from protocol documents in the Follicular Lymphoma Analysis of Surrogacy Hypothesis (FLASH) database, which consists of 21 multicenter phase 3 randomized clinical trials in patients with previously untreated FL published after 1990. The FLASH database was distinct from the clinicaltrials.gov studies described above.14 We additionally abstracted detailed clinical trial eligibility criteria from E2408,15 GALLIUM,16 and RELEVANCE17 trials. Eligibility criteria were aggregated and summarized into post hoc broad categories based on the most common criteria specifications in reviewed clinical trials. Within eligibility criteria categories, certain eligibility criteria were divided into 2 and sometimes 3 criteria specifications depending on common criteria specifications across the various clinical trials (eg, creatinine < 1.5 mg/dL vs < 2.0 mg/dL as 2 separate criteria specifications for the eligibility criteria category of renal function).
Patient cohort comparisons
We used baseline data on a cohort of patients with untreated FL derived from the Weill Cornell Medicine (WCM) Lymphoma Cohort, a prospective cohort study of patients with non-Hodgkins lymphoma (NHL) that started in 2010 and evaluated the impact of clinical trial eligibility criteria derived from clinicaltrials.gov on these patients.
For further comparison, we used data on patients enrolled in the Molecular Epidemiology Resource (MER) of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (SPORE) and the Lymphoma Epidemiology of Outcomes (LEO) cohort studies. The MER cohort study is a prospective cohort study of patients newly diagnosed with NHL enrolled between 1 September 2002 and 30 June 2015 at both Mayo Clinic Rochester and the University of Iowa.18 The LEO cohort study expanded the MER cohort study to 6 additional centers and began enrollment on 1 July 2015 of patients newly diagnosed with NHL at Cornell University, Emory University, Mayo Clinic, MD Anderson, University of Iowa, University of Miami, University of Rochester, and Washington University in St. Louis.19 For both the MER and LEO cohort studies, patients were included in this study if they were diagnosed with stages II to IV FL and received some form of immunochemotherapy as their first-line treatment. Patients diagnosed with stage I FL were not eligible. Conversely, patients with stage IIIb disease were not excluded from these analyses, given that <5% of patients in either cohort had stage IIIb disease, and exclusion of patients with stage IIIb disease did not substantially impact our analyses. Notably, all patients from WCM enrolled in the LEO cohort study are also represented in the WCM Lymphoma Cohort. Sensitivity analyses were performed excluding the patients from WCM from the LEO cohort to examine the effect of double evaluation. We excluded patients who underwent observation as their first line of management to minimize the time between baseline data and initiation of therapy. Sensitivity analyses were performed comparing patients receiving first-line therapy and patients undergoing observation to better characterize our decision to exclude patients undergoing observation. Patient characteristics available for evaluation included stage, renal function, hepatic function, performance status, serious health conditions, prior cancer diagnosis, WBC requirement, platelet requirement, hemoglobin requirement, and time from diagnosis to treatment. The remaining eligibility criteria could not be evaluated either because of sample size (too few patients being excluded) or because of data availability. With the exception of serious health conditions, all of the aforementioned criteria are derived from physician report (eg, stage, performance status), laboratory data at time of diagnosis (eg, renal function, hepatic function, WBC requirement, platelet requirement, hemoglobin requirement), or chart review (eg, prior cancer diagnosis, time from diagnosis to treatment). The serious health conditions eligibility criterion was derived from patient self-report, with health conditions including heart disease, congestive heart failure, myocardial infarction, emphysema, chronic bronchitis, pulmonary embolism, osteoporosis, depression, and so on.
Data analysis
Descriptive statistics were performed as counts and percentages among the patients in the WCM Lymphoma Database, the MER cohort, and the LEO cohort separately. Two-sample t tests and χ2 tests were used as tests for significance for continuous and categorical variables, respectively. Within the WCM Lymphoma Database, baseline patient characteristics were compared with baseline patient characteristics of patients in the various clinical trials. Within both the MER cohort and the LEO cohort, various eligibility criteria specifications were used to divide the cohorts into the included and excluded populations, consisting of patients who met the eligibility criteria specification and patients who did not meet the specification, respectively. Descriptive statistics were similarly performed among patients subdivided into included and excluded populations in both the MER cohort and the LEO cohort separately. Two-sample t tests were used as tests for significance to compare age across included and excluded populations. To evaluate the relative contribution of individual criteria to number of patients potentially excluded from trial participation, a stepwise approach was used to quantify additional patients excluded with individual criteria after applying other criteria. Using this approach, we examined the added effect of an eligibility criterion by evaluating the additional number of patients excluded after applying that eligibility criterion beyond the number of patients already excluded prior to applying that eligibility criterion. Similarly, to evaluate the relative contribution of individual criteria specification stringency (defined by the number of patients theoretically excluded based on the criteria specification, with more patients theoretically excluded with more stringent criteria specifications), a stepwise approach was also used to quantify additional patients included after liberalizing individual criteria after applying other criteria.
For survival analyses, event-free survival (EFS) was calculated from time of FL diagnosis until disease progression or relapse, unplanned retreatment for lack of efficacy, or death from any cause. We used EFS as an end point instead of overall survival, given that most trials in FL have used progression-free survival as a primary end point, and the goal of our analyses is to show that trial objectives are similar. Survival analyses were only performed in MER given insufficient follow-up data in LEO. Log-rank tests were used to statistically evaluate differences between EFS curves across comparisons.
Given the possibility of patients in both MER and LEO cohorts who were treated with single-agent rituximab as first-line treatment being systematically different from patients who were treated with other combination regimens, additional sensitivity analyses were performed to quantify the impact of these differences. Demographic and clinical characteristics were compared between patients who were treated with single-agent rituximab as first-line treatment and patients who were treated with other combination regimens in both MER and LEO cohorts. Finally, all analyses detailed above were repeated in the MER and LEO cohorts after exclusion of patients receiving single-agent rituximab as first-line treatment. All analyses were performed using R software 3.6.3. This research was approved by the relevant institutional review boards.
Results
Eligibility criteria
Eligibility criteria from 10 trials identified in clinicaltrials.gov and criteria derived from actual protocol documents for 24 first-line phase 3 FL clinical trials are in Table 1. Among the 24 first-line phase 3 FL clinical trials, the most common eligibility criteria category included stage (with 21 [88%] clinical trials including some form of specification for the category of stage), renal function (n = 21, 88%), HIV/AIDS status (n = 20, 83%), performance status (n = 19, 79%), and history of other malignancy (n = 19, 79%); conversely, the least common eligibility criteria categories (all <15%) included no major surgery within 28 days of trial registration, tissue diagnosis within 12 months of trial registration, and histologic exam within 6 months of trial registration. Among the 10 trials identified in clinicaltrials.gov, the most common eligibility criteria included stage (N = 9, 90%), platelet requirement (N = 8, 80%), pregnancy status (n = 8, 80%), performance status (n = 7, 70%), and HIV/AIDS status (n = 6, 60%).
Eligibility criteria summarized across 24 first-line phase 3 clinical trials and 10 clinicaltrials.gov trials in follicular lymphoma
| Variables . | Phase 3 clinical trials, n (%) . | Phase 2-3 clinicaltrials.gov trials, n (%) . |
|---|---|---|
| Stage/extent of disease | 21 (88) | 9 (90) |
| Renal function | 21 (88) | 3 (30) |
| HIV/AIDS status | 20 (83) | 6 (60) |
| Performance status | 19 (79) | 7 (70) |
| History of other malignancies | 19 (79) | 4 (40) |
| Hepatic function | 19 (79) | 3 (30) |
| Cardiac function | 17 (71) | 1 (10) |
| Treatment required for disease | 15 (63) | |
| Grade | 14 (58) | |
| Pregnancy status | 13 (54) | 8 (80) |
| Neuro/psych function | 13 (54) | |
| Metabolic disease | 12 (50) | |
| Birth control use | 12 (50) | |
| Pulmonary function | 12 (50) | |
| Self-reported serious medical conditions | 12 (50) | 5 (50) |
| CNS involvement | 11 (46) | |
| WBC requirement | 11 (46) | |
| HBV status | 10 (42) | 3 (30) |
| Platelet requirement | 10 (42) | 8 (80) |
| Absence of FL transformation | 10 (42) | |
| Measurable disease | 9 (38) | |
| CD20 positivity | 9 (38) | |
| HCV status | 8 (33) | 2 (20) |
| Active infection status | 8 (33) | 3 (30) |
| Life expectancy | 8 (33) | |
| Breastfeeding status | 8 (33) | |
| No concurrent trial registration | 5 (21) | 5 (50) |
| Hemoglobin requirement | 4 (17) | 1 (10) |
| On study within 3 mo of diagnosis | 3 (13) | |
| No major surgery within 28 d | 3 (13) | |
| Tissue diagnosis within 12 mo | 2 (8) | |
| Histologic exam within 6 mo | 1 (4) |
| Variables . | Phase 3 clinical trials, n (%) . | Phase 2-3 clinicaltrials.gov trials, n (%) . |
|---|---|---|
| Stage/extent of disease | 21 (88) | 9 (90) |
| Renal function | 21 (88) | 3 (30) |
| HIV/AIDS status | 20 (83) | 6 (60) |
| Performance status | 19 (79) | 7 (70) |
| History of other malignancies | 19 (79) | 4 (40) |
| Hepatic function | 19 (79) | 3 (30) |
| Cardiac function | 17 (71) | 1 (10) |
| Treatment required for disease | 15 (63) | |
| Grade | 14 (58) | |
| Pregnancy status | 13 (54) | 8 (80) |
| Neuro/psych function | 13 (54) | |
| Metabolic disease | 12 (50) | |
| Birth control use | 12 (50) | |
| Pulmonary function | 12 (50) | |
| Self-reported serious medical conditions | 12 (50) | 5 (50) |
| CNS involvement | 11 (46) | |
| WBC requirement | 11 (46) | |
| HBV status | 10 (42) | 3 (30) |
| Platelet requirement | 10 (42) | 8 (80) |
| Absence of FL transformation | 10 (42) | |
| Measurable disease | 9 (38) | |
| CD20 positivity | 9 (38) | |
| HCV status | 8 (33) | 2 (20) |
| Active infection status | 8 (33) | 3 (30) |
| Life expectancy | 8 (33) | |
| Breastfeeding status | 8 (33) | |
| No concurrent trial registration | 5 (21) | 5 (50) |
| Hemoglobin requirement | 4 (17) | 1 (10) |
| On study within 3 mo of diagnosis | 3 (13) | |
| No major surgery within 28 d | 3 (13) | |
| Tissue diagnosis within 12 mo | 2 (8) | |
| Histologic exam within 6 mo | 1 (4) |
Patient characteristics
From the WCM Lymphoma Database, we identified 196 patients with untreated stages II to IV FL. A total of 615 patients with newly diagnosed untreated stages II to IV FL from the MER cohort and 583 patients with newly diagnosed untreated stages II to IV FL from the LEO cohort were included in these analyses. Baseline demographics of patients in the MER and LEO cohorts at time of diagnosis are shown in Table 2. The majority of patients in MER and LEO initiated treatment within 3 months of diagnosis (95% in MER, 91% in LEO); equal proportions of patients in both cohorts received cyclophosphamide, doxorubicin, vincristine, and prednisone-containing regimens and rituximab alone for first-line treatments, with a large proportion of patients in the LEO cohort also receiving bendamustine-containing regimens compared with a large proportion of patients in the MER cohort also receiving cyclophosphamide, vincristine, and prednisone-containing regimens, consistent with practice pattern changes in the United States. In the MER cohort, patients receiving combination therapies (n = 413) had bulkier disease, fewer self-reported serious health conditions, fewer prior cancer diagnoses, and received more first-line cycles compared with patients receiving single-agent rituximab (n = 202). Similarly, in the LEO cohort, patients receiving combination therapies (n = 430) had bulkier disease, worse performance status, and received more first-line cycles compared with patients receiving single-agent rituximab (n = 153). However, in both the MER and LEO cohorts, there were no differences in terms of age, sex, race/ethnicity, stage at diagnosis, or hematologic/chemistry parameters (supplemental Table 2). Compared with patients undergoing observation as their first-line management, patients receiving treatment at the time of diagnosis were enriched for bulky disease and disease with splenic involvement, but otherwise did not differ in terms of performance status, self-reported serious health conditions, prior cancer diagnoses, and hematologic/chemistry parameters, with the exception of hemoglobin (supplemental Table 3).
Characteristics of patients with FL receiving first-line treatment in the WCM cohort (n = 196), MER cohort (n = 615), and LEO cohort (n = 583)
| Variables . | WCM cohort* . | MER cohort . | LEO cohort† . | |||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | |
| Age‡ | 62.6 ± 0.9 | 58.4 ± 0.5 | 59.5 ± 0.5 | |||
| Sex | ||||||
| Male | 95 | 48.5 | 329 | 53.5 | 293 | 50.3 |
| Female | 101 | 51.5 | 286 | 46.5 | 290 | 49.7 |
| Race | ||||||
| White | 156 | 79.6 | 546 | 88.8 | 510 | 87.5 |
| Other | 13 | 6.6 | 10 | 1.6 | 64 | 11.0 |
| Unknown | 27 | 13.8 | 59 | 9.6 | 9 | 1.5 |
| Ethnicity | ||||||
| Non-Hispanic | 159 | 81.1 | 492 | 80.0 | 507 | 87.0 |
| Hispanic | 19 | 9.7 | 9 | 1.5 | 63 | 10.8 |
| Unknown | 18 | 9.2 | 114 | 18.5 | 13 | 2.2 |
| Ann Arbor stage | ||||||
| II | 26 | 13.3 | 105 | 17.1 | 82 | 14.1 |
| III | 56 | 28.6 | 176 | 28.6 | 150 | 25.7 |
| IV | 46 | 23.5 | 325 | 52.8 | 321 | 55.1 |
| Unknown | 68 | 34.7 | 9 | 1.5 | 30 | 5.1 |
| Performance status | ||||||
| 0 | 143 | 73.0 | 403 | 65.5 | 338 | 58.0 |
| 1 | 51 | 26.0 | 178 | 28.9 | 177 | 30.4 |
| 2+ | 2 | 1.0 | 31 | 5.0 | 37 | 6.3 |
| Unknown | 0 | 0.0 | 3 | 0.5 | 31 | 5.3 |
| Splenic involvement | ||||||
| Yes | 89 | 14.5 | 128 | 22.0 | ||
| No | 522 | 84.9 | 420 | 72.0 | ||
| Unknown | 4 | 0.7 | 35 | 6.0 | ||
| Bulky disease | ||||||
| Yes | 8 | 4.1 | 56 | 9.1 | 76 | 13.0 |
| No | 119 | 60.7 | 548 | 89.1 | 447 | 76.7 |
| Unknown | 69 | 35.2 | 11 | 1.8 | 60 | 10.3 |
| WBC‡ | 7.3 ± 0.2 | 19.0 ± 11.5 | 7.7 ± 0.4 | |||
| ANC‡ | 4.8 ± 0.2 | 4.6 ± 0.1 | ||||
| Platelets‡ | 218 ± 8.5 | 241 ± 4.2 | 232 ± 3.8 | |||
| Hemoglobin‡ | 13.7 ± 0.1 | 13.4 ± 0.1 | 13.3 ± 0.1 | |||
| Creatinine‡ | 0.9 ± 0.02 | 1.0 ± 0.02 | 1.0 ± 0.01 | |||
| Bilirubin‡ | 0.7 ± 0.04 | 0.6 ± 0.01 | 0.6 ± 0.02 | |||
| Self-reported serious health conditions | ||||||
| Yes | 54 | 27.6 | 96 | 15.6 | 179 | 30.7 |
| No | 142 | 72.4 | 519 | 84.4 | 404 | 69.3 |
| Prior cancer diagnosis | ||||||
| Yes | 22 | 11.2 | 58 | 9.4 | 57 | 9.8 |
| Yes excluding exceptions§ | 22 | 3.6 | 37 | 6.3 | ||
| No | 174 | 88.8 | 557 | 90.6 | 526 | 90.2 |
| Number of first-line cycles‡ | 4.9 ± 0.19 | 6.0 ± 0.3 | ||||
| First-line treatmentǁ | ||||||
| CHOP-containing regimen | 168 | 27.3 | 131 | 22.5 | ||
| CVP-containing regimen | 143 | 23.3 | 4 | 0.7 | ||
| Bendamustine-containing regimen | 58 | 9.4 | 186 | 31.9 | ||
| Rituximab | 202 | 32.8 | 153 | 26.2 | ||
| Other | 44 | 7.2 | 109 | 18.7 | ||
| Time from diagnosis to treatment | ||||||
| ≤3 mo | 583 | 94.8 | 533 | 91.4 | ||
| ≤6 and >3 mo | 27 | 4.4 | 50 | 8.6 | ||
| >6 mo | 5 | 0.8 | 0 | 0.0 | ||
| Variables . | WCM cohort* . | MER cohort . | LEO cohort† . | |||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | |
| Age‡ | 62.6 ± 0.9 | 58.4 ± 0.5 | 59.5 ± 0.5 | |||
| Sex | ||||||
| Male | 95 | 48.5 | 329 | 53.5 | 293 | 50.3 |
| Female | 101 | 51.5 | 286 | 46.5 | 290 | 49.7 |
| Race | ||||||
| White | 156 | 79.6 | 546 | 88.8 | 510 | 87.5 |
| Other | 13 | 6.6 | 10 | 1.6 | 64 | 11.0 |
| Unknown | 27 | 13.8 | 59 | 9.6 | 9 | 1.5 |
| Ethnicity | ||||||
| Non-Hispanic | 159 | 81.1 | 492 | 80.0 | 507 | 87.0 |
| Hispanic | 19 | 9.7 | 9 | 1.5 | 63 | 10.8 |
| Unknown | 18 | 9.2 | 114 | 18.5 | 13 | 2.2 |
| Ann Arbor stage | ||||||
| II | 26 | 13.3 | 105 | 17.1 | 82 | 14.1 |
| III | 56 | 28.6 | 176 | 28.6 | 150 | 25.7 |
| IV | 46 | 23.5 | 325 | 52.8 | 321 | 55.1 |
| Unknown | 68 | 34.7 | 9 | 1.5 | 30 | 5.1 |
| Performance status | ||||||
| 0 | 143 | 73.0 | 403 | 65.5 | 338 | 58.0 |
| 1 | 51 | 26.0 | 178 | 28.9 | 177 | 30.4 |
| 2+ | 2 | 1.0 | 31 | 5.0 | 37 | 6.3 |
| Unknown | 0 | 0.0 | 3 | 0.5 | 31 | 5.3 |
| Splenic involvement | ||||||
| Yes | 89 | 14.5 | 128 | 22.0 | ||
| No | 522 | 84.9 | 420 | 72.0 | ||
| Unknown | 4 | 0.7 | 35 | 6.0 | ||
| Bulky disease | ||||||
| Yes | 8 | 4.1 | 56 | 9.1 | 76 | 13.0 |
| No | 119 | 60.7 | 548 | 89.1 | 447 | 76.7 |
| Unknown | 69 | 35.2 | 11 | 1.8 | 60 | 10.3 |
| WBC‡ | 7.3 ± 0.2 | 19.0 ± 11.5 | 7.7 ± 0.4 | |||
| ANC‡ | 4.8 ± 0.2 | 4.6 ± 0.1 | ||||
| Platelets‡ | 218 ± 8.5 | 241 ± 4.2 | 232 ± 3.8 | |||
| Hemoglobin‡ | 13.7 ± 0.1 | 13.4 ± 0.1 | 13.3 ± 0.1 | |||
| Creatinine‡ | 0.9 ± 0.02 | 1.0 ± 0.02 | 1.0 ± 0.01 | |||
| Bilirubin‡ | 0.7 ± 0.04 | 0.6 ± 0.01 | 0.6 ± 0.02 | |||
| Self-reported serious health conditions | ||||||
| Yes | 54 | 27.6 | 96 | 15.6 | 179 | 30.7 |
| No | 142 | 72.4 | 519 | 84.4 | 404 | 69.3 |
| Prior cancer diagnosis | ||||||
| Yes | 22 | 11.2 | 58 | 9.4 | 57 | 9.8 |
| Yes excluding exceptions§ | 22 | 3.6 | 37 | 6.3 | ||
| No | 174 | 88.8 | 557 | 90.6 | 526 | 90.2 |
| Number of first-line cycles‡ | 4.9 ± 0.19 | 6.0 ± 0.3 | ||||
| First-line treatmentǁ | ||||||
| CHOP-containing regimen | 168 | 27.3 | 131 | 22.5 | ||
| CVP-containing regimen | 143 | 23.3 | 4 | 0.7 | ||
| Bendamustine-containing regimen | 58 | 9.4 | 186 | 31.9 | ||
| Rituximab | 202 | 32.8 | 153 | 26.2 | ||
| Other | 44 | 7.2 | 109 | 18.7 | ||
| Time from diagnosis to treatment | ||||||
| ≤3 mo | 583 | 94.8 | 533 | 91.4 | ||
| ≤6 and >3 mo | 27 | 4.4 | 50 | 8.6 | ||
| >6 mo | 5 | 0.8 | 0 | 0.0 | ||
CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CVP, cyclophosphamide, vincristine, and prednisone.
Blank entries correspond to not available data at time of chart review.
From LEO, 43 patients (7.4%) were enrolled from Cornell, 36 (6.2%) were from Emory, 37 (6.3%) were from Iowa, 143 (24.5%) were from Mayo Clinic, 181 (31.0%) were from MD Anderson, 49 (8.4%) were from Miami, 62 (10.6%) were from URMC, and 32 (5.5%) were from Washington University.
Categorical variables are presented as sample size (n) and column percentage (%); continuous variables are presented as mean ± standard error of the mean. Superscripts are added to the continuous variables.
Exceptions consist of non-melanoma skin cancer (eg, squamous cell carcinoma, basal cell carcinoma), breast cancer, cervical cancer in situ, and uterine cancer in situ.
Other first-line treatments consist of acalabrutinib, ABVD, CEPP, EPOCH (with and without rituximab), lenalidomide, cyclophosphamide, chlorabucil, bortezomib, fludarabine, ibritumomab, obinutuzumab, methotrexate, and prednisone.
Comparison of clinicaltrials.gov eligibility criteria and patients in the WCM lymphoma database
Eligibility criteria from the 10 phase 2 and 3 FL clinical trials identified in clinicaltrials.gov were compared with baseline characteristics of patients with newly diagnosed untreated FL from the WCM Lymphoma Database (Table 2). A total of 39.8% (n = 78) of patients in the WCM Lymphoma Database had 1 or more values that potentially excluded them from clinical trial participation. Among eligibility criteria from clinicaltrials.gov, self-reported serious health conditions (n = 42, 21.4%) was responsible for excluding the vast majority of patients, followed by stage (n = 26, 13.3%), prior cancer diagnosis (n = 22, 11.2%), and both serum creatinine (n = 5, 2.6%) and bilirubin level (n = 5, 2.6%) requirements.
Comparison of FLASH eligibility criteria and patients in the MER/LEO cohorts
Using a stepwise approach, relative contribution of various eligibility criteria to the number of patients excluded from potential trial participation was evaluated by examining individual criteria after applying other criteria (Table 3). The only eligibility criteria that consistently impacted sample size regardless of criteria stringency were stage and self-reported serious health conditions.
Relative impact of individual eligibility criteria on number of additional people excluded when all other eligibility criteria are also applied
| Eligibility criteria . | MER cohort . | LEO cohort . | ||
|---|---|---|---|---|
| Relaxed (n = 362) . | Stringent (n = 220) . | Relaxed (n = 276) . | Stringent (n = 171) . | |
| Stage | 60 (21.4) | 33 (16.2) | ||
| Renal function (creatinine) | 21 (5.5) | 20 (8.3) | 2 (0.7) | 4 (2.3) |
| Performance status | 7 (1.9) | 15 (6.4) | 16 (5.5) | 15 (8.1) |
| Prior malignancy | 22 (6.0) | 23 (9.5) | 14 (4.8) | 12 (6.6) |
| Hepatic function (total bilirubin) | 21 (5.5) | 20 (8.3) | 10 (3.5) | 4 (2.3) |
| Self-reported serious health conditions | 58 (13.9) | 32 (12.7) | 115 (29.4) | 62 (26.6) |
| Hemoglobin | 3 (0.8) | 3 (1.3) | 0 (0.0) | 3 (1.7) |
| WBC | 3 (0.8) | 10 (4.3) | 4 (1.4) | 12 (6.6) |
| Platelet | 3 (0.8) | 23 (9.5) | 3 (1.1) | 27 (13.6) |
| Eligibility criteria . | MER cohort . | LEO cohort . | ||
|---|---|---|---|---|
| Relaxed (n = 362) . | Stringent (n = 220) . | Relaxed (n = 276) . | Stringent (n = 171) . | |
| Stage | 60 (21.4) | 33 (16.2) | ||
| Renal function (creatinine) | 21 (5.5) | 20 (8.3) | 2 (0.7) | 4 (2.3) |
| Performance status | 7 (1.9) | 15 (6.4) | 16 (5.5) | 15 (8.1) |
| Prior malignancy | 22 (6.0) | 23 (9.5) | 14 (4.8) | 12 (6.6) |
| Hepatic function (total bilirubin) | 21 (5.5) | 20 (8.3) | 10 (3.5) | 4 (2.3) |
| Self-reported serious health conditions | 58 (13.9) | 32 (12.7) | 115 (29.4) | 62 (26.6) |
| Hemoglobin | 3 (0.8) | 3 (1.3) | 0 (0.0) | 3 (1.7) |
| WBC | 3 (0.8) | 10 (4.3) | 4 (1.4) | 12 (6.6) |
| Platelet | 3 (0.8) | 23 (9.5) | 3 (1.1) | 27 (13.6) |
Relaxed criteria consist of less stringent specifications of the eligibility criteria, including stage II, III, and IV, creatinine < 2, performance status < 3, no prior malignancies with exceptions, bilirubin < 2, no self-reported health conditions, hemoglobin ≥ 8, WBC ≥ 3, and platelets ≥ 75 000. Stringent criteria consist of stringent specifications of the eligibility criteria, including stage III and IV, creatinine < 1.5, performance status < 2, no prior malignancies, bilirubin < 1.5, no self-reported health conditions, hemoglobin ≥ 9, WBC ≥ 4, and platelets ≥ 150 000.
Impact of eligibility criteria stringency
The differential contribution of relaxed versus stringent criteria specifications, with relaxed defined as criteria specifications excluding fewer patients than stringent criteria specifications (ie, for renal function, creatinine < 1.5 mg/dL was the stringent criteria specification compared with <2.0 mg/dL, which was the relaxed criteria specification), was additionally evaluated in Table 3. With relaxed criteria specifications, self-reported serious health conditions excluded 13.9% of additional patients in MER and 29.4% of additional patients in LEO. Similarly, with stringent criteria specifications, self-reported serious health conditions excluded 12.7% of additional patients in MER and 26.6% of additional patients in LEO. In contrast, hematologic parameters, with the exception of platelet count, and prior malignancy were responsible for excluding very few additional patients in either stringent or relaxed criteria specifications. With stringent criteria specifications, platelet count excluded 9.5% and 13.6% of additional patients in MER and LEO, respectively, compared with 0.8% and 1.1% of additional patients in MER and LEO, respectively, with relaxed criteria specifications
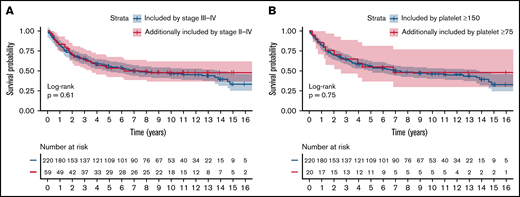
The impact of criteria stringency on percentage of patients excluded, sociodemographic composition of the resulting patient pool, and EFS was additionally evaluated (Table 4). In the MER cohort, liberalizing stage criteria specification from stage III to IV disease to stage II to IV disease and liberalizing platelet requirement specification from ≥150 000 to ≥75 000 increased potential patient accrual by 21.4% and 8.3%, respectively. Similarly, in the LEO cohort, liberalizing stage criteria specification and platelet requirement specification increased potential patient eligibility by 16.2% and 12.8%, respectively. In contrast, liberalization of the other criteria had less impact on percentage of patients included. Additional patients eligible by liberalizing stage criteria specification and platelet criteria specification did not differ significantly from patients included by the respective stringent specifications in terms of EFS in the MER cohort (Figure 1; log-rank tests, P = .61 and .75, respectively).
Relative impact of eligibility criteria specification stringency on number (%) of patients excluded when all other eligibility criteria are also applied
| Eligibility criteria switch . | MER cohort (n = 220) . | LEO cohort (n = 171) . |
|---|---|---|
| Stage (III-IV to II-IV) | 60 (21.4) | 33 (16.2) |
| Renal function (creatinine <2 to <1.5) | 11 (4.8) | 4 (2.3) |
| Performance status (0-2 to 0-1) | 9 (3.9) | 9 (5.0) |
| Hepatic function (total bilirubin <2 to <1.5) | 2 (0.9) | 1 (0.6) |
| Prior malignancy (with exceptions to no exceptions) | 6 (2.7) | 5 (2.8) |
| Hemoglobin (≥8 to ≥9) | 1 (0.5) | 3 (1.7) |
| WBC requirement (≥3 to ≥4) | 8 (3.5) | 8 (4.5) |
| Platelet requirement (≥75 to ≥150) | 20 (8.3) | 25 (12.8) |
| Eligibility criteria switch . | MER cohort (n = 220) . | LEO cohort (n = 171) . |
|---|---|---|
| Stage (III-IV to II-IV) | 60 (21.4) | 33 (16.2) |
| Renal function (creatinine <2 to <1.5) | 11 (4.8) | 4 (2.3) |
| Performance status (0-2 to 0-1) | 9 (3.9) | 9 (5.0) |
| Hepatic function (total bilirubin <2 to <1.5) | 2 (0.9) | 1 (0.6) |
| Prior malignancy (with exceptions to no exceptions) | 6 (2.7) | 5 (2.8) |
| Hemoglobin (≥8 to ≥9) | 1 (0.5) | 3 (1.7) |
| WBC requirement (≥3 to ≥4) | 8 (3.5) | 8 (4.5) |
| Platelet requirement (≥75 to ≥150) | 20 (8.3) | 25 (12.8) |
Eligibility criteria include stage II, III, and IV, creatinine < 2, performance status < 3, no prior malignancies with exceptions, bilirubin < 2, no self-reported health conditions, treated within 3 mo of diagnosis, hemoglobin ≥ 8, WBC ≥ 3, and platelets ≥ 100 000.
Impact of liberalizing eligibility criteria. (A) Stage from stage III to IV disease to stage II to IV disease and (B) platelet requirement from platelets ≥150 000 to platelets ≥75 000 on EFS in the MER cohort. Blue lines represent patients included by the stringent specifications of stage and platelet requirements. Yellow lines represent patients additionally included by liberalizing stage and platelet requirements. P values are obtained from log-rank tests comparing the 2 survival curves shown in each plot.
Impact of liberalizing eligibility criteria. (A) Stage from stage III to IV disease to stage II to IV disease and (B) platelet requirement from platelets ≥150 000 to platelets ≥75 000 on EFS in the MER cohort. Blue lines represent patients included by the stringent specifications of stage and platelet requirements. Yellow lines represent patients additionally included by liberalizing stage and platelet requirements. P values are obtained from log-rank tests comparing the 2 survival curves shown in each plot.
Demographic characteristics of patients in LEO/MER excluded by eligibility criteria
Each category of eligibility criteria was further subclassified into common criteria specifications (eg, creatinine < 1.5 mg/dL vs creatinine < 2.0 mg/dL as 2 separate criteria specifications for the category of renal function). Among the various criteria specifications studied, none of the populations of patients excluded by the criteria specifications differed significantly from the populations of patients included in terms of sex or race/ethnicity distribution (supplemental Table 4). In contrast, several eligibility criteria, namely renal function, prior malignancy, and self-reported serious health conditions, excluded populations of patients that were significantly older than patients included (Table 5). For example, the criteria specification of creatinine < 1.5 mg/dL for the criteria category of renal function excluded 24 patients (3.9%) from potential trial participation in MER and 26 patients (4.5%) in LEO. Patients excluded in MER had a median age of 70 compared with 59 among patients not excluded (P < .001). Similarly, patients excluded in LEO had a median age of 68 years compared with 60 years among patients not excluded (P < .01).
Age of patients included and excluded by various eligibility criteria
| Variables . | MER cohort . | LEO cohort . | ||||
|---|---|---|---|---|---|---|
| n . | Age . | P2 . | n . | Age . | P2 . | |
| Age, y | ||||||
| 18-60 | 336 | 50.0 (8.7) | **** | 299 | 51.0 (8.5) | **** |
| >60 | 279 | 68.0 (7.0) | 284 | 69.0 (7.0) | ||
| 18-75 | 556 | 58.0 (11.5) | **** | 518 | 58.0 (11.4) | **** |
| >75 | 59 | 80 (4.2) | 65 | 80.0 (3.9) | ||
| Stage | ||||||
| III-IV | 501 | 59.0 (13.7) | 471 | 60.0 (13.3) | ||
| II | 105 | 60.0 (11.7) | 82 | 60.5 (13.3) | ||
| Renal function (creatinine) | ||||||
| <1.5 mg/dL | 487 | 59.0 (13.1) | *** | 459 | 60.0 (13.2) | ** |
| ≥1.5 mg/dL | 24 | 70.0 (13.6) | 26 | 68.0 (14.1) | ||
| <2 mg/dL | 508 | 60.0 (13.2) | 478 | 60.0 (13.2) | ** | |
| ≥2 mg/dL | 3 | 72.0 (22.5) | 7 | 74.0 (11.3) | ||
| Performance status | ||||||
| 0-1 | 581 | 59.0 (13.2) | 515 | 59.0 (13.0) | *** | |
| >1 | 31 | 60.0 (14.1) | 68 | 64.5 (12.7) | ||
| 0-2 | 605 | 59.0 (13.3) | 552 | 60.0 (13.2) | ||
| >2 | 7 | 66.0 (12.7) | 31 | 62.0 (11.9) | ||
| Prior malignancy | ||||||
| No previous malignancy | 557 | 58.0 (13.2) | **** | 526 | 59.0 (13.1) | **** |
| Any previous malignancy | 58 | 66.0 (10.9) | 57 | 68.0 (11.1) | ||
| No previous malignancy w/exceptions* | 575 | 59.0 (13.3) | *** | 546 | 60.0 (13.1) | **** |
| Any exception* | 36 | 66.5 (10.2) | 37 | 68.0 (11.8) | ||
| Hepatic function (total bilirubin) | ||||||
| <1.5 mg/dL | 495 | 60.0 (13.3) | 462 | 60.0 (13.3) | ||
| ≥1.5 mg/dL | 12 | 54.0 (11.2) | 6 | 62.5 (6.5) | ||
| <2 mg/dL | 504 | 60.0 (13.2) | 465 | 60.0 (13.3) | ||
| ≥2 mg/dL | 3 | 59.0 (19.2) | 3 | 61.0 (5.5) | ||
| Self-reported serious health conditions | ||||||
| None | 519 | 58.0 (13.4) | **** | 404 | 59.0 (13.3) | ** |
| Any serious health conditions | 96 | 66.0 (10.2) | 179 | 63.0 (12.5) | ||
| Hemoglobin | ||||||
| ≥9 g/dL | 544 | 60.0 (13.1) | 495 | 60.0 (13.3) | ||
| <9 g/dL | 13 | 61.0 (15.6) | 9 | 63.0 (10.0) | ||
| ≥8 g/dL | 550 | 60.0 (13.1) | not applicable | |||
| <8 g/dL | 7 | 49.0 (14.2) | not applicable | |||
| WBC | ||||||
| ≥3000/μL | 547 | 60.0 (13.1) | 495 | 60.0 (13.3) | ||
| <3000/μL | 9 | 61.0 (15.1) | 7 | 64.0 (9.5) | ||
| ≥4000/μL | 524 | 60.0 (13.2) | 474 | 60.0 (13.4) | ||
| <4000/μL | 32 | 55.5 (10.9) | 28 | 61.5 (9.5) | ||
| Platelet | ||||||
| ≥75 000/μL | 544 | 60.0 (13.1) | 495 | 60.0 (13.2) | * | |
| <75 000/μL | 11 | 60.0 (15.0) | 6 | 66.0 (9.4) | ||
| ≥150 000/μL | 489 | 60.0 (13.0) | 434 | 60.0 (12.9) | ||
| <150 000/μL | 66 | 59.5 (13.8) | 67 | 63.0 (14.8) | ||
| Variables . | MER cohort . | LEO cohort . | ||||
|---|---|---|---|---|---|---|
| n . | Age . | P2 . | n . | Age . | P2 . | |
| Age, y | ||||||
| 18-60 | 336 | 50.0 (8.7) | **** | 299 | 51.0 (8.5) | **** |
| >60 | 279 | 68.0 (7.0) | 284 | 69.0 (7.0) | ||
| 18-75 | 556 | 58.0 (11.5) | **** | 518 | 58.0 (11.4) | **** |
| >75 | 59 | 80 (4.2) | 65 | 80.0 (3.9) | ||
| Stage | ||||||
| III-IV | 501 | 59.0 (13.7) | 471 | 60.0 (13.3) | ||
| II | 105 | 60.0 (11.7) | 82 | 60.5 (13.3) | ||
| Renal function (creatinine) | ||||||
| <1.5 mg/dL | 487 | 59.0 (13.1) | *** | 459 | 60.0 (13.2) | ** |
| ≥1.5 mg/dL | 24 | 70.0 (13.6) | 26 | 68.0 (14.1) | ||
| <2 mg/dL | 508 | 60.0 (13.2) | 478 | 60.0 (13.2) | ** | |
| ≥2 mg/dL | 3 | 72.0 (22.5) | 7 | 74.0 (11.3) | ||
| Performance status | ||||||
| 0-1 | 581 | 59.0 (13.2) | 515 | 59.0 (13.0) | *** | |
| >1 | 31 | 60.0 (14.1) | 68 | 64.5 (12.7) | ||
| 0-2 | 605 | 59.0 (13.3) | 552 | 60.0 (13.2) | ||
| >2 | 7 | 66.0 (12.7) | 31 | 62.0 (11.9) | ||
| Prior malignancy | ||||||
| No previous malignancy | 557 | 58.0 (13.2) | **** | 526 | 59.0 (13.1) | **** |
| Any previous malignancy | 58 | 66.0 (10.9) | 57 | 68.0 (11.1) | ||
| No previous malignancy w/exceptions* | 575 | 59.0 (13.3) | *** | 546 | 60.0 (13.1) | **** |
| Any exception* | 36 | 66.5 (10.2) | 37 | 68.0 (11.8) | ||
| Hepatic function (total bilirubin) | ||||||
| <1.5 mg/dL | 495 | 60.0 (13.3) | 462 | 60.0 (13.3) | ||
| ≥1.5 mg/dL | 12 | 54.0 (11.2) | 6 | 62.5 (6.5) | ||
| <2 mg/dL | 504 | 60.0 (13.2) | 465 | 60.0 (13.3) | ||
| ≥2 mg/dL | 3 | 59.0 (19.2) | 3 | 61.0 (5.5) | ||
| Self-reported serious health conditions | ||||||
| None | 519 | 58.0 (13.4) | **** | 404 | 59.0 (13.3) | ** |
| Any serious health conditions | 96 | 66.0 (10.2) | 179 | 63.0 (12.5) | ||
| Hemoglobin | ||||||
| ≥9 g/dL | 544 | 60.0 (13.1) | 495 | 60.0 (13.3) | ||
| <9 g/dL | 13 | 61.0 (15.6) | 9 | 63.0 (10.0) | ||
| ≥8 g/dL | 550 | 60.0 (13.1) | not applicable | |||
| <8 g/dL | 7 | 49.0 (14.2) | not applicable | |||
| WBC | ||||||
| ≥3000/μL | 547 | 60.0 (13.1) | 495 | 60.0 (13.3) | ||
| <3000/μL | 9 | 61.0 (15.1) | 7 | 64.0 (9.5) | ||
| ≥4000/μL | 524 | 60.0 (13.2) | 474 | 60.0 (13.4) | ||
| <4000/μL | 32 | 55.5 (10.9) | 28 | 61.5 (9.5) | ||
| Platelet | ||||||
| ≥75 000/μL | 544 | 60.0 (13.1) | 495 | 60.0 (13.2) | * | |
| <75 000/μL | 11 | 60.0 (15.0) | 6 | 66.0 (9.4) | ||
| ≥150 000/μL | 489 | 60.0 (13.0) | 434 | 60.0 (12.9) | ||
| <150 000/μL | 66 | 59.5 (13.8) | 67 | 63.0 (14.8) | ||
Age is represented by median (SD). P values via 2-sample t tests for continuous variables and χ2 tests for categorical variables: *<.05, **<.01, ***<.001, ****<00001.
Exceptions consist of nonmelanoma skin cancer, breast cancer, cervical cancer in situ, and uterine cancer in situ.
Sensitivity analyses
Analyses using the LEO cohort after patients enrolled in WCM were removed yielded results that were unchanged from analyses using the LEO cohort without removal of WCM patients (data not shown). Similarly, exclusion of patients receiving single-agent rituximab as first-line treatment in both MER and LEO cohorts did not impact results (supplemental Tables 5-8; supplemental Figure 1).
Discussion
In this study, we describe the impact of front-line FL clinical trial eligibility criteria on resulting trial demographics, patient numbers, and patient outcomes. In an ideal world, the population of clinical trial participants would closely mirror the population of patients who have the disease, with a possible exception for enrichment of some populations of special interest. Similarly, eligibility criteria should be designed in a way that does not hamper study completion. Unfortunately, we found neither of these to be true. Clinical trial eligibility from a large number of front-line trials clearly resulted in selection of a patient population that skewed younger than the general FL population, and they limited the pool of patients available for study participation, potentially prolonging recruitment to a study.
Our findings have potential implications in how future clinical trials in FL are designed and conducted. Older patients are underrepresented in clinical trials despite accounting for most new cancer diagnoses.20-23 Desire to participate in trials may have a partial role in this distinction, but our results suggest that eligibility criteria may also play an important rule. Compared with clinical trial populations (using GALLIUM and RELEVANCE as examples), the median ages of patients in the LEO/MER cohorts are not significantly different (59 in MER and 60 in LEO compared with 60 and 58 in the intervention and control arms of GALLIUM,16 respectively, and 59 in both the intervention and control arms of RELEVANCE17 ); however, we found that exclusion of patients with renal dysfunction, prior malignancy, and self-reported serious health conditions may reduce participation of older individuals, thus potentially reducing participation of older individuals in actual clinical trial settings. In a study of 5922 patients with FL from 18 randomized controlled trials in the FLASH database, the median overall survival of patients > 70 years was significantly shorter than that of patients ≤ 70 years of age (7.4 years vs 15.7 years, respectively).14 These data suggest that by selecting a population of patients who are significantly younger than the general FL population, trial results may not be generalizable to older patients in terms of efficacy. Furthermore, given that older patients are also more likely to experience toxicity as a result of their treatment (and potentially stop treatment),24 the safety profile suggested by clinical trials may not be applicable in older populations. These findings should give us all pause when applying the results of clinical trials to older or frailer patients that we see in our daily clinics, but they unfortunately leave us with little data on which to make our decisions. Given that older adults already experience difficulties when it comes to clinical trial enrollment,25 if eligibility criteria must remain restrictive for reasons of patient safety, then separate trials that focus on these patients (about one-third of all FL patients according to our data) should be considered.
Our finding that eligibility criteria had no significant impact on sex and race/ethnicity of the included vs excluded populations, but it should be noted that patient populations in the WCM, MER, and LEO databases were predominantly non-Hispanic White. Data collection for the LEO version 2.0 cohort is currently ongoing, with additional efforts to enroll a diverse patient population. It has been noted that Black patients remain underrepresented as a whole in clinical trials,26 despite similar rates of participation when offered a clinical trial.27 Further work needs to be done to elucidate reasons for this disparity.
We additionally found that liberalizing eligibility criteria specifications for stage and platelet requirement can increase the pool of eligible patients, more so than liberalizing performance status, prior malignancy, other hematologic parameters, and other organ function-based criteria specifications. In a recent meta-analysis of studies that examined structural, clinical, and physician and patient barriers to clinical trial participation, investigators found that for 55.6% of patients in the summarized trials, no trial was available for the patient’s cancer type and stage; additionally, a further 21.5% of patients were not eligible for an available trial.28 These findings are consistent with our findings that liberalizing stage may increase the eligible pool by 21% and that liberalizing platelet requirement may increase the pool by 13%. Fortunately, more recent trials appear to have partially taken this lesson to heart (eg, the minimum platelet requirements in both GALLIUM16 and RELEVANCE17 was 75 000). Importantly, we found that patients potentially included by liberalizing platelet criteria did not have worse EFS compared with patients already included; this suggests that liberalizing platelet criteria do not unnecessarily include patients who are at any increased risk of poor outcomes and that certain eligibility criteria may indeed be practically arbitrary.
We performed a set of sensitivity analyses to better understand the impact of first-line treatment decisions on our findings. Given that the eligibility criteria examined in this study were derived largely from trials that studied combination regimens as first-line treatment options in FL (eg, bendamustine and rituximab in E2408,15 rituximab and lenalidomide in RELEVANCE,17 cyclophosphamide, doxorubicin, vincristine, and prednisone and tositumomab in SWOG S001629 ), systematic bias may exist because of inclusion of a significant number of patients treated with single-agent rituximab (32.8% and 26.2% of patients in MER and LEO cohorts, respectively). This may especially be the case given that single-agent rituximab is often reserved for low-stage disease or for patients with poorer ability to tolerate combination treatments with more toxic side effect profiles.30 In our analyses, although we identified certain significant clinical differences between patients receiving single-agent rituximab and patients receiving combination treatments in both MER and LEO cohorts, these differences did not translate into differences in study results and conclusions. Patients who received combination regimens had predictably slightly worse disease and slightly worse performance status compared with patients who received single-agent rituximab but did not differ in terms of hematologic/chemistry parameters and demographic characteristics. These results allow us to generalize our findings to patients treated not only with combination regimens, but also with single-agent rituximab.
A significant limitation in our study is use of the term “serious health conditions,” whose use as an exclusion criterion was found to select for younger patients. The term was self-reported in questionnaires administered to participants in LEO/MER cohorts. Examples of “serious health conditions” provided to patients included congestive heart failure, pulmonary embolism, and myocardial infarction, whereas common, but less significant health conditions (eg, hypertension, diabetes, osteoporosis) were specifically accounted for in the questionnaire. We included the term here and believe that the conditions included therein are likely significant, but we recognize the potential for bias. Yet another form of bias comes in the form of reliance on individual providers to interpret clinical trial eligibility criteria that arbitrarily define “serious health conditions” (eg, exclusion criterion of “Any condition, including the presence of laboratory abnormalities, which places the subject at unacceptable risk if he/she were to participate in the study, or which confounds the ability to interpret data from the study” in RELEVANCE17 ). Another major limitation is the fact that the majority of patients in both the MER and LEO cohorts were on treatment within 3 months following diagnosis, which is not necessarily consistent with the natural history of FL. In sensitivity analyses comparing patients who received first-line treatment to patients who underwent observation, patients who received treatment had predictable reasons for receiving treatment (eg, bulky disease, splenic involvement); furthermore, patients who received treatment did not differ from patients who underwent observation in terms of other clinical characteristics, making it unlikely that this limitation affected the conclusions from this study. Furthermore, we were unable to differentiate between contiguous and non-ontiguous stage II disease in our cohorts; given that treatment algorithms differ between the 2 and that noncontiguous stage II disease is treated similarly to stages III and IV disease, further study should examine the impact of eligibility criteria with regard to contiguous and noncontiguous stage II disease. Finally, certain eligibility criteria could not be evaluated because they were not collected as part of the MER and LEO cohort studies, including HIV/AIDS status (included in LEO, but not included in MER), hepatitis B status, and hepatitis C status. Especially with evidence of patients living with HIV still being excluded from most oncology trials,31 despite new guidelines encouraging increased enrollment of such patients,32 further studies are needed to address these important eligibility criteria.
Ideally, optimal design of clinical trials will simultaneously protect study participants, minimize unnecessary delays in study conduct, and maximize the probability that trial results will be relevant to the intended population. Patient selection is among the most critical aspects of study design, and the role that eligibility criteria play in study results is worthy of further attention. Future clinical trials can clearly benefit from an expanded population of potential participants, which also provides a broader basis for generalizability of trial findings. When criteria must remain restrictive for purposes of participant protection, the potential impact of individual criteria should be carefully considered and presented, and alternative trials should be designed to benefit those groups.
Authorship
Contribution: D.L., T.F., A.T., and P.M. conceived and designed the study; C.R.F., B.L., J.W.F., J.B.C., B.K., I.S.L., L.N., M.J.M., J.R.C., and P.M. provided study material or patients; all authors collected and assembled the data; D.L., T.F., A.T., and P.M. analyzed and interpreted the data; and all authors wrote and approved the manuscript and are accountable for all aspects of the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Martin, 1305 York Ave, Rm Y-735, New York, NY 10021; e-mail: pem9019@med.cornell.edu.
References
Author notes
Data may be requested by e-mailing the corresponding author at pem9019@med.cornell.edu.
The full-text version of this article contains a data supplement.