Key Points
TMLI at 2000 cGy for HaploHCT with PTCy was determined to be safe in patients with high-risk leukemia and MDS.
At 2000 cGy, a 1-year relapse rate of 17% was achieved without increasing GVHD or transplant-related mortality.
Abstract
Posttransplant cyclophosphamide (PTCy) platform has shown low rates of graft-versus-host disease (GVHD) and nonrelapse mortality (NRM) after haploidentical hematopoietic cell transplantation (HaploHCT). However, because of the limited disease control, relapse rate remains a major cause of treatment failure in high-risk patients. Total marrow and lymphoid irradiation (TMLI) allows for delivery of high radiation to bone marrow and other targeted structures, without increasing off-target radiation exposure and toxicity to end organs. In this phase 1 trial, 31 patients with high-risk and/or active primary refractory leukemias or myelodysplastic syndrome underwent peripheral blood stem cell HaploHCT with TMLI, fludarabine, and cyclophosphamide as the conditioning regimen. Radiation dose was escalated in increments of 200 cGy (1200-2000 cGy). GVHD prophylaxis was PTCy with tacrolimus/mycophenolate mofetil. Grade 2 toxicities by the Bearman scale were mucositis (n = 1), hepatic (n = 3), gastrointestinal (n = 5), and cardiac (n = 2). One patient (1800 cGy) experienced grade 3 pulmonary toxicity (dose-limiting toxicity). At a follow-up duration of 23.9 months for the whole cohort; 2-year NRM was 13%. Cumulative incidence of day 100 grade 2 to 4 and 3 to 4 acute GVHD was 52% and 6%, respectively. Chronic GVHD at 2 years was 35%. For patients treated with 2000 cGy, with a median follow-up duration of 12.3 months, 1-year relapse/progression, progression-free survival, and overall survival rates were 17%, 74%, and 83%, respectively. In conclusion, HaploHCT-TMLI with PTCy was safe and feasible in our high-risk patient population with promising outcomes.
Introduction
Haploidentical hematopoietic cell transplantation (haploHCT) offers a potentially curative therapy for patients with hematologic malignancies lacking a fully matched donor, making transplant available to nearly all eligible patients.1,2 An approach using unmanipulated bone marrow (BM)3 or peripheral blood stem cells (PBSCs)4 followed by immunosuppression with high-dose posttransplant cyclophosphamide (PTCy) as graft-versus-host disease (GVHD) prophylaxis has demonstrated acceptably low incidence of nonrelapse mortality (NRM) and GVHD. Yet, a shortcoming of this regimen continues to be graft failure and the relatively high rates of relapse.3,5
In a recent retrospective study by the Center for International Blood and Marrow Transplant Research, myeloablative regimens for haploHCT were shown to be associated with lower relapse rate and better disease-free survival (DFS) compared with reduced intensity conditioning (RIC), at least in younger patients with acute leukemias and myelodysplastic syndrome (MDS).6 An incremental increase in radiation dose in total body irradiation (TBI) results in a lower risk of disease progression/relapse after HCT; however, the beneficial impact of higher doses is offset by higher regimen-related toxicities and deaths.7,8 Furthermore, there has been a large heterogeneity in the dose coverage to lungs using conventional TBI.9
We have pioneered and developed total marrow and lymphoid irradiation (TMLI) that allows for precise delivery of increased intensity treatment through sculpting radiation to sites with high disease burden or higher risk for disease involvement or relapse while sparing healthy tissues.10-14 We have shown that TMLI at 2000 cGy in combination with chemotherapy (cyclophophamide and etoposide) is safe and results in a relatively low NRM rate and no increased risk of GVHD in patients with relapsed/refractory acute leukemias undergoing HCT in the match donor setting,11,15 but no data are available in the setting of haploHCT.
In this study, we investigated the safety and feasibility of dose escalation of TMLI when combined with fludarabine and cyclophosphamide for conditioning before T cell–replete haploHCT with PTCy in patients with high-risk acute myeloid or lymphoblastic leukemia (AML or ALL, respectively) and MDS.
Subjects and methods
Clinical protocol
This single-institution phase 1 trial with expansion cohort tested escalating doses of TMLI when given as conditioning regimen for haploHCT. Our primary objectives were to establish the recommended phase 2 dose (RP2D) of TMLI in this regimen and to describe the toxicities at each dose level (DL). Secondary objectives included estimation of overall survival (OS), DFS, cumulative incidence of relapse/progression (RP), NRM, acute/chronic GVHD, and an assessment of radiation dose to target and off-target organs.
This trial was registered with clinicaltrials.gov (#NCT02446964) and approved by the City of Hope institutional review board. An assurance was filed with and approved by the Department of Health and Human Services. Informed consent was obtained for all study participants in compliance with the Declaration of Helsinki.
Patients with high-risk and/or active primary refractory or relapsed AML (classified per Döhner et al),16 ALL (classified per Moorman et al),17 or MDS (classified per Revised International Prognostic Scoring System [IPSS-R])18 aged 12 to 60 years with adequate organ function and an available haploidentical donor were eligible. Patients with prior history of radiation therapy of more than 20% of BM-containing areas or any area exceeding 2000 cGy were excluded. Graft source was PBSCs for all patients.
TMLI
TMLI was delivered at 8 to 10 planned doses ranging from 1200 to 2000 cGy, administered in twice daily fraction over 4 to 5 days, as published previously.10,11,19 Briefly, all patients underwent computed tomography simulation and were treated on a TomoTherapy system (Accuray, Sunnyvale, CA). For treatment planning purposes, the target organs included the bone and BM, major lymph node chains, spleen, testes, liver, and brain. All other organs (such as lungs, heart, small and large intestine, kidneys, eyes, lenses, oral cavity, bladder, parotid glands, stomach, and esophagus) were identified as organs at risk, and efforts were made to minimize dose to these organs. Because of dose sparing of the gastrointestinal (GI) tract and the oral cavity, mesenteric lymph nodes, Waldeyer ring lymph nodes, and the mandible were not included as target regions. Doses to BM, major lymph node chains, and testes were escalated up to 2000 cGy, but doses to liver, porta hepatis, and spleen were kept at 1200 cGy. Brain was treated to 1200 cGy only in patients with ALL with a history of positive cerebrospinal fluid while the skull was considered a targeted organ.
Preparative regimen and GVHD prophylaxis
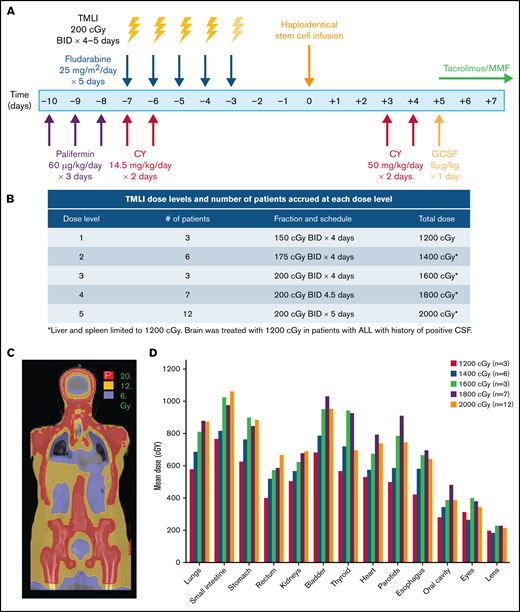
The study schema is depicted in Figure 1A. Palifermin (60 µg/kg per day) was administered from day −10 to day −8 before HCT.20 Patients received cyclophosphamide (14.5 mg/kg per day) on days −7 and −6. Escalating doses of TMLI (Figure 1B) were delivered concurrently with fludarabine (25 mg/m2 per day) from day −7 to day −3. GVHD prophylaxis consisted of high-dose PTCy (50 mg/kg per day) on days +3 and +4, followed by granulocyte colony stimulating factor (5 µg/kg) on day +5 until absolute neutrophil count > 1500. Tacrolimus and mycophenolate mofetil were initiated on day +5 and continued until day +180 and +35, respectively, if no active GVHD was developed.
TMLI radiation therapy for haploidentical hematopoietic cell transplantation. (A) Study schema; patients received palifermin (60 µg/kg per day) for 3 days, starting on day −10 before stem cell infusion. PTCy was administered at 14.5 mg/kg per day on days −7 and −6. TMLI at the scheduled dose was delivered concurrently with fludarabine (25 mg/m2 per day) for 5 days from day −7 to day −3. GvHD prophylaxis consisted of posttransplant cyclophosphamide (50 mg/kg per day) on days +3 and +4, GCSF (5 µg/kg) starting on day +5 and continued until >1500, and tacrolimus and mycophenolate mofetil starting on day +5 and continued until days +90 and +35, respectively. (B) TMLI dose levels and number of patients accrued at each dose level. (C) TMLI dose distribution colorwash map in a representative patient with AML who was treated at a TMLI dose of 2000 cGy. (D) Mean organ doses at each TMLI dose level for all patients (n = 31). G-CSF granulocyte colony stimulating factor
TMLI radiation therapy for haploidentical hematopoietic cell transplantation. (A) Study schema; patients received palifermin (60 µg/kg per day) for 3 days, starting on day −10 before stem cell infusion. PTCy was administered at 14.5 mg/kg per day on days −7 and −6. TMLI at the scheduled dose was delivered concurrently with fludarabine (25 mg/m2 per day) for 5 days from day −7 to day −3. GvHD prophylaxis consisted of posttransplant cyclophosphamide (50 mg/kg per day) on days +3 and +4, GCSF (5 µg/kg) starting on day +5 and continued until >1500, and tacrolimus and mycophenolate mofetil starting on day +5 and continued until days +90 and +35, respectively. (B) TMLI dose levels and number of patients accrued at each dose level. (C) TMLI dose distribution colorwash map in a representative patient with AML who was treated at a TMLI dose of 2000 cGy. (D) Mean organ doses at each TMLI dose level for all patients (n = 31). G-CSF granulocyte colony stimulating factor
Flow cytometry and GVHD biomarkers
Peripheral blood was collected on days 30, 100, and 180 after HCT. For Treg staining, PBMCs were surface stained for CD3, CD4, CD25, and CD127 (eBioscience) and intracellularly stained for Foxp3 using Foxp3/Transcription Factor Staining Buffer Set (eBioscience). All other antibodies were purchased from eBioscience. Flow cytometry was performed using a BD FACSCelesta (BD Biosciences), and data were analyzed using Flowjo software (Tree Star).
Serum samples were obtained on weekly basis for 4 weeks and were analyzed for GVHD biomarkers using the Luminex FlexMap 3D bead array technology. Multiplex kits were purchased from R&D Systems, and assays were performed according to the manufacturer’s instructions in the analytical Pharmacology Core Facility.
BM examinations
Trephine biopsies were obtained before TMLI and at 1 year after transplant. Samples were prospectively analyzed by immunohistochemistry staining with CD34 antibody (Ventana Roche). Marrow samples were also evaluated for cellularity and vessel density and quantified by a certified pathologist.
Statistical considerations
Dose-limiting toxicity (DLT) was defined as any of the following that were assigned an attribution level of at least possibly related to TMLI: For nonhematologic toxicities, any regimen-related grade 3 or 4 toxicity per Bearman toxicity grading scale,21 and for nonhematologic toxicities that are not part of the Bearman toxicity, any ≥grade 4 toxicity per NCI Common Terminology Criteria for Adverse Events (CTCAE) v4.03, except for metabolic/electrolyte disturbances and vomiting controlled by medical management. For hematologic toxicities, per NCI Common Terminology Criteria for Adverse Event (CTCAE) v4.03, any grade 4 neutropenia associated with fever or infection and lasting for more than 21 days or grade 4 neutropenia lasting for more than 28 days (engraftment failure) was counted as DLT.
This study used a modified, more conservative version of the rolling 6 design of Skolnik et al.22 See supplemental Material for details of the study design and outcome definitions. Supplemental Table 1 shows dose escalation rules. Descriptive statistics were used to summarize adverse event data: observed incidence by severity and type of toxicity. All evaluable patients were included in the analysis, in terms of NRM, GVHD, and toxicities/complications, and patients who received TMLI at 2000 cGy were evaluated for survival, response, and RP. Survival estimates were calculated based on the Kaplan-Meier product-limit method; 95% confidence intervals (CIs) were calculated using the logit transformation and the Greenwood variance estimate.23 Cumulative incidence estimates were generated for NRM and RP in the competing risks setting, given that death and RP events were in competition. The cumulative incidence of NRM, RP, and acute/chronic GVHD was calculated using the method described by Gooley et al.24
To be evaluable in the context of dose escalation, a patient had to start treatment and be observed for at least 30 days after the completion of transplantation procedure or experience a DLT. Hematologic toxicities were evaluated from day 0. Other DLTs were evaluated from day −9 to day +30. Engraftment was defined as the first of 3 consecutive days in which the absolute neutrophil count exceeded 0.5 × 109/L. GVHD grading was scored according to published criteria.25-27 Clinical response was assessed according to published data,16-18 with blood draws and BM biopsies at regularly scheduled visits.
Results
Patient and disease characteristics
We accrued 31 eligible patients from June 2015 to October 2018. Patient and transplant characteristics are summarized in Table 1. Median age at the time of HCT was 37 years (range, 21-58 years), with 5 (16%) patients being older than 50 years old at the time of HCT. All patients had Karnofsky performance status (KPS) of ≥80. HCT comorbidity index was ≥3 in 26% of patients (range, 0-6). Two patients presented with pre-HCT extramedullary disease (EMD); 1 at the left distal humerus and the other at the mediastinal mass. EMD at the time of HCT was recorded only in 1 patient with mediastinal mass-EMD. Patients were transplanted for AML (n = 17), ALL (n = 13), or MDS (n = 1). At the time of HCT, patients with AML and ALL were in first complete remission (CR) with poor risk cytogenetics (n = 14), or CR2/CR3 (n = 9), or had active disease (n = 7). The disease risk index was high/very high in 62% of treated patients (n = 19).
Patient, disease and transplant characteristics
| Variable . | Median (range) or N (%) . |
|---|---|
| Patient sex | |
| Female | 12 (39) |
| Male | 19 (61) |
| Patient’s age at transplant (y) | 37 (21-58) |
| Donor’s age at transplant (y), n = 30 | 29 (12-68) |
| Sex of donor/recipient | |
| Female/male | 7 (23) |
| Other | 24 (77) |
| Number of mismatches | 5 (2-6) |
| Disease diagnosis | |
| AML | 17 (55) |
| ALL | 13 (42) |
| MDS | 1 (3) |
| Cytogenetic risk at diagnosis | |
| AML* | |
| Intermediate | 8 (26) |
| Advance | 9 (29) |
| ALL† | |
| Favorable | 3 (10) |
| Unfavorable | 10 (32) |
| MDS‡ | |
| High risk | 1 (3) |
| Disease status before conditioning | |
| AML/ALL | |
| CR1 with poor risk cytogenetics | 14 (45) |
| CR2/CR3 | 9 (29.5) |
| Active disease | 7 (22.5) |
| MDS | |
| High risk relapsed | 1 (3) |
| Time from diagnosis to transplant (mo) | 7.1 (2.7-62.7) |
| Donor CMV status | |
| Negative | 11 (35) |
| Positive | 20 (65) |
| Patient CMV status | |
| Negative | 4 (13) |
| Positive | 27 (87) |
| HCT comorbidity index | 1 (0-6) |
| 0 | 14 (45) |
| 1-2 | 9 (29) |
| ≥3 | 8 (26) |
| Disease risk iIndex | |
| Low | 1 (3) |
| Intermediate | 11 (35) |
| High | 12 (39) |
| Very high | 7 (23) |
| Karnofsky performance status at HCT | |
| ≥80 | 31 (100) |
| Variable . | Median (range) or N (%) . |
|---|---|
| Patient sex | |
| Female | 12 (39) |
| Male | 19 (61) |
| Patient’s age at transplant (y) | 37 (21-58) |
| Donor’s age at transplant (y), n = 30 | 29 (12-68) |
| Sex of donor/recipient | |
| Female/male | 7 (23) |
| Other | 24 (77) |
| Number of mismatches | 5 (2-6) |
| Disease diagnosis | |
| AML | 17 (55) |
| ALL | 13 (42) |
| MDS | 1 (3) |
| Cytogenetic risk at diagnosis | |
| AML* | |
| Intermediate | 8 (26) |
| Advance | 9 (29) |
| ALL† | |
| Favorable | 3 (10) |
| Unfavorable | 10 (32) |
| MDS‡ | |
| High risk | 1 (3) |
| Disease status before conditioning | |
| AML/ALL | |
| CR1 with poor risk cytogenetics | 14 (45) |
| CR2/CR3 | 9 (29.5) |
| Active disease | 7 (22.5) |
| MDS | |
| High risk relapsed | 1 (3) |
| Time from diagnosis to transplant (mo) | 7.1 (2.7-62.7) |
| Donor CMV status | |
| Negative | 11 (35) |
| Positive | 20 (65) |
| Patient CMV status | |
| Negative | 4 (13) |
| Positive | 27 (87) |
| HCT comorbidity index | 1 (0-6) |
| 0 | 14 (45) |
| 1-2 | 9 (29) |
| ≥3 | 8 (26) |
| Disease risk iIndex | |
| Low | 1 (3) |
| Intermediate | 11 (35) |
| High | 12 (39) |
| Very high | 7 (23) |
| Karnofsky performance status at HCT | |
| ≥80 | 31 (100) |
TMLI radiation therapy
TMLI was delivered to sculpt radiation dose to lymph nodes and bone/BM in each patient. (Figure 1C) Mean organ doses at each DL for each organ is shown in Figure 1D. The mean organ doses of TMLI at all DLs compared favorably with those reported with standard TBI of 1200 to 1320 cGy, where mean lung doses are approximately 900 cGy28 (Table 2).
Mean organ dose (cGy; n = 31)
| . | Mean . | Minimum . | Maximum . |
|---|---|---|---|
| Lung | 808 | 551 | 980 |
| Intestine | 969 | 682 | 1412 |
| Stomach | 836 | 517 | 1236 |
| Rectum | 589 | 363 | 811 |
| Kidney | 646 | 480 | 793 |
| Bladder | 918 | 615 | 1303 |
| Thyroid | 770 | 486 | 1316 |
| Heart | 697 | 501 | 908 |
| Parotids | 735 | 462 | 1213 |
| Esophagus | 630 | 372 | 781 |
| Oral cavity | 394 | 270 | 606 |
| Eyes | 344 | 204 | 524 |
| Lens | 215 | 144 | 288 |
| . | Mean . | Minimum . | Maximum . |
|---|---|---|---|
| Lung | 808 | 551 | 980 |
| Intestine | 969 | 682 | 1412 |
| Stomach | 836 | 517 | 1236 |
| Rectum | 589 | 363 | 811 |
| Kidney | 646 | 480 | 793 |
| Bladder | 918 | 615 | 1303 |
| Thyroid | 770 | 486 | 1316 |
| Heart | 697 | 501 | 908 |
| Parotids | 735 | 462 | 1213 |
| Esophagus | 630 | 372 | 781 |
| Oral cavity | 394 | 270 | 606 |
| Eyes | 344 | 204 | 524 |
| Lens | 215 | 144 | 288 |
Safety of TMLI for Haplo-HCT
Bearman toxicities for each DL are detailed in Table 3. At DL-5 (2000 cGy), 8 patients developed grade 1 and 1 patient developed grade 2 mucositis (stomatitis per Bearman toxicity scale),21 attributed to radiation and chemotherapy. Pulmonary toxicities were seen in 2 patients: 1 patient at DL-3 (1600 cGy) had grade 1, and the other patient at DL-4 had grade 3 pulmonary toxicity that was considered DLT. Six patients developed cardiac toxicities (4 had grade 1, and 2 had grade 2), 17 developed GI toxicities (12 had grade 1, and 5 had grade 2), and 8t developed hepatic toxicities (5 had grade 1, and 3 had grade 2).
Toxicities by dose level per Bearman scale21
| . | Grade . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DL1 (1200 cGy;n = 3) . | DL2 (1400 cGy;n = 6) . | DL3 (1600 cGy;n = 3) . | DL4 (1800 cGy;n = 7) . | DL5 (2000 cGy;n = 12) . | |||||||
| Organ assessed . | 1 . | 2 . | 1 . | 2 . | 1 . | 2 . | 1 . | 2 . | 3 . | 1 . | 2 . |
| Bladder | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cardiac | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| CNS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| GI | 1 | 1 | 1 | 0 | 0 | 2 | 3 | 0 | 0 | 7 | 2 |
| Hepatic | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 0 | 0 | 2 | 0 |
| Pulmonary | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1* | 0 | 0 |
| Renal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stomatitis | 0 | 2 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 8 | 1 |
| . | Grade . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DL1 (1200 cGy;n = 3) . | DL2 (1400 cGy;n = 6) . | DL3 (1600 cGy;n = 3) . | DL4 (1800 cGy;n = 7) . | DL5 (2000 cGy;n = 12) . | |||||||
| Organ assessed . | 1 . | 2 . | 1 . | 2 . | 1 . | 2 . | 1 . | 2 . | 3 . | 1 . | 2 . |
| Bladder | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cardiac | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| CNS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| GI | 1 | 1 | 1 | 0 | 0 | 2 | 3 | 0 | 0 | 7 | 2 |
| Hepatic | 1 | 1 | 1 | 2 | 0 | 0 | 1 | 0 | 0 | 2 | 0 |
| Pulmonary | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1* | 0 | 0 |
| Renal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stomatitis | 0 | 2 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 8 | 1 |
Dose-limiting toxicity.
Engraftment and HCT outcomes
Neutrophil recovery was achieved in 97% (95% CI: 91-100) of patients by day 28. All patients achieved neutrophil recovery at a median of 17 days (range, 13-38 days). Platelet recovery was achieved in 93% (n = 28/30) of patients at a median of 28 days (range, 16-133 days), and 1 patient died at day +34 and therefore was not evaluable. This patient died of sinusoidal obstruction and was heavily pretreated for refractory AML with 5 prior salvage lines of therapy, including CD33 antibodies-drug conjugate.
Cumulatively, there were no deaths within the first 30 days after transplant and only 1 between days +30 and +100. The cumulative incidence of NRM at 100 days and 2 years were 3% (95% CI: 0.5-22) and 13% (95% CI: 5-33), respectively. Causes of death were disease relapse/progression (n = 8), multiple organ failure (n = 3, including the patient with sinusoidal obstruction), infection (n = 1), and GVHD (n = 1).
With the median follow-up of 23.9 months of the surviving patients, 12 patients relapsed, of whom only 4 received TMLI at the 2000-cGy dose. Extramedullary relapse was detected in the central nervous system without BM involvement in 1 patient at 29 months after HCT. None of the patients with pre–HCT-EMD presented with extramedullary relapse. All patients who were treated with 2000 cGy (n = 12) achieved CR by day 30. With a median follow-up duration of 12.3 months among the surviving patients at DL-5, 1-year NRM, relapse, DFS, and OS rates were 9% (95% CI: 1-60), 17% (95% CI: 5-59), 74% (95% CI: 39-91), and 83% (95% CI: 46-95), respectively. See supplemental Table 2 for overall rates of survival (progression-free and overall), relapse/progression, and NRM in all patients at 1 and 2 years after HCT.
GVHD outcomes
The cumulative incidence of grade 2 to 4 and 3 to 4 acute GVHD (aGVHD) at day +100 were 52% (95% CI: 37-73) and 6% (95% CI: 2-25), respectively, with a median time to onset of 35 days (range, 4-112 days). Of the 23 patients who developed aGVHD, 87% were responsive to steroids.
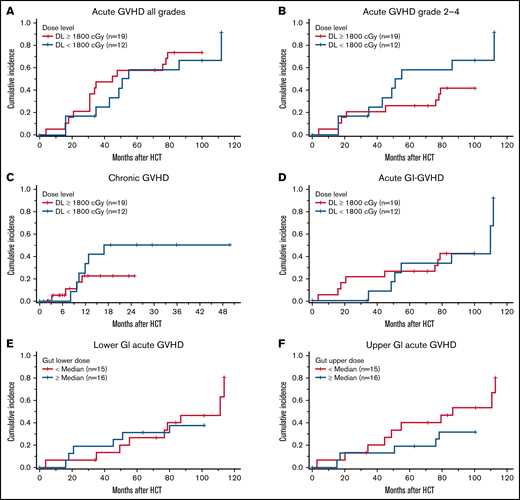
Chronic GVHD (cGvHD) occurred in 10 of the 30 patients surviving beyond 100 days, with 4 patients having moderate to severe (2 moderate and 2 severe) disease by the National Institutes of Health consensus grading.29 By day +365, the cumulative incidence of cGVHD and moderate to severe cGVHD was 23% (95% CI: 12-45) and 10% (95% CI: 3-30), with the median time to onset being 308 days (range, 98-516 days). At the time of this report, none of the patients died because of complications of cGVHD. Cumulative incidence of acute or cGVHD were not significantly different with higher TMLI doses when we compared ≥1800-cGy doses to <1800-cGy (aGVHD, no vs yes, P = .52; aGVHD, none-1 vs grade 2-4, P = .21; cGVHD, no vs yes, P = .25; Figure 2A-C). Specifically, there was no statistical difference in the cumulative incidence of GI aGVHD based on TMLI doses (53% vs 31%, P = .86; Figure 2D), lower GI radiation dose (47% vs 38%, P = .74; Figure 2E), or upper GI radiation dose (53% vs 31%, P = .24; Figure 2F). Supplemental Table 3 shows incidence of aCVHD and cGVHD at each dose level.
Comparison of GvHD outcomes at higher (≥1800 cGy) and lower (<1800 cGy) of TMLI. (A) Cumulative incidence of acute GVHD all grades. (B) Cumulative incidence of acute GVHD grades 2 to 4. (C) Cumulative incidence of chronic GVHD. (D) Cumulative incidence of acute GI GVHD. (E-F) Cumulative incidence of acute lower and upper GI GvHD.
Comparison of GvHD outcomes at higher (≥1800 cGy) and lower (<1800 cGy) of TMLI. (A) Cumulative incidence of acute GVHD all grades. (B) Cumulative incidence of acute GVHD grades 2 to 4. (C) Cumulative incidence of chronic GVHD. (D) Cumulative incidence of acute GI GVHD. (E-F) Cumulative incidence of acute lower and upper GI GvHD.
Immune reconstitution, cytokine release syndrome, and infections
Cellular immune recovery was measured on days 30, 100, and 180 after HCT by flow cytometry (supplemental Figure 1). Cellular immune reconstitution was not impacted by the dose of radiation to the marrow/lymph nodes when we compared higher dose (≥1800 cGy) to lower dose (<1800 cGy) for each lymphocyte subset at the 3e abovementioned time points (supplemental Figure 1). Cytokine release syndrome (CRS) at any grade was developed in 30 patients (97%). None of the patients developed grade 3 to 4 CRS, 13 patients (43%) had grade 2, and the rest (57%) had grade 1 CRS.
Infectious complications were recorded from day −9 to day +100 and graded per The Blood and Marrow Transplant Clinical Trials Network (BMT CTN), version 4.03 (Table 4). Cytomegalovirus (CMV) viremia was detected in 21 patients (7 patients had G2 and 14 had G1). Other viral infections included respiratory infections (n = 9), BK virus cystitis (n = 7; all had grade 1), and Human Herpesvirus 6 (HHV-6) (n = 7; all but 1 has G1). Gram-positive and -negative bacterial infection were detected in 10 and 5 patients, respectively, and only 7 patients (all grade 1) developed C. difficile colitis.
Infection outcomes per CTN grading
| . | <1800 cGy . | ≥1800 cGy . | Total . | ||||
|---|---|---|---|---|---|---|---|
| G1 . | G2 . | G3 . | G1 . | G2 . | G3 . | ||
| Bacterial | |||||||
| Clostridioides difficile | 2 | 0 | 0 | 5 | 0 | 0 | 7 |
| Gram negative | 1 | 1 | 0 | 1 | 2 | 0 | 5 |
| Gram positive | 4 | 1 | 0 | 1 | 4 | 0 | 10 |
| Viral | |||||||
| BK | 3 | 0 | 0 | 4 | 0 | 0 | 7 |
| CMV | 7 | 3 | 0 | 7 | 4 | 0 | 21 |
| HHV6 | 2 | 0 | 1 | 4 | 0 | 0 | 7 |
| HSV | 1 | 0 | 0 | 1 | 0 | 0 | 2 |
| Respiratory | 1 | 3 | 1 | 0 | 3 | 1 | 9 |
| Fungal | |||||||
| Mold | 0 | 1 | 0 | 0 | 0 | 1 | 2 |
| Yeast | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| . | <1800 cGy . | ≥1800 cGy . | Total . | ||||
|---|---|---|---|---|---|---|---|
| G1 . | G2 . | G3 . | G1 . | G2 . | G3 . | ||
| Bacterial | |||||||
| Clostridioides difficile | 2 | 0 | 0 | 5 | 0 | 0 | 7 |
| Gram negative | 1 | 1 | 0 | 1 | 2 | 0 | 5 |
| Gram positive | 4 | 1 | 0 | 1 | 4 | 0 | 10 |
| Viral | |||||||
| BK | 3 | 0 | 0 | 4 | 0 | 0 | 7 |
| CMV | 7 | 3 | 0 | 7 | 4 | 0 | 21 |
| HHV6 | 2 | 0 | 1 | 4 | 0 | 0 | 7 |
| HSV | 1 | 0 | 0 | 1 | 0 | 0 | 2 |
| Respiratory | 1 | 3 | 1 | 0 | 3 | 1 | 9 |
| Fungal | |||||||
| Mold | 0 | 1 | 0 | 0 | 0 | 1 | 2 |
| Yeast | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
GVHD biomarker analysis
Of the 6 GVHD biomarkers, levels of Reg3A30,31 were longitudinally evaluated and compared in patients undergoing TMLI at a higher dose (≥1800 cGy, n = 16) vs lower dose (<1800 cGy, n = 10). We observed no statistically significant difference in the Reg3A levels between high- and low-TMLI doses; however, there was a trend toward greater Reg3A levels in higher vs lower TMLI dose group on day 21 (median, 11 504.1; range, 2836.4-37 427.4 vs 8058.6; range, 2468.5-10 742.4; P = .09; supplemental Figure 2).
We next examined the relationship between the TMLI dose and the dose delivered to the GI tract and found a positive dose correlation for the lower GI tract (P = .03) and rectum dose (P = .04) and a trend for the upper GI tract (P = .06; supplemental Figure 3). We observed no significant association between the gut radiation dose and Reg3a levels at any time point.
BM recovery
At 1-year after HCT, BM cellularity was in a normal range (∼50%) in most cases (n = 5/7). There were no dose-dependent changes in BM cellularity. No obvious change was detected in vessel density between pre- and postirradiated marrows highlighted by CD34-IHC at 1200 and 1400 cGy; however, vessel density was increased between pre- and postirradiated marrows at higher DLs (1600, 1800, and 2000 cGy; supplemental Figure 4).
Discussion
In this phase 1 trial, consistent with our earlier experience with TMLI for matched related donor HCTs,11 we demonstrated that TMLI dose-sparing nonhematopoietic and uninvolved organs can be safely escalated to 2000 cGy when combined with haploHCT followed by PTCy and result in low rates of NRM on day 100 and 2 years. The rationale for not exceeding 2000 cGy was based on lung doses at this level, which were comparable to that of standard TBI using 50% lung transmission blocks. Our data indicated that 2000 cGy (RP2D) was clinically tolerable, with median doses delivered to the nontargeted healthy organs remaining below the corresponding doses delivered by TBI, leading to low incidence and grade of pulmonary, cardiac, GI, and hepatic toxicity.
Our study population was enriched with patients with high-risk disease characteristics including active disease (26%), high/very high disease risk index (62%), and unfavorable/adverse leukemia risk by cytogenetic and molecular markers (65%); however, 1-year relapse rate in the R2PD cohort was promising. EMD relapse is a potential concern with TMLI, particularly in patients with extramedullary disease before transplant. In our study, only 1 patient (3%), who did not have pre-HCT-EMD, was presented with EMD relapse. CRS rate in this trial was consistent with our institutional rate.32 Despite of the short follow-up period, short-term outcomes indicated that optimizing the conditioning regimen intensity could overcome the arguably higher rate of relapse described in earlier reports in this population.3,4
The escalated radiation dose to the BM from 1200 to 2000 cGy did not impact the recovery of hematopoiesis, confirmed by excellent engraftment rates. When we compared patients receiving lower and higher TMLI doses, immune cell subsets recovery was not significantly impacted by TMLI dose. Immune reconstitution and infection rates/severity were similar between our study and what has been reported in patients undergoing haploHCT with PTCy.33
Gut radiation dose has been linked to severe GI tissue destruction, resulting in more antigen presentation and higher incidence and severity of GVHD.34 In our study, with increasing TMLI doses, there was a subsequent increase in gut radiation dose. However, the incidence and severity of GvHD was comparable to published data in PBSC-haploHCT with PTCy platform.4,35 These comparable outcomes could be because of the sparing/lower dose of radiation to the gut during conditioning in our study. In fact, when we measured plasma levels of Reg3A as a marker of gut injury and GVHD, we could not find a correlation between levels of Reg3A and TMLI or gut dose.
Although increasing BM targeted radiation may increase therapeutic benefit, potential damage and delayed recovery of BM environment elements (eg, hematopoietic stem cells and vasculature) become essential as reported earlier.36 In this cohort, we evaluated BM cellularity at 1 year, most cases were found to be as expected after transplant and according to patient age, with increased vessel density when higher doses of TMLI were administered, which was of unknown significance/impact. This secondary evaluation of trephine biopsy sample, although limited, suggest normal BM recovery. Further studies are in process to measure details of BM recovery with a larger sample size. Moreover, immune reconstitution, infection rates, and severity were similar to what has been reported in this population using PTCy as GVHD prophylaxis.33 In addition, recovery of immune cellular subsets were not significantly impacted by dose escalation for TMLI when we compared patients receiving lower and higher TMLI doses.
In conclusion, our data indicate that TMLI-based conditioning regimen is safe and feasible in the setting of PBSC HaploHCT. We successfully delivered higher doses of radiation to the marrow without increasing the organ toxicity. In this high-risk patient population, promising results with low disease relapse was achieved without increasing rates of GVHD or TRM compared with standard preparative regimens. Given the small sample size of our phase 1 trial (n = 31), we did not have the power to investigate the benefit of our TMLI regimen according to the disease (AML vs ALL). However, based on confirmed safely profile of TMLI and short-term HCT outcomes of efficacy, we are currently accruing patients on a phase 2 study (#NCT04262843), using our RP2D of 2000 cGy in the setting HaploHCT with PTCy. Our phase 2 trial is designed to investigate the efficacy of TMLI treatment at 20 Gy in 2 separate arms of (1) patients with AML or MDS and (2) patients with ALL.
Last, the delivery of TMLI requires intensity-modulated radiation therapy technology, specifically arc or helical delivery of intensity-modulated radiation therapy. These technologies are installed in almost all hospital-based radiation oncology programs and are also in community-based practices. At City of Hope, we deliver TMLI using equipment from Accuray, Inc. (Tomotherapy) and Varian, Inc. (TrueBeam), which is available in the United States, Europe, Asia, Central America, and South America. Efforts are currently being made to extend the TMLI platform to other centers at national and international levels.
Acknowledgments
The authors thank City of Hope staff and nurses, as well as the patients and their families, without whom this work would not be possible; they also thank Golnaz Namdar (clinical research coordinator), Becky Barney, and Cynthia Slape (clinical research nurses) for their hard work.
This trial was funded by an intramural Chairs Discretionary grant and National Institutes of Health grant P30 CA033572 (Biostatistics Core).
Authorship
Contribution: M.M.A.M., J.P., J.Y.C.W., and J.R. contributed to study concept and design and data interpretation; N.-C.T. and J.P. were the study biostatisticians and did the statistical analysis; S.M. drafted the report and provided feedback to the report; and all authors contributed to critical revision of the manuscript for intellectual content and read and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Monzr M. Al Malki, Department of Hematology and Hematopoietic Cell Transplantation, City of Hope National Medical Center, 1500 E. Duarte Rd, Duarte, CA 91010; e-mail: malmalki@coh.org.
References
Author notes
J.Y.C.W. and J.R. contributed equally to this study.
For original data, please contact malmalki@coh.org. Individual participant data will not be shared.
The full-text version of this article contains a data supplement.